Synthesis of Nano-Magnetite from Industrial Mill Chips for the Application of Boron Removal: Characterization and Adsorption Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Nano-Magnetite (Fe3O4) from Industrial Milled Chips
2.3. Characterization of Synthesised Nano-Magnetite
2.4. Batch Adsorption Study
2.5. Adsorption Isotherm
2.5.1. Langmuir Isotherm
2.5.2. Freundlich Isotherm
2.5.3. Re-Usability
2.6. Analytical Method
3. Results and Discussion
3.1. Characterization of Synthesized Nano-Magnetite
3.1.1. Functional Group Analysis
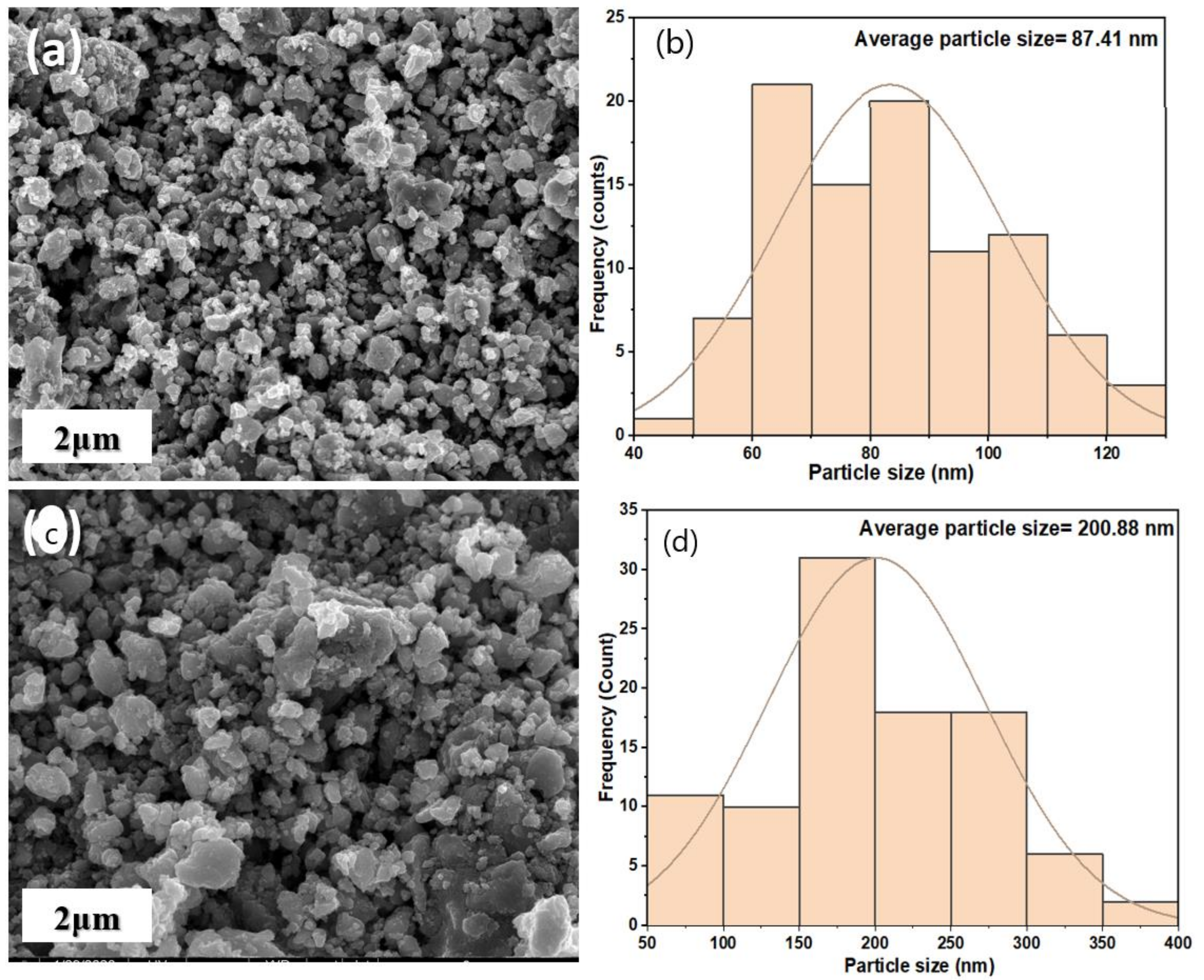
3.1.2. Morphological Analysis of Synthesized Nano-Magnetite
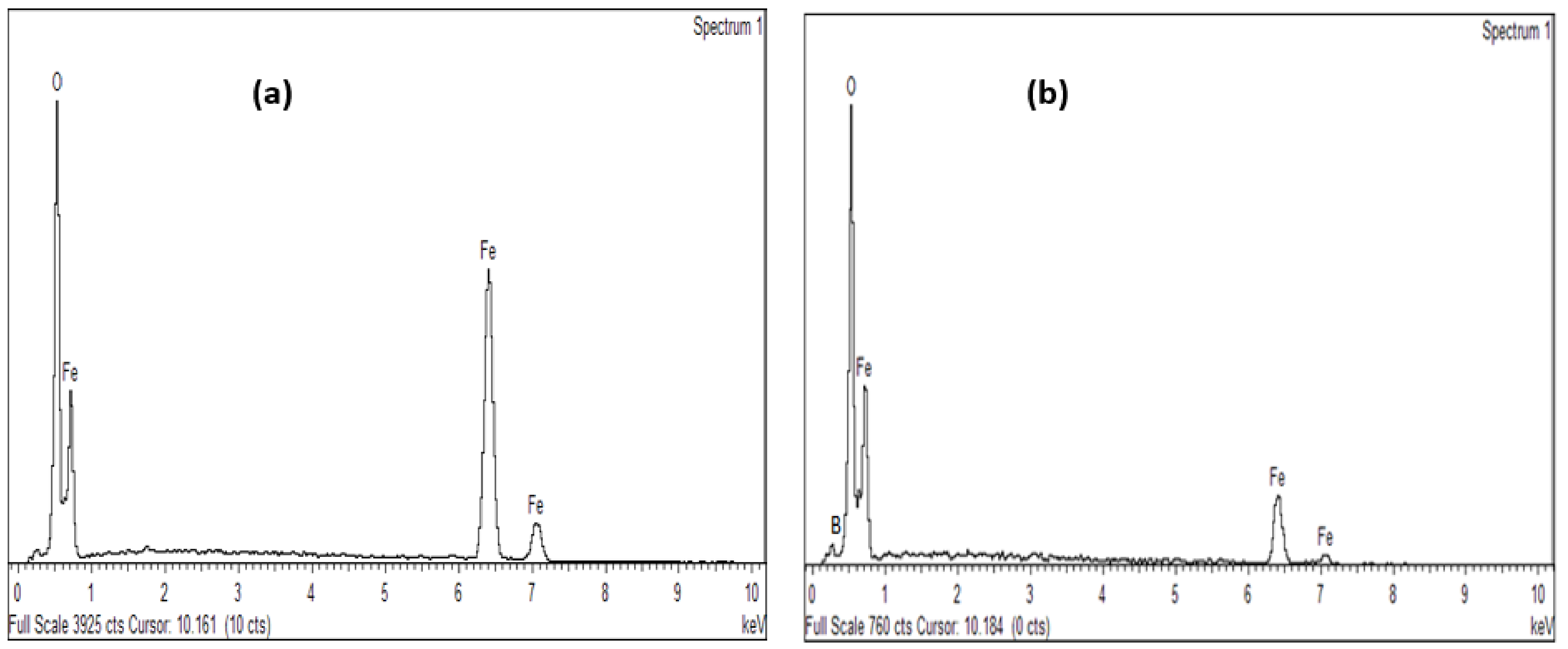
3.1.3. Elemental Analysis of Synthesized Magnetite
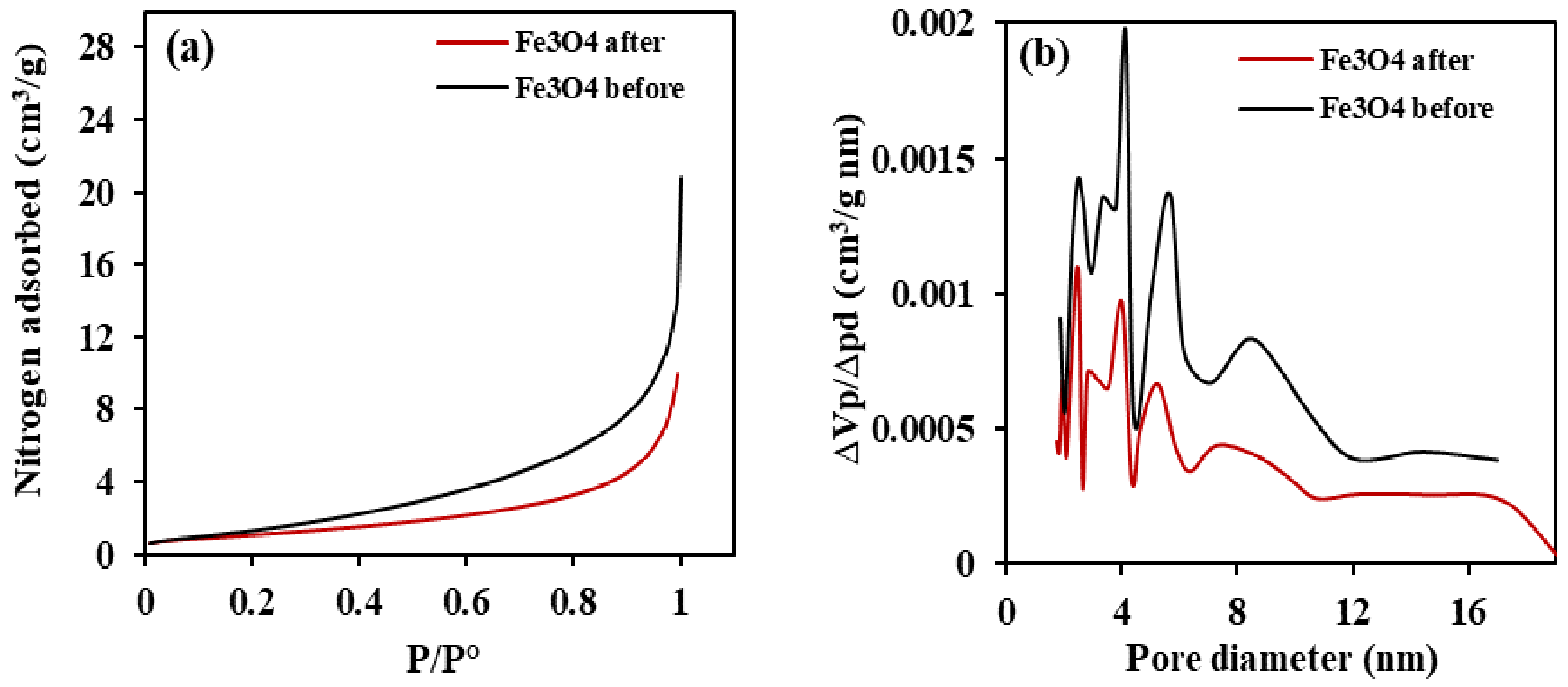
3.1.4. Assessment of Specific Surface Area of Synthesized Nano-Magnetite
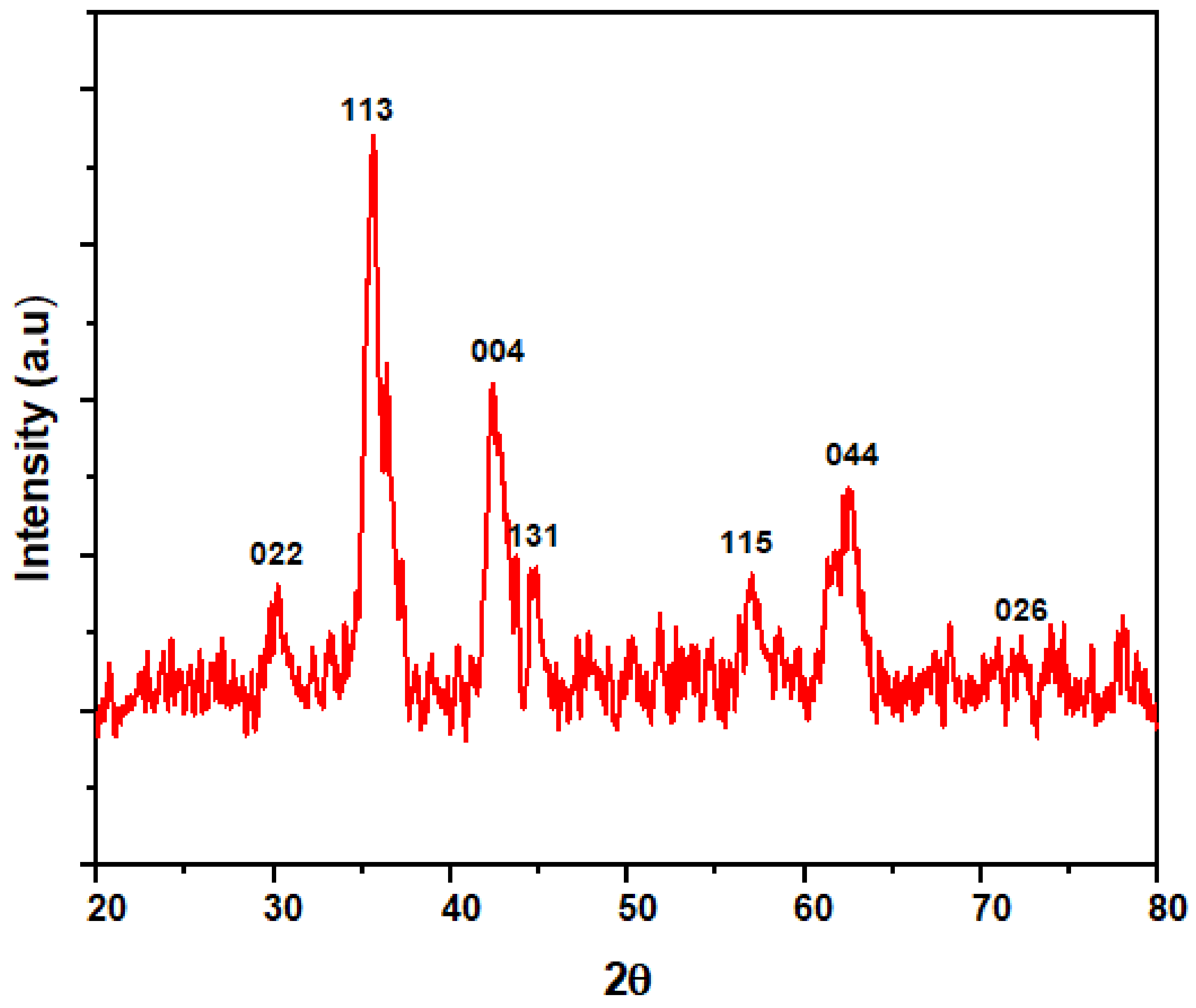
3.1.5. X-ray Diffraction Pattern
3.2. Batch Adsorption Analyses
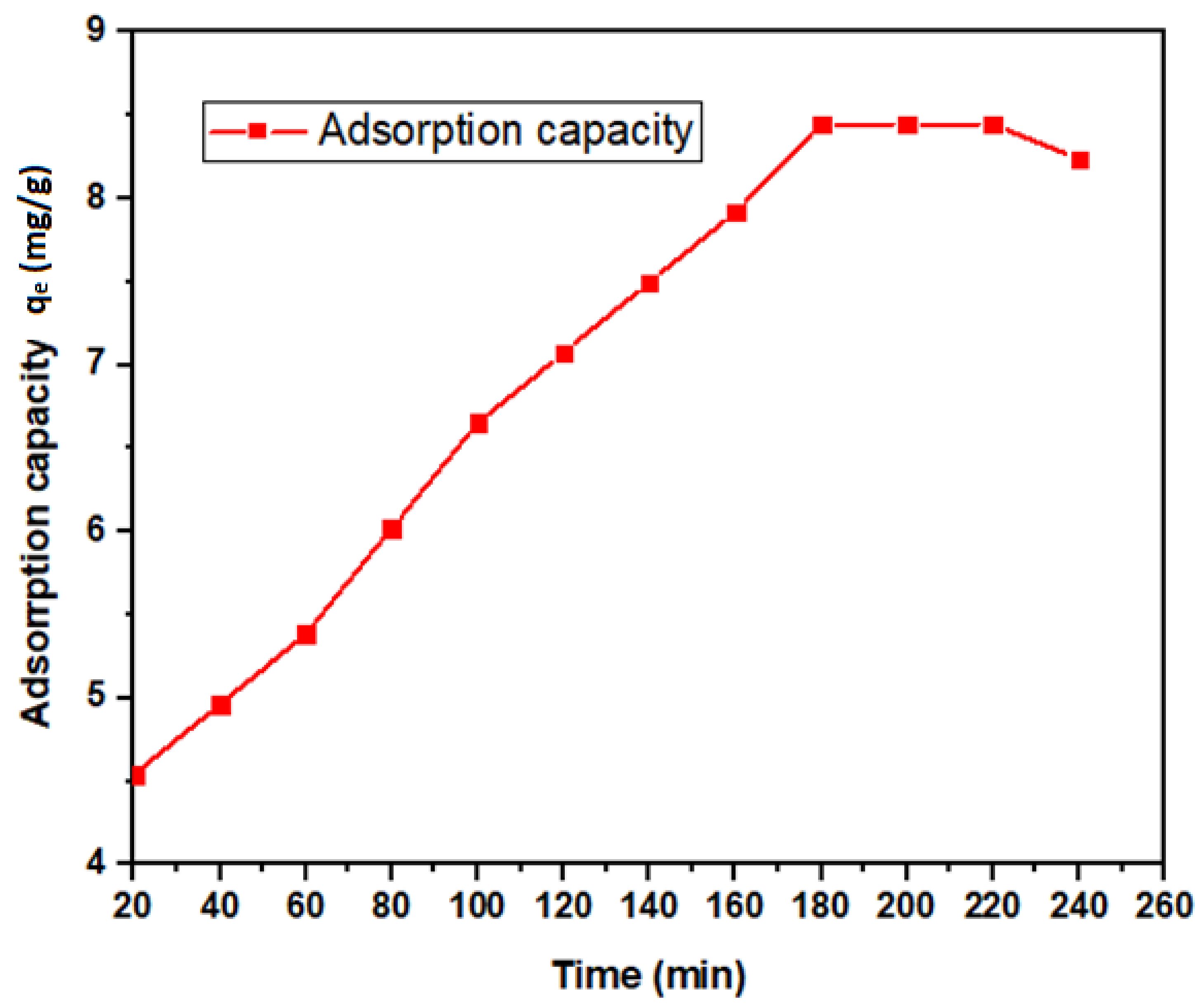
3.2.1. Contact Time Dependency
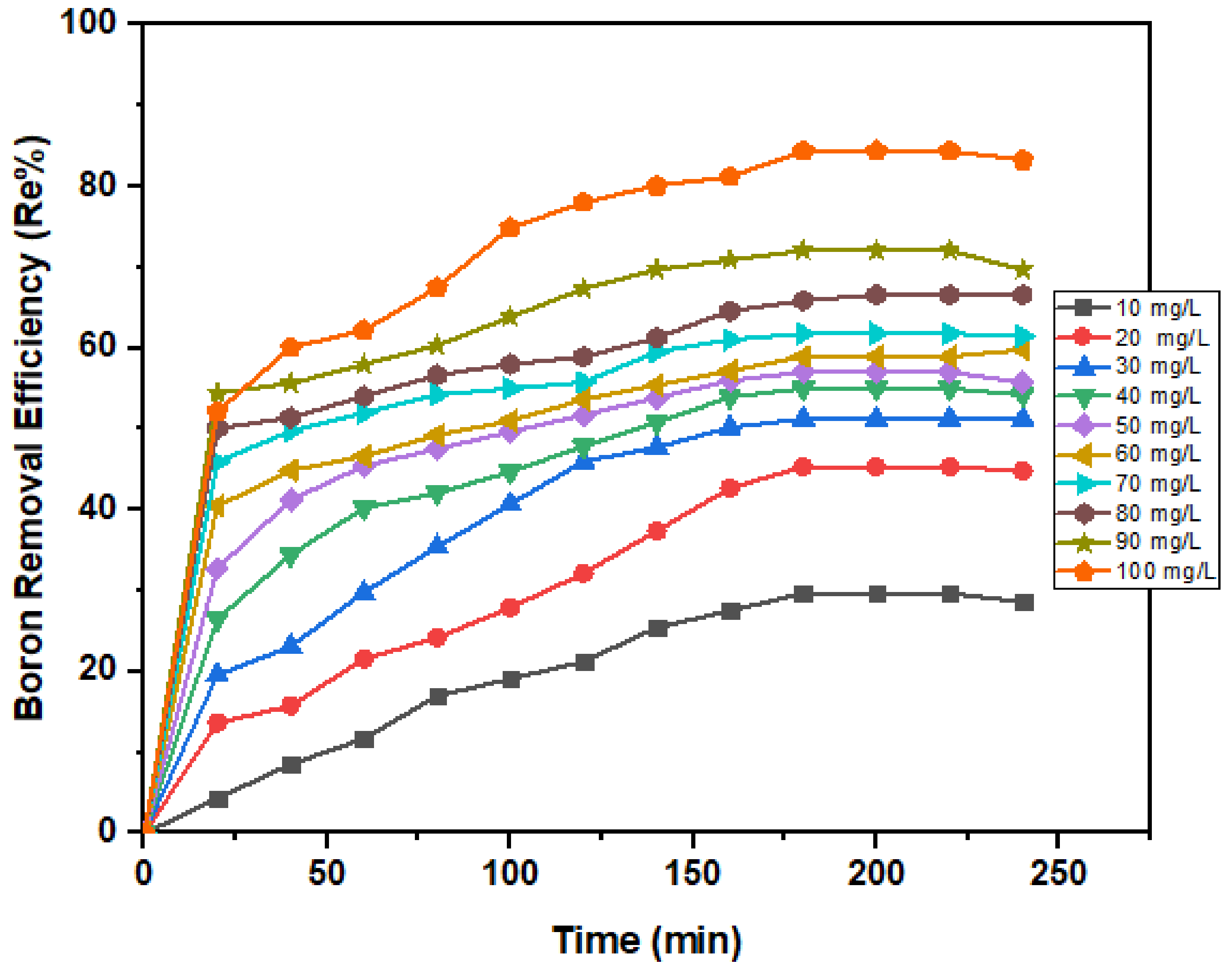
3.2.2. Initial Concentration Dependency
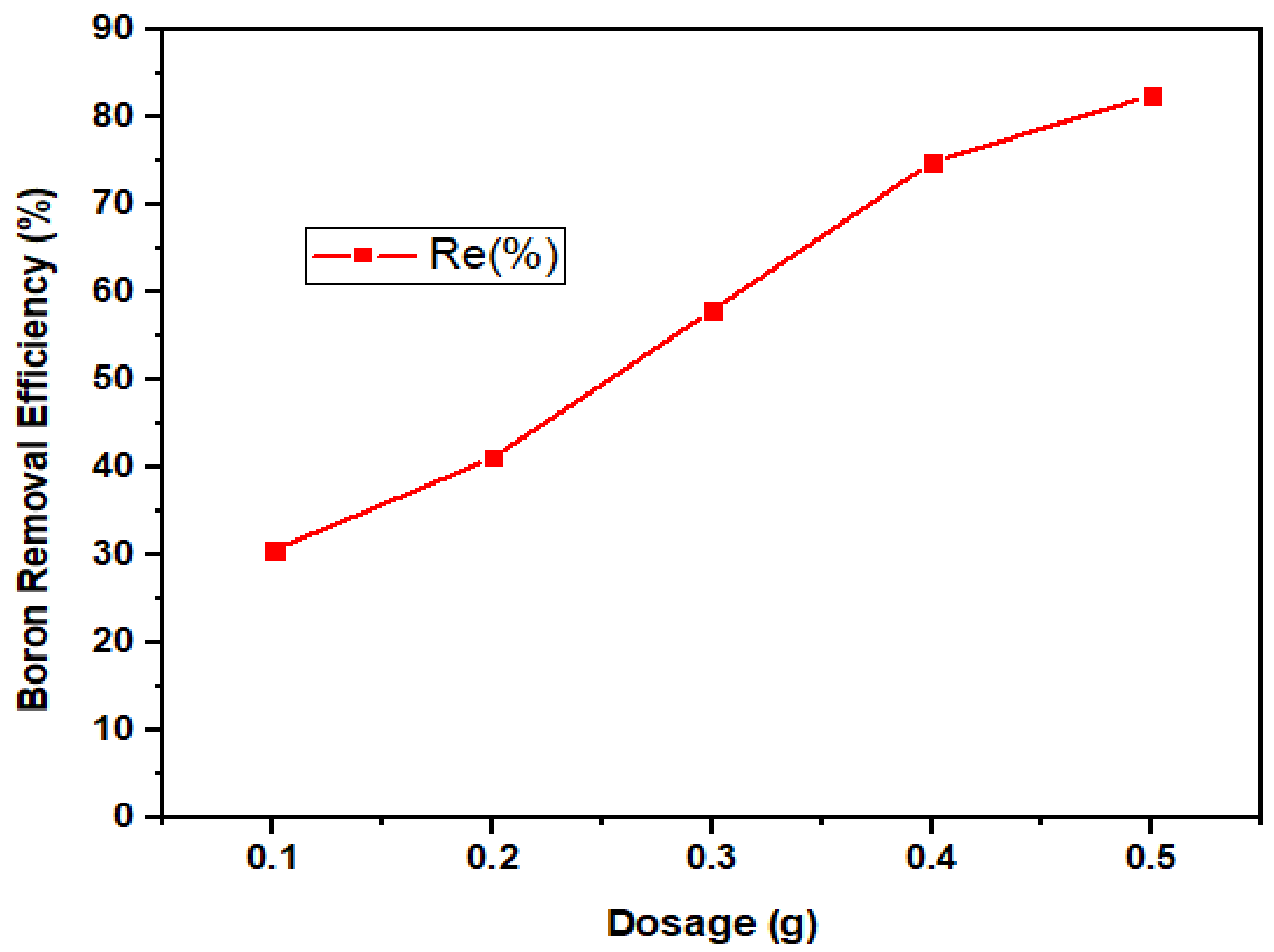
3.2.3. Dosage Dependency
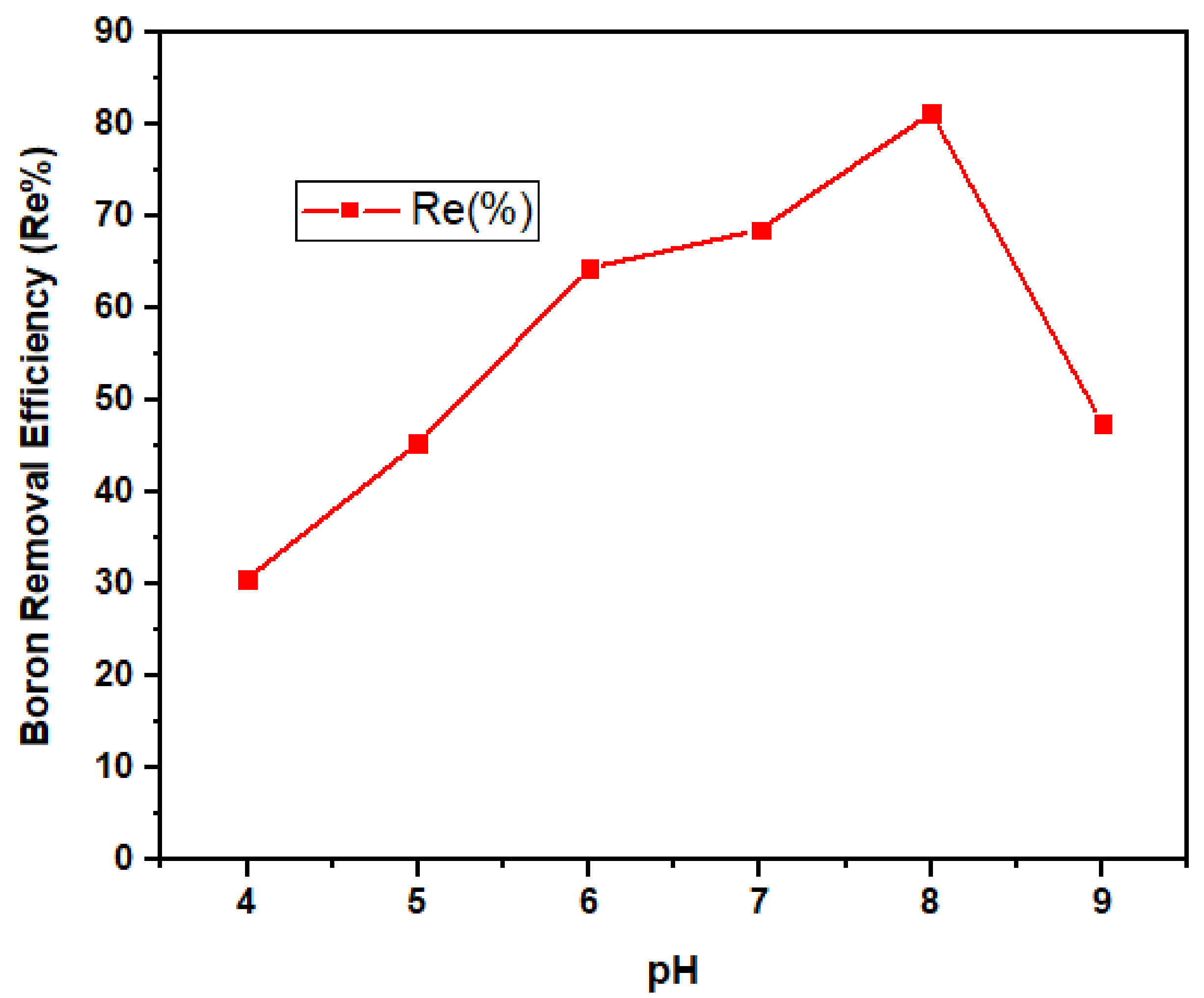
3.2.4. pH Dependency
3.3. Regeneration Study
3.4. Boron Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Che Jamin, N.; Mahmood, N.Z. Scheduled Waste Management in Malaysia: An Overview. Adv. Mater. Res. 2015, 1113, 841–846. [Google Scholar] [CrossRef]
- Lotti, P.; Gatta, G.D.; Demitri, N.; Guastella, G.; Rizzato, S.; Ortenzi, M.A.; Magrini, F.; Comboni, D.; Guastoni, A.; Fernandez-Diaz, M.T. Crystal chemistry and temperature behavior of the natural hydrous borate colemanite, a mineral commodity of boron. Phys. Chem. Miner. 2018, 45, 405–422. [Google Scholar] [CrossRef]
- Liu, H.; Qing, B.; Ye, X.; Li, Q. Boron adsorption by composite magnetic particles. Chem. Eng. J. 2009, 151, 235–240. [Google Scholar] [CrossRef]
- Mar, M.; Fuente, D.; Mufioz, E. Boron removal from industrial wastewaters by ion exchange: An analytical control parameter. Desalination 2005, 181, 207–216. [Google Scholar] [CrossRef]
- Taylor, P. Desalination and Water Treatment The removal of boron from the aquatic environment—State of the art. Desalin. Water Treat. 2015, 37–41. [Google Scholar] [CrossRef]
- Hossini, H.; Rezaee, A.; Ayati, B.; Mahvi, A.H. Optimizing ammonia volatilization by air stripping from aquatic solutions using response surface methodology (RSM). Desalin. Water Treat. 2016, 57, 11765–11772. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Ruya, K.; Sahin, Y.; Ozlem, S.; Refiye, Y.; Aysen, Y.; Fikrettin, S. Boron concentrations in tap water in many cities of Turkey. Toxicol. Environ. Chem. 2020. [Google Scholar] [CrossRef]
- Turkez, H.; Geyikoglu, F.; Tatar, A.; Keles, M.S. Experimental and Toxicologic Pathology the effects of some boron compounds against heavy metal toxicity in human blood. Exp. Toxicol. Pathol. 2012, 64, 93–101. [Google Scholar] [CrossRef]
- Patrick, H.; Hening, H. Does only a boron play a structural role in the growing tissues of higher plants. Plant Soil 1997, 196, 211–215. [Google Scholar]
- Fujita, Y.; Hata, T.; Nakamaru, M.; Iyo, T. A study of boron adsorption onto activated sludge. Bioresour. Technol. 2005, 96, 1350–1356. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, P.; Cao, J.; Zhang, Y.; Cheng, B.; Meng, J. A novel mixed matrix membrane allowing for flow-through removal of boron. Chem. Eng. J. 2017, 308, 557–567. [Google Scholar] [CrossRef]
- Ilker Kıpcak, M.Ö. Removal of boron from aqueous solution using calcined magnesite tailing. Chem. Eng. J. 2012, 190, 68–74. [Google Scholar] [CrossRef]
- Yoji Taguchi, Y.; Takahashi, A.; Tomoko Iwakura, S.B.T.Y. Coagulative Removal Precipitation of Boron from and the Adsorption Wastewater. J. Environ. Chem. 2001, 11, 557–565. [Google Scholar] [CrossRef]
- Bryjak, M.; Wolska, J.; Soroko, I.; Kabay, N. Adsorption-membrane filtration process in boron removal from first stage seawater RO permeate. Desalination 2009, 241, 127–132. [Google Scholar] [CrossRef]
- González, A.G.; Pokrovsky, O.S.; Santana-Casiano, J.M.; González-Dávila, M. Bioadsorption of heavy metals. Prospect. Challenges Algal Biotechnol. 2017, 4, 233–255. [Google Scholar] [CrossRef]
- Bouguerra, W.; Mnif, A.; Hamrouni, B.; Dhahbi, M. Boron removal by adsorption onto activated alumina and by reverse osmosis. Desalination 2008, 223, 31–37. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. Afr. J. Range Forage Sci. 2010, 22, 73–74. [Google Scholar] [CrossRef]
- Okay, O.; Ggs, H.; Soner, E.; Scientific, M.; Kocaeli, G. Boron pollution in the Simav River, Turkey and various methods of boron removal. Water Res 1985, 857–862. [Google Scholar] [CrossRef]
- Goldberg, S. Reanalysis of Boron Adsorption on Soils and Soil Minerals Using the Constant Capacitance Model. Sci. Soc. Am. J. 1999, 63, 823–829. [Google Scholar] [CrossRef]
- Polat, H.; Vengoshb, A.; Pankratovb, I.; Polat, M. A new methodology for removal of boron fro by coal and fly ash. Desalination 2004, 164, 173–188. [Google Scholar] [CrossRef]
- Boncukcuoglu, R.; Yllmaza, A.E.; Kocakerimb, M.M.; Copurb, M. An empirical model for kinetics of boron removal from boron- containing wastewaters by ion exchange in a batch reactor. Desalination 2004, 160, 159–166. [Google Scholar] [CrossRef]
- Okafor, P.C.; Okon, P.U.; Daniel, E.F.; Ebenso, E.E. Adsorption capacity of coconut (Cocos nucifera L.) shell for lead, copper, cadmium and arsenic from aqueous solutions. Int. J. Electrochem. Sci. 2012, 7, 12354–12369. [Google Scholar]
- Abdulsalam, M.; Man, H.C.; Idris, A.I.; Abidin, Z.Z.; Yunos, K.F. The pertinence of microwave irradiated coconut shell bio-sorbent for wastewater decolourization: Structural morphology and adsorption optimization using the response surface method (RSM). Int. J. Environ. Res. Public Health 2018, 15, 2200. [Google Scholar] [CrossRef]
- Kluczka, J.; Korolewicz, T.; Zołotajkin, M.; Adamek, J. Boron removal from water and wastewater using new polystyrene-based resin grafted with glycidol. Water Resour. Ind. 2015, 11, 46–57. [Google Scholar] [CrossRef]
- Kluczka, J.; Tórz, A.; Łącka, D.; Kazek, A. Boron Removal by Adsorption on Cobalt (II) Doped Chitosan Bio- composite. J. Polym. Environ. 2017. [Google Scholar] [CrossRef]
- Kluczka, J.; Pudło, W.; Krukiewicz, K. Boron adsorption removal by commercial and modified activated carbons. Chem. Eng. Res. Des. 2019, 147, 30–42. [Google Scholar] [CrossRef]
- Man, H.C.; Chin, W.H.; Zadeh, M.R.; Yusof, M.R.M. Adsorption potential of unmodified rice husk for boron removal. BioResources 2012, 7, 3810–3822. [Google Scholar]
- Babiker, E.; Al-ghouti, M.A.; Zouari, N.; Mckay, G.P.T. Removal of boron from water using adsorbents derived from waste tire rubber. Biochem. Pharmacol. 2019, 7, 102948. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Zhang, L.; Zheng, S.; Zhang, Y. Ultrasonics Sonochemistry Enhanced boron adsorption onto synthesized MgO nanosheets by ultrasonic method. Ultrason. Sonochem. 2017, 34, 938–946. [Google Scholar] [CrossRef]
- Kashif, M.; Phearom, S.; Choi, Y. Chemosphere Synthesis of magnetite from raw mill scale and its application for arsenate adsorption from contaminated water. Chemosphere 2018, 203, 90–95. [Google Scholar] [CrossRef]
- Chun, H.C.Y. A Study on the Mill Scale Pretreatment and Magnetite Production for Phosphate Adsorption. J. Korean Soc. Environ. Eng. 2015, 37, 246–252. [Google Scholar] [CrossRef]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe 3O 4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; San, S.; Young, P.; Choi, G. Adsorption of arsenic (V) on magnetite-enriched particles separated from the mill scale. Environ. Earth Sci. 2019, 78, 1–11. [Google Scholar] [CrossRef]
- Kumkrong, P.; LeBlanc, K.L.; Mercier, P.H.J.; Mester, Z. Selenium analysis in waters. Part 1: Regulations and standard methods. Sci. Total Environ. 2018, 640–641, 1611–1634. [Google Scholar] [CrossRef]
- Nazri, N.A.A.; Azis, R.S.; Mustaffa, M.S.; Shaari, A.H.; Ismail, I.; Man, H.C.; Saiden, N.M.; Abdullah, N.H. Magnetite nanoparticles (MNPs) used as cadmium metal removal from the aqueous solution from mill scales waste sources. Sains Malays. 2020, 49, 847–858. [Google Scholar] [CrossRef]
- Velásquez, A.A.; Marín, C.C.; Urquijo, J.P. Synthesis and characterization of magnetite-maghemite nanoparticles obtained by the high-energy ball milling method. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Azis, R.S.; Hashim, M.; Yahya, N.; Saiden, N.M. A Study of Sintering Tempratures Variation on Microstructure Developement of Strontium Hexaferrite Millscale-Derived. Pakistan J. Appl. Sci. 2002, 2. [Google Scholar] [CrossRef][Green Version]
- Öztürk, N.; Kavak, D. Adsorption of Boron from Aqueous Solutions by Sepiolite using Full Factorial Design: I. Batch Studies. In Proceedings of the Uluslararası Bor Sempozyumu, Eskisehir, Turkey, 23–25 September 2004; pp. 23–25. [Google Scholar]
- Elmorsi, T.M. Equilibrium Isotherms and Kinetic Studies of Removal of Methylene Blue Dye by Adsorption onto Miswak Leaves as a Natural Adsorbent. J. Environ. Prot. 2011, 02, 817–827. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Da̧browski, A. Adsorption-From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Ayawei, N.; Angaye, S.S.; Wankasi, D.; Dikio, E.D. Synthesis, Characterization and Application of Mg/Al Layered Double Hydroxide for the Degradation of Congo Red in Aqueous Solution. Open J. Phys. Chem. 2015, 5, 56–70. [Google Scholar] [CrossRef]
- Ayawei, N.; Ekubo, A.T.; Wankasi, D.; Dikio, E.D. Adsorption of congo red by Ni/Al-CO3: Equilibrium, thermodynamic and kinetic studies. Orient. J. Chem. 2015, 31, 1307–1318. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ali, M.; Choong, T.S.Y. Kinetic and isotherm studies for lead adsorption from aqueous phase on carbon coated monolith. Chem. Eng. J. 2013, 217, 248–255. [Google Scholar] [CrossRef]
- Choong, T.S.Y.; Chuah, T.G.; Yunus, R.; Yap, Y.H.T. Adsorption of ˇ-carotene onto mesoporous carbon coated monolith in isopropyl alcohol and n-hexane solution: Equilibrium and thermodynamic study. Chem. Eng. J. 2010, 164, 178–182. [Google Scholar] [CrossRef]
- Song, W.; Gao, B.; Xu, X.; Xing, L.; Han, S.; Duan, P.; Song, W.; Jia, R. Bioresource Technology Adsorption—Desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater. Bioresour. Technol. 2016, 210, 123–130. [Google Scholar] [CrossRef]
- Ngomsik, A.F.; Bee, A.; Draye, M.; Cote, G.; Cabuil, V. Magnetic nano- and microparticles for metal removal and environmental applications: A review. Comptes Rendus Chim. 2005, 8, 963–970. [Google Scholar] [CrossRef]
- Taylor, P.; Demetriou, A.; Pashalidis, I.; Demetriou, A.; Pashalidis, I. Desalination and Water Treatment Adsorption of boron on iron-oxide in aqueous solutions. Taylor Fr. Group 2012, 37–41. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, W.; Wang, H.; Meng, X.; Zhang, L. A novel polyamine-type starch/glycidyl methacrylate copolymer for adsorption of Pb (II), Cu (II), Cd (II) and Cr (III) ions from aqueous solutions. R. Soc. Open Sci. 2018, 5, 180281. [Google Scholar] [CrossRef]
- Yu, D.; Morisada, S.; Kawakita, H.; Ohto, K.; Inoue, K. Selective Cesium Adsorptive Removal on Using Crosslinked Tea Leaves. Processes 2019, 7, 412. [Google Scholar] [CrossRef]
- Huang, X.; Chen, T.; Zou, X.; Zhu, M.; Chen, D.; Pan, M. The adsorption of Cd(II) on manganese oxide investigated by batch and modeling techniques. Int. J. Environ. Res. Public Health 2017, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Shirivastava, D.S.S.V.S. Adsorptive removal of heavy metals by magnetic nanoadsorbent: An equilibrium and thermodynamic study. Appl. Nanosci. 2015, 5, 927–935. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Seo, D.J.; Song, H.J.; Park, S.S.; Ha, C.S. Magnetic mesoporous silica hybrid nanoparticles for highly selective boron adsorption. J. Mater. Chem. A 2013, 1, 12485–12496. [Google Scholar] [CrossRef]
- Liu, H.; Ye, X.; Li, Q.; Kim, T.; Qing, B.; Guo, M.; Ge, F.; Wu, Z.; Lee, K. Colloids and Surfaces A: Physicochemical and Engineering Aspects Boron adsorption using a new boron-selective hybrid gel and the commercial resin D564. Colloids Surfaces A Physicochem. Eng. Asp. J. 2009, 341, 118–126. [Google Scholar] [CrossRef]
- Karahan, S.; Yurdakoç, M.; Yurdakoç, K. Removal of boron from aqueous solution by clays and modified clays. J. Colloid Interface Sci. 2006, 293, 36–42. [Google Scholar] [CrossRef]
- Pistorius, C.W.F.T. Potential Function of Boric Acid. J. Chem. Phys. 1970, 1454, 10–14. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman, C.U.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B. Bin Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Chang, Y.; Yang, J.; Singh, J.; Choi, E.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 2016. [Google Scholar] [CrossRef]
- Nishi, Y.; Inagaki, M. Gas Adsorption/Desorption Isotherm for Pore Structure Characterization; Tsinghua University Press Limited: Cambridge, MA, USA, 2016; ISBN 9780128052563. [Google Scholar]
- Sari, A.; Tuzen, M. Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 160, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fu, Y.; Xu, X.; Huang, Y.; Hu, J.; Chen, Q.; Wu, Y. Preparation of new diatomite—chitosan composite materials and their adsorption properties and mechanism of Hg (II). R. Soc. Open Sci. 2017, 4, 170829. [Google Scholar]
- Tuzen, M.; Sari, A. Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies. Chem. Eng. J. 2010, 158, 200–206. [Google Scholar] [CrossRef]
- Hao, Y.M.; Man, C.; Hu, Z.B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef]
- Taylor, P.; Köse, T.E.; Demiral, H. Desalination and Water Treatment Adsorption of boron from aqueous solutions using activated carbon prepared from olive bagasse Adsorption of boron from aqueous solutions using activated carbon prepared from olive bagasse. Desalin. Water Treat. 2012, 37–41. [Google Scholar] [CrossRef]
- Feng, B.; Shen, W.; Shi, L.; Qu, S. Adsorption of hexavalent chromium by porous carbon from aqueous solution. R. Soc. Open Sci. 2018, 5, 171662. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, F.; Pang, Z.; Yang, G. Efficient and selective adsorption of multi-metal ions using sulfonated cellulose as adsorbent. Carbohydr. Polym. 2016, 151, 230–236. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Effects of humic acid and suspended solids on the removal of heavy metals from water by adsorption onto granular activated carbon. Int. J. Environ. Res. Public Health 2015, 12, 10475–10489. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Silas, K.; Azlina, W.; Ab, W.; Ghani, K.; Shean, T.; Choong, Y.; Rashid, U. Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NOx removal from flue gas. Fuel Process. Technol. 2018, 180, 155–165. [Google Scholar] [CrossRef]
- Zohdi, N.; Mahdavi, F.; Abdullah, L.C.; Choong, T.S.Y.Y. Removal of boron from aqueous solution using magnetic carbon nanotube improved with tartaric acid. J. Environ. Heal. Sci. Eng. 2014, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Q.; Lyu, J.; Bai, P.; Guo, X. Boron removal and reclamation by magnetic magnetite (Fe3O4) nanoparticle: An adsorption and isotopic separation study. Sep. Purif. Technol. 2020, 231, 115930. [Google Scholar] [CrossRef]
- Mercado-Borrayo, B.; Schouwenaars, R.; Litter, M.; Ramirez-Zamora, R. Adsorption of boron by metallurgical slag and iron nanoparticles. Adsorpt. Sci. Technol. 2014, 32, 117–123. [Google Scholar] [CrossRef]




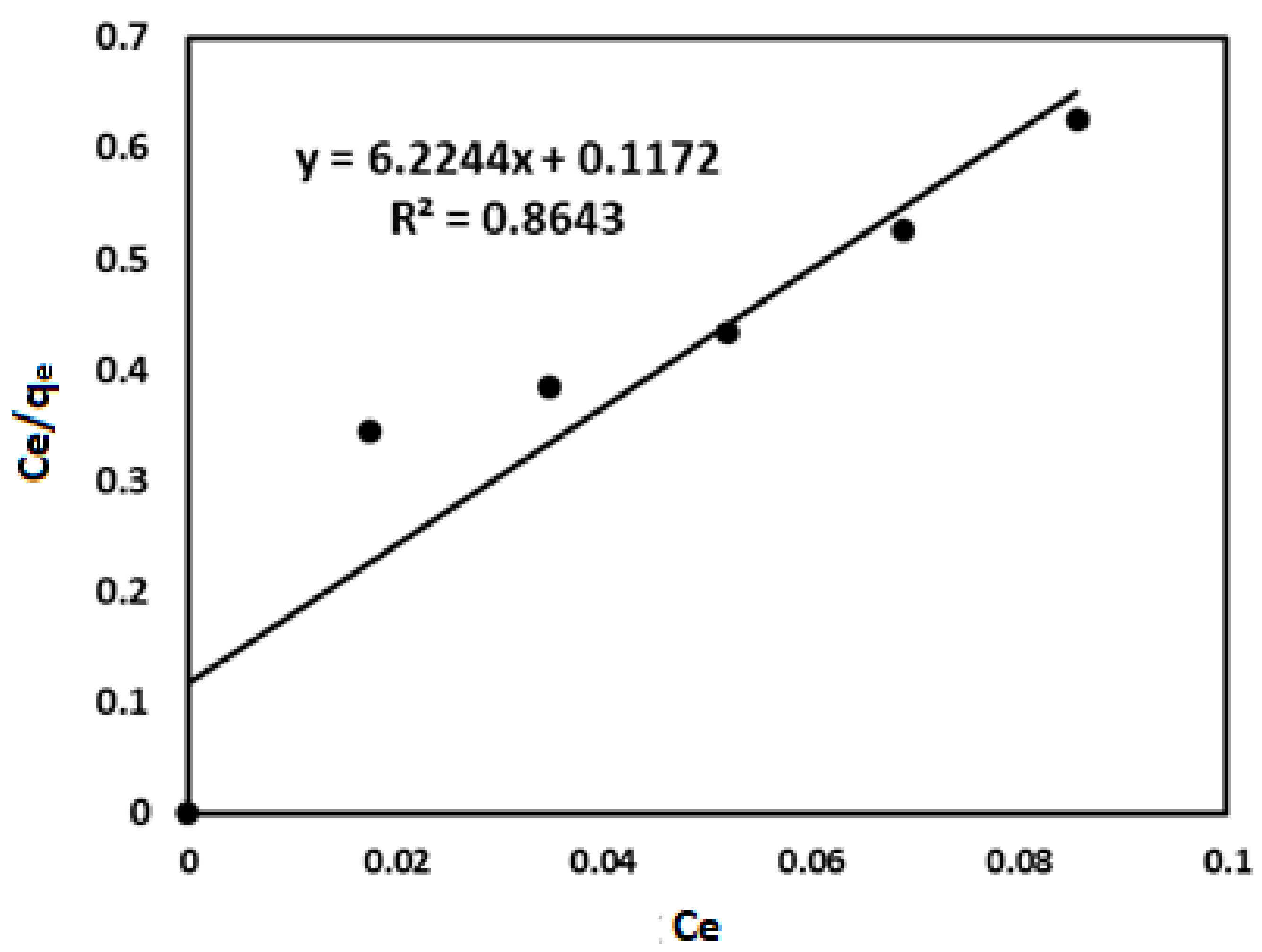
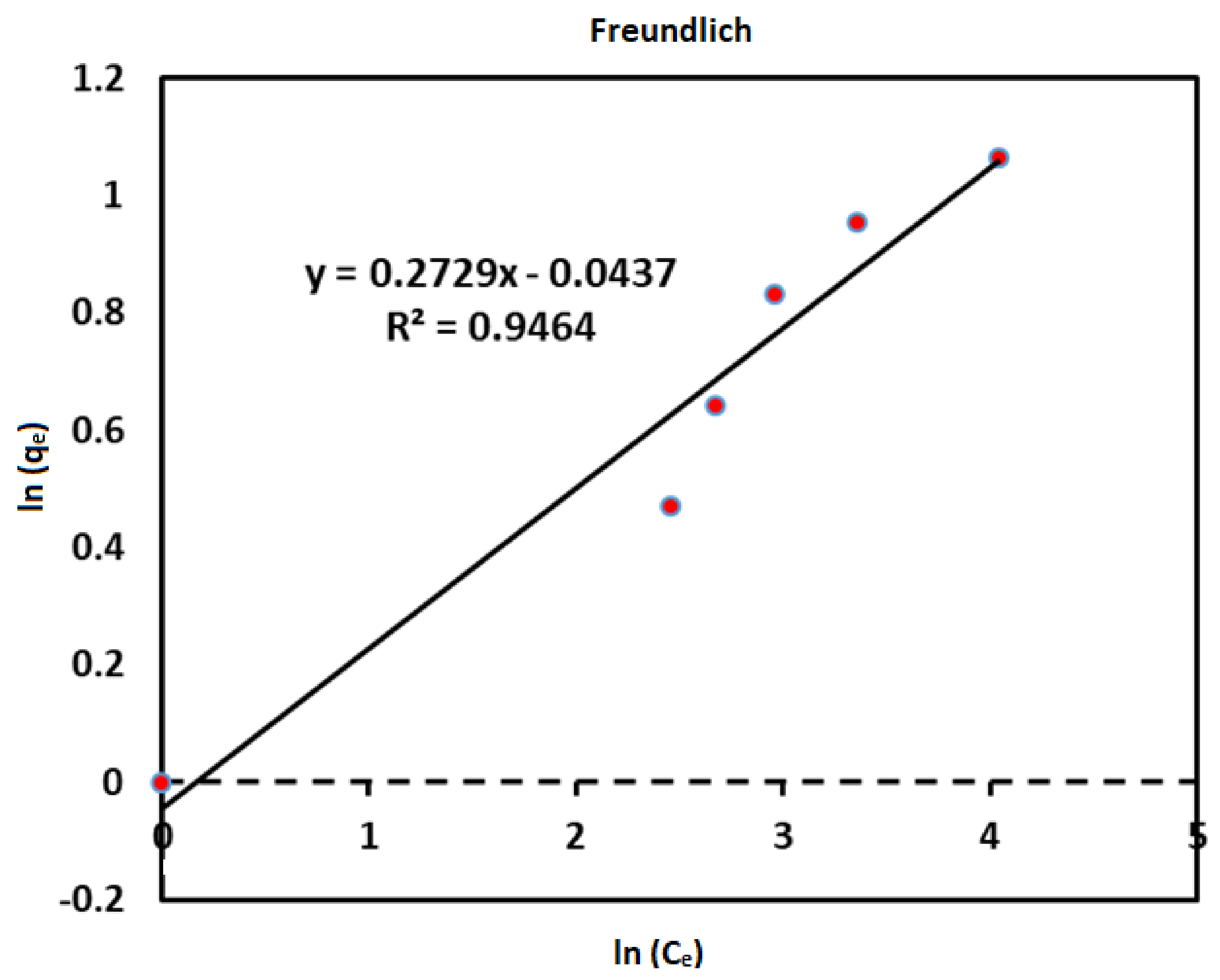
| Isotherm models | Parameters | Values |
|---|---|---|
| Freundlich | n | 0.2729 |
| 1/n | 3.6643 | |
| (mg/g)(l/mg)1/n | 2.7183 | |
| R2 | 0.9464 | |
| Langmuir | (mg/g) | 0.1607 |
| (L/mg) | 0.7293 | |
| R2 | 0.8643 |
| Adsorbents | pH | qe (mg/g) | Re (%) | References |
|---|---|---|---|---|
| Magnetic nanoparticles improved with tartaric acid | 6 | 1.97 | ND | [73] |
| Magnetic magnetite (Fe3O4) nanoparticle | 7 | 1.07 | ND | [74] |
| Metallurgical slag and iron nanoparticles | 8 | NA | 93.33 | [75] |
| Boron-selective gel and commercial resin | 8 | 1.15 | ND | [56] |
| Gly-resin | 9 | 1.60 | 96.90 | [25] |
| Activated carbon | 5.5 | 3.50 | 35 | [68] |
| Iron oxide | 8 | 6.94 | ND | [50] |
| Cobalt (11)-doped chitosan bio-composite | 8.5 | 2.50 | ND | [26] |
| Activated alumina | 8.5 | --- | 65 | [17] |
| Unmodified rice husk | 5 | 4.23 | [28] | |
| Fe3O4 | 8 | 8.44 | 84 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abba, M.U.; Che Man, H.; Syahidah Azis, R.; Idris, A.I.; Hazwan Hamzah, M.; Abdulsalam, M. Synthesis of Nano-Magnetite from Industrial Mill Chips for the Application of Boron Removal: Characterization and Adsorption Efficacy. Int. J. Environ. Res. Public Health 2021, 18, 1400. https://doi.org/10.3390/ijerph18041400
Abba MU, Che Man H, Syahidah Azis R, Idris AI, Hazwan Hamzah M, Abdulsalam M. Synthesis of Nano-Magnetite from Industrial Mill Chips for the Application of Boron Removal: Characterization and Adsorption Efficacy. International Journal of Environmental Research and Public Health. 2021; 18(4):1400. https://doi.org/10.3390/ijerph18041400
Chicago/Turabian StyleAbba, Mohammed Umar, Hasfalina Che Man, Raba’ah Syahidah Azis, Aida Isma Idris, Muhammad Hazwan Hamzah, and Mohammed Abdulsalam. 2021. "Synthesis of Nano-Magnetite from Industrial Mill Chips for the Application of Boron Removal: Characterization and Adsorption Efficacy" International Journal of Environmental Research and Public Health 18, no. 4: 1400. https://doi.org/10.3390/ijerph18041400
APA StyleAbba, M. U., Che Man, H., Syahidah Azis, R., Idris, A. I., Hazwan Hamzah, M., & Abdulsalam, M. (2021). Synthesis of Nano-Magnetite from Industrial Mill Chips for the Application of Boron Removal: Characterization and Adsorption Efficacy. International Journal of Environmental Research and Public Health, 18(4), 1400. https://doi.org/10.3390/ijerph18041400






