The Combined Effects of Fine Particulate Matter and Temperature on Preterm Birth in Seoul, 2010–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Birth Data
2.2. Exposure Assessment
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Margerison-Zilko, C.E.; Li, Y.; Luo, Z. Economic Conditions During Pregnancy and Adverse Birth Outcomes Among Singleton Live Births in the United States, 1990–2013. Am. J. Epidemiol. 2017, 186, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, Y.; Namiiro, F.; Nankunda, J.; Mugalu, J.; Vaucher, Y. Mortality among very low birth weight infants after hospital discharge in a low resource setting. BMC Pediatr. 2018, 18, 239. [Google Scholar] [CrossRef]
- Ko, H.S.; Wie, J.H.; Choi, S.K.; Park, I.Y.; Park, Y.G.; Shin, J.C. Multiple birth rates of Korea and fetal/neonatal/infant mortality in multiple gestation. PLoS ONE 2018, 13, e0202318. [Google Scholar] [CrossRef]
- Chang, L.S.; Cho, A.; Park, H.; Nam, K.; Kim, D.; Hong, J.H.; Song, C.K. Human-model hybrid Korean air quality forecasting system. J. Air Waste Manag. Assoc. 2016, 66, 896–911. [Google Scholar] [CrossRef]
- Gawda, A.; Majka, G.; Nowak, B.; Marcinkiewicz, J. Air pollution, oxidative stress, and exacerbation of autoimmune diseases. Cent. Eur. J. Immunol. 2017, 42, 305–312. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, H.; Yang, T.; Rui, W.; Liu, F.; Zhang, F.; Zhao, Y.; Ding, W. Direct effects of airborne PM2.5 exposure on macrophage polarizations. Biochim. Biophys. Acta 2016, 1860, 2835–2843. [Google Scholar] [CrossRef]
- Wei, T.; Tang, M. Biological effects of airborne fine particulate matter PM. Environ. Toxicol. Pharmacol. 2018, 60, 195–201. [Google Scholar] [CrossRef]
- Mikrut, M.; Regiel-Futyra, A.; Samek, L.; Macyk, W.; Stochel, G.; van Eldik, R. Generation of hydroxyl radicals and singlet oxygen by particulate matter and its inorganic components. Environ. Pollut. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Kim, D.; Chen, Z.; Zhou, L.F.; Huang, S.X. Air pollutants and early origins of respiratory diseases. Chronic. Dis. Transl. Med. 2018, 4, 75–94. [Google Scholar] [CrossRef]
- Gilman-Sachs, A.; Dambaeva, S.; Salazar Garcia, M.D.; Hussein, Y.; Kwak-Kim, J.; Beaman, K. Inflammation induced preterm labor and birth. J. Reprod. Immunol. 2018, 129, 52–58. [Google Scholar] [CrossRef]
- Hallman, M.; Haapalainen, A.; Huusko, J.M.; Karjalainen, M.K.; Zhang, G.; Muglia, L.J.; Rämet, M. Spontaneous premature birth as a target of genomic research. Pediatr. Res. 2018, 85, 422–431. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Liu, P.; Xia, B.; Zhu, Q.; Wang, X.; Song, Q.; Kan, H.; Zhang, Y. Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Sci. Total Environ. 2017, 607–608, 1103–1108. [Google Scholar] [CrossRef]
- Stower, H. Predicting preterm birth. Nat. Med. 2018, 24, 1088. [Google Scholar] [CrossRef]
- Hartig, T.; Catalano, R. Cold summer weather, constrained restoration, and very low birth weight in Sweden. Health Place 2013, 22, 68–74. [Google Scholar] [CrossRef]
- Arroyo, V.; Diza, J.; Carmona, R.; Ortiz, C.; Linares, C. Impact of air pollution and temperature on adverse birth outcomes: Madrid, 2001–2009. Environ. Pollut. 2016, 218, 1154–1161. [Google Scholar] [CrossRef]
- Han, C.; Kim, S.; Lim, Y.H.; Bae, H.J.; Hong, Y.C. Spatial and Temporal Trends of Number of Deaths Attributable to Ambient PM. J. Korean Med. Sci. 2018, 33, e193. [Google Scholar] [CrossRef]
- Leem, J.H.; Kim, S.T.; Kim, H.C. Public-health impact of outdoor air pollution for 2(nd) air pollution management policy in Seoul metropolitan area, Korea. Ann. Occup. Environ. Med. 2015, 27, 7. [Google Scholar] [CrossRef]
- Hakami, A.; Henze, D.K.; Seinfeld, J.H.; Singh, K.; Sandu, A.; Kim, S.; Byun, D.; Li, Q. The adjoint of CMAQ. Environ. Sci. Technol. 2007, 41, 7807–7817. [Google Scholar] [CrossRef]
- Suh, Y.J.; Kim, H.; Seo, J.H.; Park, H.; Kim, Y.J.; Hong, Y.C.; Ha, E.H. Different effects of PM10 exposure on preterm birth by gestational period estimated from time-dependent survival analyses. Int. Arch. Occup. Environ. Health 2009, 82, 613–621. [Google Scholar] [CrossRef]
- Mathew, S.; Mathur, D.; Chang, A.B.; McDonald, E.; Singh, G.R.; Nur, D.; Gerritsen, R. Examining the Effects of Ambient Temperature on Pre-Term Birth in Central Australia. Int. J. Environ. Res. Public Health 2017, 14, 147. [Google Scholar] [CrossRef]
- Appenheimer, M.M.; Evans, S.S. Temperature and adaptive immunity. Handb. Clin. Neurol. 2018, 156, 397–415. [Google Scholar] [PubMed]
- Assibey-Mensah, V.; Christopher Glantz, J.; Hopke, P.K.; Jusko, T.A.; Thevenet-Morrison, K.; Chalupa, D.; Rich, D.Q. Ambient wintertime particulate air pollution and hypertensive disorders of pregnancy in Monroe County, New York. Environ. Res. 2019, 168, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, D.; Naghshineh, E.; Sarsangi, A.; Zare Sakhvidi, M.J. Environmental extreme temperature and daily preterm birth in Sabzevar, Iran: A time-series analysis. Environ. Health Prev. Med. 2019, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Beghetti, I.; Martini, S.; Faldella, G.; Corvaglia, L. Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk. Oxid. Med. Cell Longev. 2018, 2018, 7397659. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Block, C.L.; Bolton, J.L.; Hanamsagar, R.; Tran, P.K. Beyond infection-Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2018, 299, 241–251. [Google Scholar] [CrossRef]
- Michikawa, T.; Morokuma, S.; Fukushima, K.; Kato, K.; Nitta, H.; Yamazaki, S. Maternal exposure to air pollutants during the first trimester and foetal growth in Japanese term infants. Environ. Pollut. 2017, 230, 387–393. [Google Scholar] [CrossRef]
- Majali-Martinez, A.; Barth, S.; Lang, U.; Desoye, G.; Cervar-Zivkovic, M. Temporal changes of the endothelin system in human cytotrophoblasts during the first trimester of pregnancy. Physiol. Res. 2018, 67, S247–S255. [Google Scholar] [CrossRef]
- Yadav, A.K.; Chaudhari, H.; Shah, P.K.; Madan, T. Expression and localization of collectins in feto-maternal tissues of human first trimester spontaneous abortion and abortion prone mouse model. Immunobiology 2016, 221, 260–268. [Google Scholar] [CrossRef]
- He, J.R.; Liu, Y.; Xia, X.Y.; Ma, W.J.; Lin, H.L.; Kan, H.D.; Lu, J.H.; Feng, Q.; Mo, W.J.; Wang, P.; et al. Ambient Temperature and the Risk of Preterm Birth in Guangzhou, China (2001–2011). Environ. Health Perspect. 2016, 124, 1100–1106. [Google Scholar] [CrossRef]
| Characteristic | Full-Term (GA ≥ 37 Weeks) | Premature (GA < 37 Weeks) | p | |
|---|---|---|---|---|
| n (%) or Mean ± SD | ||||
| Sex | Male | 283,774 (51.09) | 14,616 (56.56) | <0.0001 |
| Female | 271,625 (48.91) | 11,224 (43.44) | ||
| Birth weight (kg) | 3.28 ± 0.38 | 2.43 ± 0.63 | <0.0001 | |
| Gestational age at birth (weeks) | 39.03 ± 1.07 | 34.35 ± 2.57 | <0.0001 | |
| Parity of mother | 1st | 324,888 (58.61) | 14,488 (56.67) | <0.0001 |
| 2nd | 193,259 (34.86) | 9033 (35.33) | ||
| ≥3rd | 36,164 (6.52) | 2043 (7.99) | ||
| Season of birth | Spring | 142,981 (25.74) | 6300 (24.38) | <0.0001 |
| Summer | 135,175 (24.34) | 6740 (26.08) | ||
| Fall | 137,980 (24.84) | 6298 (24.37) | ||
| Winter | 139,263 (25.07) | 6502 (25.16) | ||
| Age of mother (years) | 31.90 ± 3.85 | 32.36 ± 4.22 | <0.0001 | |
| Education level of mother | None | 242 (0.04) | 17 (0.07) | <0.0001 |
| Elementary | 811 (0.15) | 61 (0.24) | ||
| Middle | 4646 (0.84) | 315 (1.23) | ||
| High | 96,978 (17.53) | 5636 (21.98) | ||
| University | 386,257 (69.82) | 16,756 (65.34) | ||
| Graduate | 64,266 (11.62) | 2860 (11.15) | ||
| Employment status of mother | No | 295,870 (53.27) | 14,393 (55.70) | <0.0001 |
| Yes | 259,529 (46.73) | 11,447 (44.30) | ||
| Age of father (years) | 34.38 ± 4.27 | 34.82 ± 4.57 | <0.0001 | |
| Education level of father | None | 187 (0.03) | 12 (0.05) | <0.0001 |
| Elementary | 788 (0.14) | 56 (0.22) | ||
| Middle | 4294 (0.78) | 252 (1.01) | ||
| High | 94,446 (17.20) | 5194 (20.75) | ||
| University | 367,083 (66.84) | 16,037 (64.08) | ||
| Graduate | 82,398 (15.00) | 3477 (13.89) | ||
| Employment status of father | No | 18,164 (3.27) | 905 (3.50) | 0.0408 |
| Yes | 537,235 (96.73) | 24,935 (96.50) | ||
| Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|
| Temperature (°C) | −14.5 | 3.4 | 14.5 | 22.8 | 31.8 |
| PM2.5 (µg/m3) | 3.5 | 18.5 | 27.2 | 37.9 | 200 |
| Stage | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
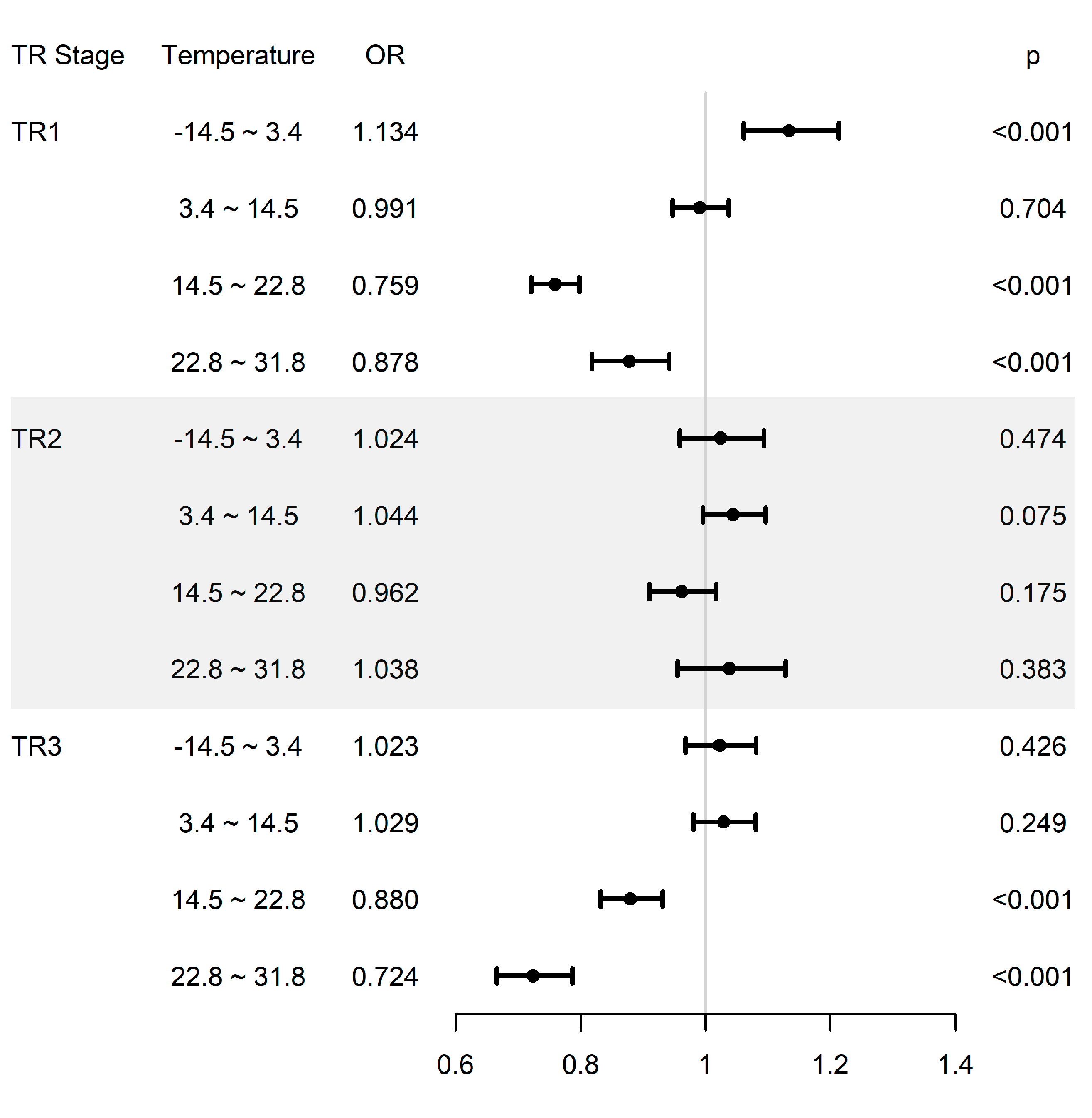
| TR1 | 0.965 | 0.920–1.012 | 0.143 | 1.134 | 1.061–1.213 | <0.001 |
| TR2 | 0.907 | 0.861–0.955 | <0.001 | 1.024 | 0.959–1.094 | 0.474 |
| TR3 | 0.947 | 0.902–0.993 | 0.025 | 1.023 | 0.968–1.081 | 0.426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwag, Y.; Kim, M.-h.; Ye, S.; Oh, J.; Yim, G.; Kim, Y.J.; Kim, E.; Lee, S.; Koh, T.K.; Ha, E. The Combined Effects of Fine Particulate Matter and Temperature on Preterm Birth in Seoul, 2010–2016. Int. J. Environ. Res. Public Health 2021, 18, 1463. https://doi.org/10.3390/ijerph18041463
Kwag Y, Kim M-h, Ye S, Oh J, Yim G, Kim YJ, Kim E, Lee S, Koh TK, Ha E. The Combined Effects of Fine Particulate Matter and Temperature on Preterm Birth in Seoul, 2010–2016. International Journal of Environmental Research and Public Health. 2021; 18(4):1463. https://doi.org/10.3390/ijerph18041463
Chicago/Turabian StyleKwag, Youngrin, Min-ho Kim, Shinhee Ye, Jongmin Oh, Gyeyoon Yim, Young Ju Kim, Eunji Kim, Semi Lee, Tai Kyung Koh, and Eunhee Ha. 2021. "The Combined Effects of Fine Particulate Matter and Temperature on Preterm Birth in Seoul, 2010–2016" International Journal of Environmental Research and Public Health 18, no. 4: 1463. https://doi.org/10.3390/ijerph18041463
APA StyleKwag, Y., Kim, M.-h., Ye, S., Oh, J., Yim, G., Kim, Y. J., Kim, E., Lee, S., Koh, T. K., & Ha, E. (2021). The Combined Effects of Fine Particulate Matter and Temperature on Preterm Birth in Seoul, 2010–2016. International Journal of Environmental Research and Public Health, 18(4), 1463. https://doi.org/10.3390/ijerph18041463






