Acute Effects of a Single Bout of Walking on Affective Responses in Patients with Major Depressive Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Procedure
2.3. Conditions
2.4. Measurements Section
2.4.1. Basic Dimensional Affective Responses
2.4.2. Rotated Dimensional Affective Responses
2.4.3. Categorical Affective Responses
2.4.4. Physical Exertion
2.5. Statistical Analyses
3. Results
3.1. Preliminary Analysis
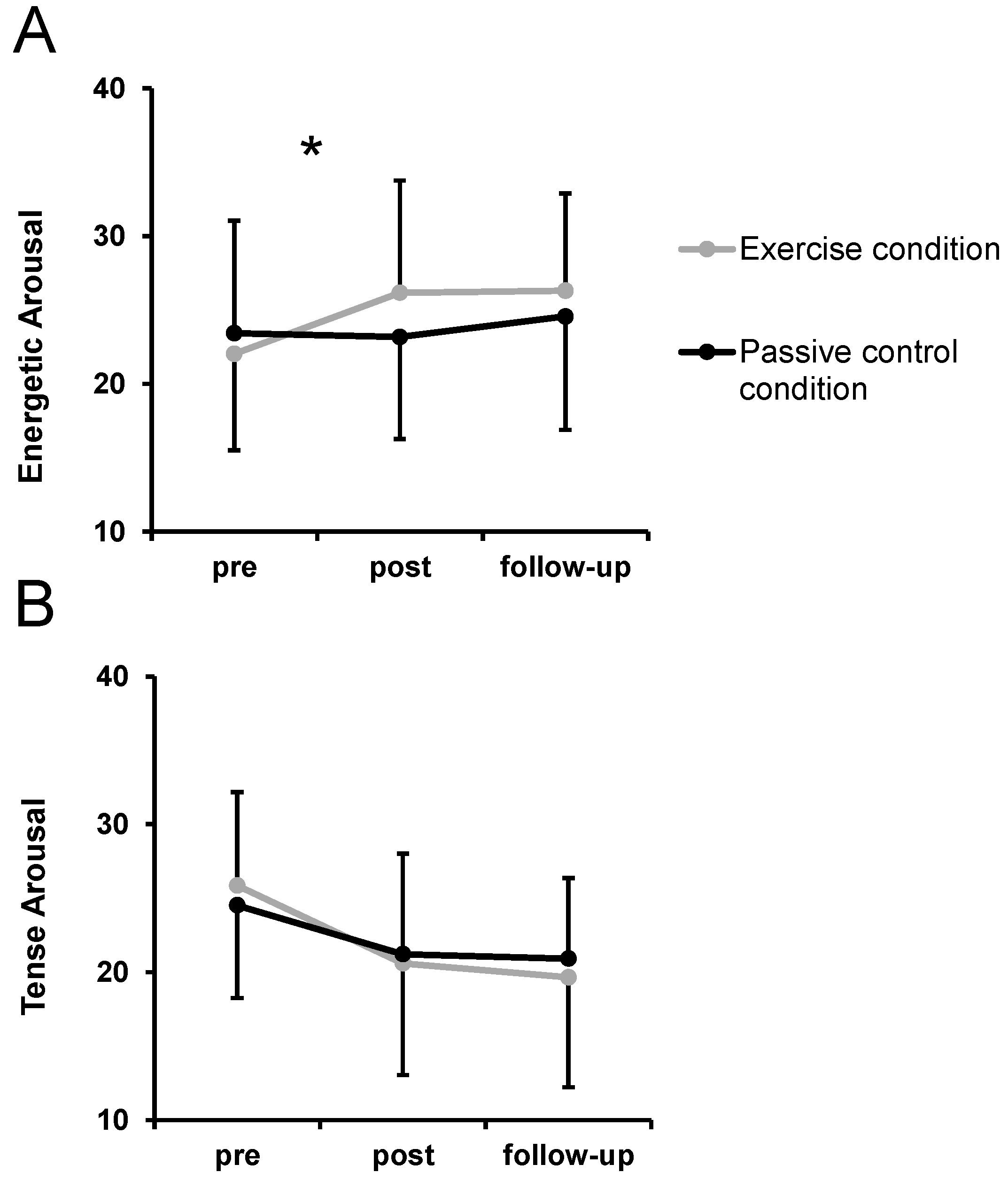
3.2. Basic Dimensional Affective Responses
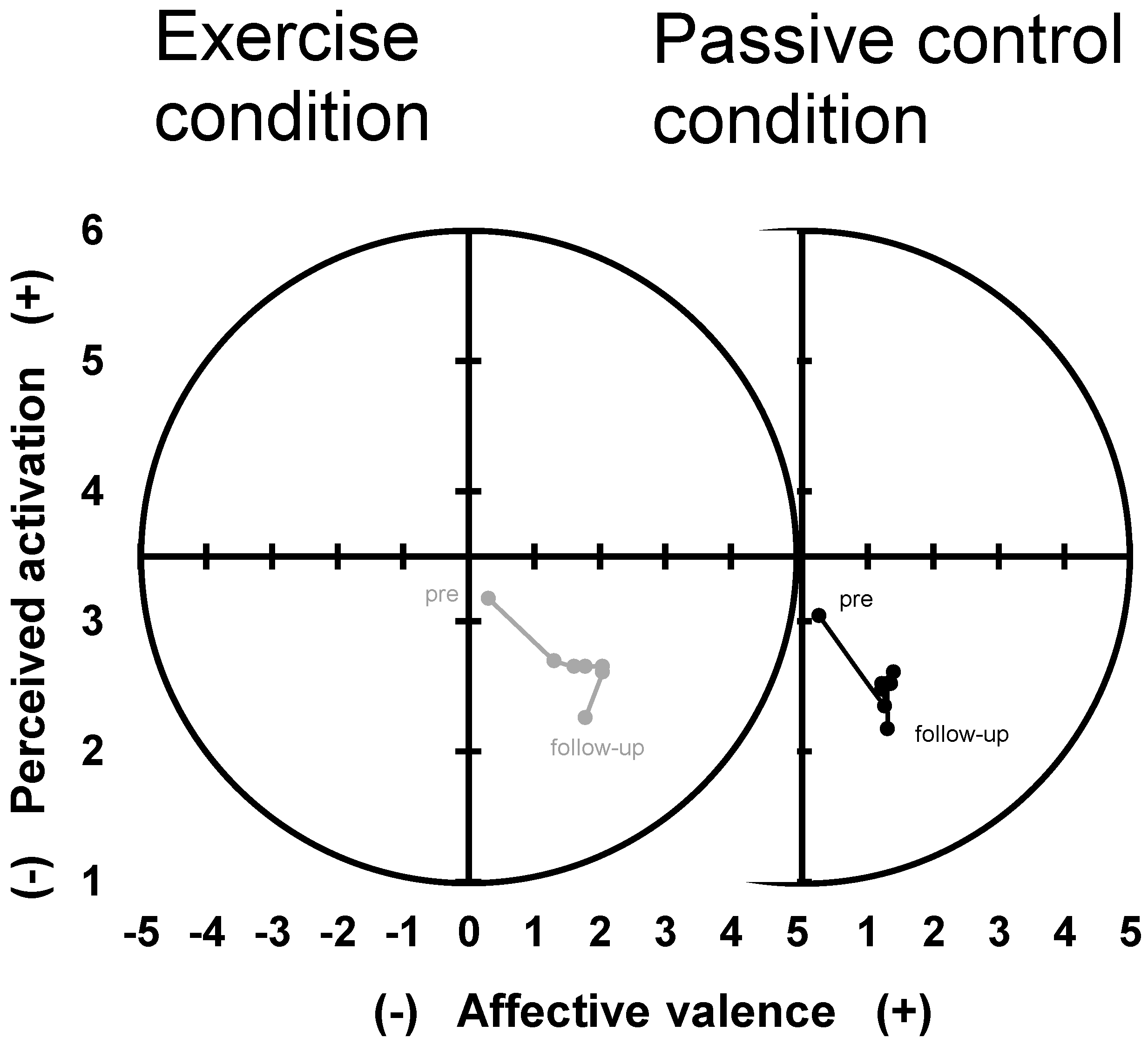
3.3. Rotated Dimensional Affective Responses
3.4. Categorical Affective Responses
4. Discussion
4.1. Affective Responses to Exercise Bouts in In-Patient Settings
4.2. Critical Reflection of Mixed Findings
4.3. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.T.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- National Institute for Health and Clinical Excellence. Depression: The Treatment and Management of Depression in Adult. Available online: www.nice.org.uk/CG90 (accessed on 14 April 2014).
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013, 9, CD004366. [Google Scholar] [CrossRef]
- Ekkekakis, P. Honey, I shrunk the pooled SMD! Guide to critical appraisal of systematic reviews and meta-analyses using the Cochrane review on exercise for depression as example. Ment. Health Phys. Act. 2015, 8, 21–36. [Google Scholar] [CrossRef]
- Kopp, M.; Fleischhacker, W.W.; Stürz, K.; Ruedl, G.; Kumnig, M.; Rumpold, G. Poor health behaviour and reduced quality of life of people treated with psychotropic drugs. Hum. Psychopharmacol. Clin. Exp. 2011, 26, 161–167. [Google Scholar] [CrossRef]
- Knubben, K.; Reischies, F.M.; Adli, M.; Schlattmann, P.; Bauer, M.; DiMeo, F. A randomised, controlled study on the effects of a short-term endurance training programme in patients with major depression. Br. J. Sports Med. 2007, 41, 29–33. [Google Scholar] [CrossRef]
- Martinsen, E.W.; Medhus, A.; Sandvik, L. Effects of aerobic exercise on depression: A controlled study. BMJ 1985, 291, 109. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vasconcelos-Moreno, M.P.; Borowsky, C.; Fleck, M.P. Exercise and severe depression: Preliminary results of an add-on study. J. Affect. Disord. 2011, 133, 615–618. [Google Scholar] [CrossRef]
- Frühauf, A.; Niedermeier, M.; Elliott, L.R.; Ledochowski, L.; Marksteiner, J.; Kopp, M. Acute effects of outdoor physical activity on affect and psychological well-being in depressed patients—A preliminary study. Ment. Health Phys. Act. 2016, 10, 4–9. [Google Scholar] [CrossRef]
- Frühauf, A.; Niedermeier, M.; Sevecke, K.; Haid-Stecher, N.; Albertini, C.; Richter, K.; Schipflinger, S.; Kopp, M. Affective responses to climbing exercises in children and adolescents during in-patient treatment for mental health disorders a pilot study on acute effects of different exercise interventions. Psychiatry Res. 2020, 291, 113245. [Google Scholar] [CrossRef]
- Bartholomew, J.B.; Morrison, D.; Ciccolo, J.T. Effects of Acute Exercise on Mood and Well-Being in Patients with Major Depressive Disorder. Med. Sci. Sports Exerc. 2005, 37, 2032–2037. [Google Scholar] [CrossRef]
- Stark, R.; Schöny, W.; Kopp, M. Auswirkungen einer moderaten Bewegungseinheit auf die psychische Befindlichkeit bei PatientInnen mit affektiven Störungen in stationär psychiatrischer Behandlung. Acute effects of a single bout of moderate exercise on psychological well-being in patients with affective disorder during hospital treatment. Neuropsychiatrie 2012, 26, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Gelhorn, H.L.; Sexton, C.C.; Classi, P.M. Patient preferences for treatment of major depressive disorder and the impact on health outcomes: A systematic review. Prim. Care Companion CNS Disord. 2011, 13, PCC.11r01161. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.; Robertson, A.; Jepson, R.; Maxwell, M. Walking for depression or depressive symptoms: A systematic review and meta-analysis. Ment. Health Phys. Act. 2012, 5, 66–75. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Petruzzello, S.J. Analysis of the affect measurement conundrum in exercise psychology: II. A conceptual and methodological critique of the Exercise-induced Feeling inventory. Psychol. Sport Exerc. 2001, 2, 1–26. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. Practical markers of the transition from aerobic to anaerobic metabolism during exercise: Rationale and a case for affect-based exercise prescription. Prev. Med. 2004, 38, 149–159. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Petruzzello, S.J. Affective, but hardly effective: A reply to Gauvin and Rejeski (2001). Psychol. Sport Exerc. 2004, 5, 135–152. [Google Scholar] [CrossRef]
- Hall, E.E.; Ekkekakis, P.; Petruzzello, S.J. The affective beneficence of vigorous exercise revisited. Br. J. Health Psychol. 2002, 7, 47–66. [Google Scholar] [CrossRef]
- Russell, J.A. A circumplex model of affect. J. Person. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Hall, E.E.; VanLanduyt, L.M.; Petruzzello, S.J. Walking in (Affective) Circles: Can Short Walks Enhance Affect? J. Behav. Med. 2000, 23, 245–275. [Google Scholar] [CrossRef]
- Thayer, R.E. Factor Analytic and Reliability Studies on the Activation-Deactivation Adjective Check List. Psychol. Rep. 1978, 42, 747–756. [Google Scholar] [CrossRef]
- Ekkekakis, P. The Measurement of Affect., Mood, and Emotion: A Guide for Health-Behavioral Research; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Sudeck, G.; Schmid, J.; Conzelmann, A. Exercise Experiences and Changes in Affective Attitude: Direct and Indirect Effects of In Situ Measurements of Experiences. Front. Psychol. 2016, 7, 900. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.; Russell, J.A.; Peterson, B.S. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 2005, 17, 715–734. [Google Scholar] [CrossRef]
- Tseng, A.; Bansal, R.; Liu, J.; Gerber, A.J.; Goh, S.; Posner, J.; Colibazzi, T.; Algermissen, M.; Chiang, I.-C.; Russell, J.A.; et al. Using the circumplex model of affect to study valence and arousal ratings of emotional faces by children and adults with autism spectrum disorders. J. Autism Dev. Disord. 2014, 44, 1332–1346. [Google Scholar] [CrossRef] [PubMed]
- Dilling, H.; Mombour, W.; Schmidt, M.H. Internationale Klassifikation Psychischer Störungen: ICD–10 Kapitel V Klinisch–Diagnostische Leitlinien; Huber: Bern, Switzerland, 2013. [Google Scholar]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can. J. Sport Sci. 1992, 17, 338–345. [Google Scholar] [PubMed]
- Ekkekakis, P.; Backhouse, S.H.; Gray, C.; Lind, E. Walking is popular among adults but is it pleasant? A framework for clarifying the link between walking and affect as illustrated in two studies. Psychol. Sport Exerc. 2008, 9, 246–264. [Google Scholar] [CrossRef]
- Kopp, M.; Steinlechner, M.; Ruedl, G.; Ledochowski, L.; Rumpold, G.; Taylor, A.H. Acute effects of brisk walking on affect and psychological well-being in individuals with type 2 diabetes. Diabetes Res. Clin. Pract. 2012, 95, 25–29. [Google Scholar] [CrossRef]
- Ledochowski, L.; Ruedl, G.; Taylor, A.H.; Kopp, M. Acute Effects of Brisk Walking on Sugary Snack Cravings in Overweight People, Affect and Responses to a Manipulated Stress Situation and to a Sugary Snack Cue: A Crossover Study. PLoS ONE 2015, 10, e0119278. [Google Scholar] [CrossRef]
- Hardy, C.J.; Rejeski, W.J. Not What, but How One Feels: The Measurement of Affect during Exercise. J. Sport Exerc. Psychol. 1989, 11, 304–317. [Google Scholar] [CrossRef]
- Svebak, S.; Murgatroyd, S. Metamotivational dominance: A multimethod validation of reversal theory constructs. J. Person. Soc. Psychol. 1985, 48, 107. [Google Scholar] [CrossRef]
- Maibach, M.; Niedermeier, M.; Sudeck, G.; Kopp, M. Erfassung unmittelbarer affektiver Reaktionen auf körperliche Aktivität. Zeitschr. Sportpsychol. 2020, 27, 4–12. [Google Scholar] [CrossRef]
- VanLanduyt, L.M.; Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. Throwing the Mountains into the Lakes: On the Perils of Nomothetic Conceptions of the Exercise-Affect Relationship. J. Sport Exerc. Psychol. 2000, 22, 208–234. [Google Scholar] [CrossRef]
- Imhof, M. Erprobung der deutschen version der adjektiv-checkliste nach thayer (1989) zur Erfassung der aktuellen aktiviertheit. Z. Differ. Diagn. Psychol. 1998, 19, 179–186. [Google Scholar]
- Abele-Brehm, A.; Brehm, W. Zur konzeptualisierung und messung von befindlichkeit: Die entwicklung der “befindlichkeitsskalen” (bfs). Diagnostica 1986, 32, 209–228. [Google Scholar]
- Ziemainz, H.; Peters, S. Die Messung aktuellen Wohlbefindens im Gesundheitssport. Sportwissenschaft 2010, 40, 174–181. [Google Scholar] [CrossRef]
- Niedermeier, M.; Grafetstätter, C.; Kopp, M.; Huber, D.; Mayr, M.; Pichler, C.; Hartl, A. The Role of Anthropogenic Elements in the Environment for Affective States and Cortisol Concentration in Mountain Hiking—A Crossover Trial. Int. J. Environ. Res. Public Health 2019, 16, 290. [Google Scholar] [CrossRef]
- Niedermeier, M.; Einwanger, J.; Hartl, A.; Kopp, M. Affective responses in mountain hiking—A randomized crossover trial focusing on differences between indoor and outdoor activity. PLoS ONE 2017, 12, e0177719. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.E.; Kates, A. Can the Affective Response to Exercise Predict Future Motives and Physical Activity Behavior? A Systematic Review of Published Evidence. Ann. Behav. Med. 2015, 49, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Raglin, J.S.; Morgan, W.P. Influence of vigorous exercise on mood state. Behav. Ther. 1985, 8, 179–183. [Google Scholar]
- Thompson Coon, J.; Boddy, K.; Stein, K.; Whear, R.; Barton, J.; Depledge, M.H. Does Participating in Physical Activity in Outdoor Natural Environments Have a Greater Effect on Physical and Mental Wellbeing than Physical Activity Indoors? A Systematic Review. Environ. Sci. Technol. 2011, 45, 1761–1772. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Lind, E. Exercise does not feel the same when you are overweight: The impact of self-selected and imposed intensity on affect and exertion. Int. J. Obes. 2006, 30, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Diener, E. Subjective well-being. Psychol. Bull. 1984, 95, 542–575. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, M.; Weiss, E.M.; Steidl-Müller, L.; Burtscher, M.; Kopp, M. Acute Effects of a Short Bout of Physical Activity on Cognitive Function in Sport Students. Int. J. Environ. Res. Public Health 2020, 17, 3678. [Google Scholar] [CrossRef]
- Niedermeier, M.; Grafetstätter, C.; Hartl, A.; Kopp, M. A Randomized Crossover Trial on Acute Stress-Related Physiological Responses to Mountain Hiking. Int. J. Environ. Res. Public Health 2017, 14, 905. [Google Scholar] [CrossRef]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef]
- Privitera, G.J.; Antonelli, D.E.; Szal, A.L. An Enjoyable Distraction During Exercise Augments the Positive Effects of Exercise on Mood. J. Sports Sci. Med. 2014, 13, 266–270. [Google Scholar] [PubMed]
- Ekkekakis, P.; Brand, R. Affective responses to and automatic affective valuations of physical activity: Fifty years of progress on the seminal question in exercise psychology. Psychol. Sport Exerc. 2019, 42, 130–137. [Google Scholar] [CrossRef]

| Categorical Affective States | Exercise Condition | Passive Control Condition | p-Value | η²p a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||||||||
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | Condition | Time | Interaction | Condition | Time | Interaction | |
| Activity | 11.2 | (3.7) | 13.1 | (3.8) | 12.1 | (3.9) | 11.3 | (3.5) | 0.271 | 0.329 | 0.017 | 0.05 | 0.04 | 0.23 |
| Elation | 11.0 | (3.6) | 14.1 | (4.3) | 12.6 | (4.0) | 13.1 | (4.1) | 0.420 | 0.006 | 0.053 | 0.03 | 0.30 | 0.16 |
| Calmness | 11.6 | (3.7) | 15.5 | (3.9) | 12.9 | (4.3) | 14.5 | (4.2) | 0.767 | <0.001 | 0.075 | 0.00 | 0.51 | 0.14 |
| Fatigue | 15.3 | (5.5) | 11.7 | (4.7) | 13.7 | (5.6) | 13.0 | (4.9) | 0.833 | 0.004 | 0.035 | 0.00 | 0.32 | 0.19 |
| Depression | 15.1 | (5.3) | 11.2 | (4.9) | 12.8 | (5.1) | 10.3 | (4.0) | 0.004 | <0.001 | 0.223 | 0.32 | 0.56 | 0.07 |
| Contemplation | 13.8 | (4.3) | 12.5 | (4.0) | 13.7 | (2.6) | 12.2 | (3.8) | 0.759 | 0.036 | 0.765 | 0.00 | 0.18 | 0.00 |
| Anger | 12.3 | (5.1) | 8.1 | (4.4) | 10.0 | (4.8) | 8.3 | (4.1) | 0.037 | <0.001 | 0.030 | 0.18 | 0.62 | 0.20 |
| Excitement | 14.5 | (4.9) | 10.1 | (4.3) | 12.2 | (5.0) | 9.6 | (4.6) | 0.043 | <0.001 | 0.028 | 0.17 | 0.46 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedermeier, M.; Ledochowski, L.; Leitner, H.; Zingerle, H.; Kopp, M. Acute Effects of a Single Bout of Walking on Affective Responses in Patients with Major Depressive Disorder. Int. J. Environ. Res. Public Health 2021, 18, 1524. https://doi.org/10.3390/ijerph18041524
Niedermeier M, Ledochowski L, Leitner H, Zingerle H, Kopp M. Acute Effects of a Single Bout of Walking on Affective Responses in Patients with Major Depressive Disorder. International Journal of Environmental Research and Public Health. 2021; 18(4):1524. https://doi.org/10.3390/ijerph18041524
Chicago/Turabian StyleNiedermeier, Martin, Larissa Ledochowski, Hartmann Leitner, Helmut Zingerle, and Martin Kopp. 2021. "Acute Effects of a Single Bout of Walking on Affective Responses in Patients with Major Depressive Disorder" International Journal of Environmental Research and Public Health 18, no. 4: 1524. https://doi.org/10.3390/ijerph18041524
APA StyleNiedermeier, M., Ledochowski, L., Leitner, H., Zingerle, H., & Kopp, M. (2021). Acute Effects of a Single Bout of Walking on Affective Responses in Patients with Major Depressive Disorder. International Journal of Environmental Research and Public Health, 18(4), 1524. https://doi.org/10.3390/ijerph18041524





