Incidence of Cancer and Asbestos-Related Diseases among Residents Living near Abandoned Asbestos Mines in South Korea: A Retrospective Cohort Study Using National Health Insurance Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
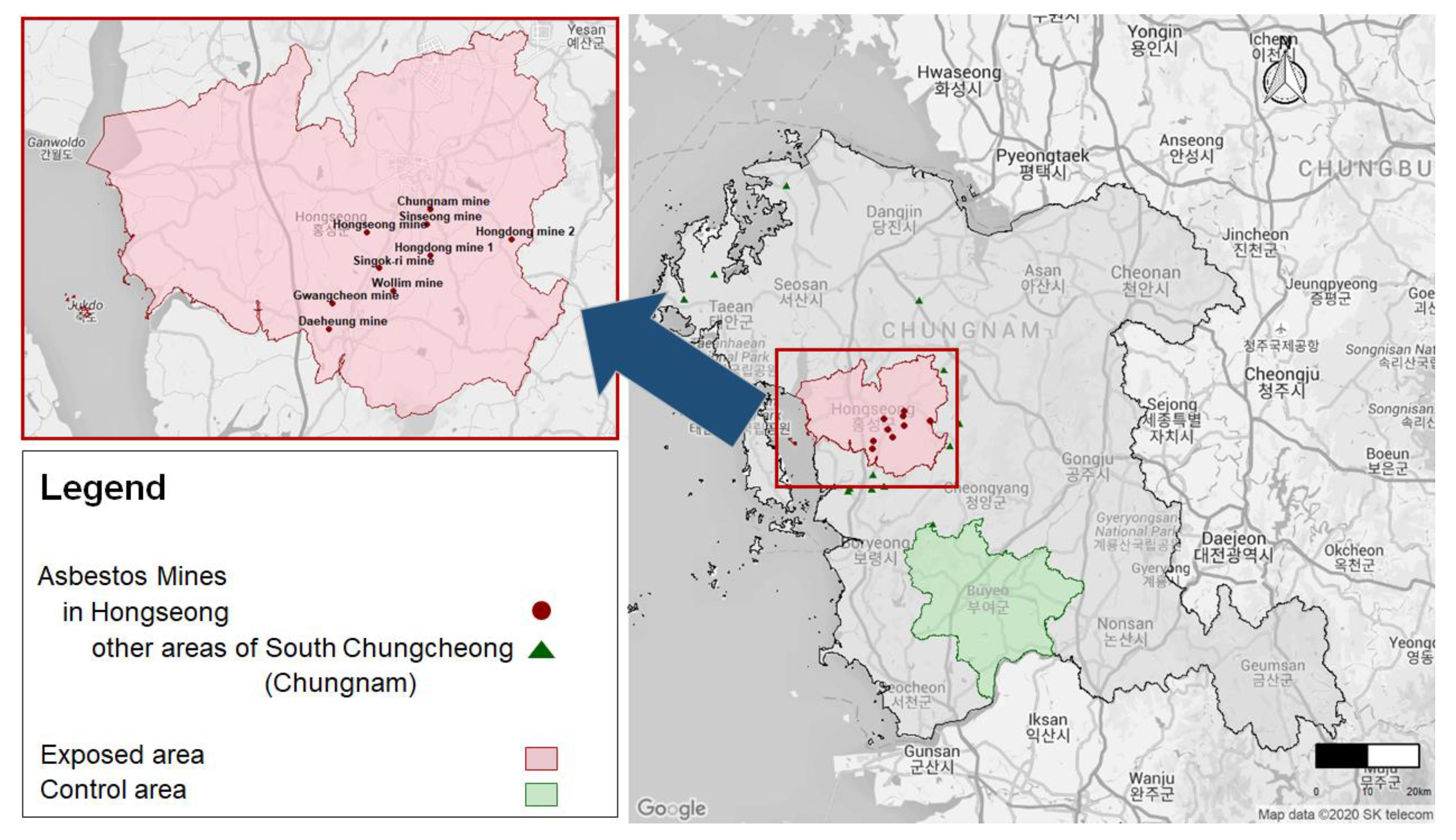
2.2. Exposed and Control Areas
2.3. Study Population
2.4. Follow-Up Period
2.5. Target Diseases
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Subjects
3.2. Incidence Rates and Survival Analyses for ARDs
3.3. Stratification Analysis for Malignant Mesothelioma, Asbestosis, and Pleural Plaques
3.4. Age-Standardized Incidence Ratios of Asbestos-Related Cancers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am. J. Respir. Crit. Care Med. 2004, 61, 691–715. [Google Scholar] [CrossRef]
- Rafferty, M.A.; Fenton, J.E.; Jones, A.S. The history, aetiology and epidemiology of laryngeal carcinoma. Clin. Otolaryngol. Allied Sci. 2001, 26, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Stayner, L.T.; Straif, K.; Reina, M.; Al-Alem, U.; Demers, P.A.; Landrigan, P.J. Occupational exposure to asbestos and ovarian cancer: A meta-analysis. Environ. Health Perspect. 2011, 119, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.M. Health effects of environmental asbestos exposure. J. Environ. Health Sci. 2009, 35, 71–77. (In Korean) [Google Scholar] [CrossRef]
- Ahn, Y.S.; Kim, H.R. Asbestosis epidemics caused by non-occupational neighborhood. J. Korean. Med. Assoc. 2009, 52, 472–481. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Khang, Y.H.; Park, J.H.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Bang, J.H.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef]
- Shin, H.R.; Won, Y.J.; Jung, K.W.; Kong, H.J.; Yim, S.H.; Lee, J.K.; Noh, H.I.; Lee, J.K.; Pisani, P.; Park, J.G. Nationwide cancer incidence in Korea, 1999–2001; first result using the national cancer incidence database. Cancer Res. Treat. 2005, 37, 325–331. [Google Scholar] [CrossRef]
- Oh, C.M.; Won, Y.J.; Jung, K.W.; Kong, H.J.; Cho, H.; Lee, J.K.; Lee, D.H.; Lee, K.H. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2013. Cancer Res. Treat. 2016, 48, 436–450. [Google Scholar] [CrossRef]
- Ameille, J.; Brochard, P.; Letourneux, M.; Paris, C.; Pairon, J.-C. Asbestos-related cancer risk in patients with asbestosis or pleural plaques. Rev. Mal. Respir. 2011, 8, e11–e17. [Google Scholar] [CrossRef]
- Hillerdal, G.; Henderson, D.W. Asbestos, asbestosis, pleural plaques and lung cancer. Scand. J. Work Environ. Health 1997, 23, 93–103. [Google Scholar] [CrossRef]
- Becklake, M.R. Asbestos-related diseases of the lung and other organs: Their epidemiology and implications for clinical practice. Am. Rev. Respir. Dis. 1976, 114, 187–227. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P. Health effects of asbestos exposure in humans: A quantitative assessment. Med. Lav 1998, 89, 471–480. [Google Scholar] [PubMed]
- Lee, Y.J.; Park, C.H.; Kim, Y.B.; Jang, E.C.; Kim, S.H.; Shin, Y.S.; Kim, J.S. The prevalence of asbestos related pleural plaque among residents living near asbestos mines in Korea. Korean J. Occup. Environ. Med. 2012, 24, 1–10. [Google Scholar] [CrossRef]
- Tossavainen, A. Asbestos, asbestosis, and cancer: The Helsinki criteria for diagnosis and attribution. Scand. J. Work Environ. Health 1997, 23, 311–316. [Google Scholar] [CrossRef]
- Camus, M.; Siemiatycki, J.; Meek, B. Nonoccupational exposure to chrysotile asbestos and the risk of lung cancer. N. Engl. J. Med. 1998, 338, 1565–1571. [Google Scholar] [CrossRef]
- Choi, J.K.; Paek, D.M.; Park, N.W. The production, the use, the number of workers and exposure level of asbestos in Korea. Korean Ind. Hyg. Assoc. J. 1998, 8, 242–253. (In Korean) [Google Scholar]
- Moon, Y.H. Epidemiological survey of asbestosis in asbestos miners and the inhabitants. Korean Cent. J. Med. 1979, 37, 169–178. [Google Scholar]
- Song, S.H.; Hwang, J.H.; Hwang, B.G.; Kim, H.W. Occurrence types and mineralogical characteristics of asbestos for the Kwangcheon area, Chungnam. J. Korean Soc. Occup. Environ. Hyg. 2008, 18, 271–281. (In Korean) [Google Scholar]
- Ministry of Environment. Investigation of Asbestos-Containing Soil, Groundwater, etc. around the Abandoned Asbestos Mine in 2010; Ministry of Environment: Sejong, Korea, 2010. (In Korean) [Google Scholar]
- Ministry of Environment. Investigation of Asbestos-Containing Soil, Groundwater, etc. around the Abandoned Asbestos Mine in 2011; Ministry of Environment: Sejong, Korea, 2011. (In Korean) [Google Scholar]
- Pasetto, R.; Comba, P.; Marconi, A. Mesothelioma associated with environmental exposures. Med. Lav. 2005, 96, 330–337. [Google Scholar]
- Ministry of Environment. Analysis on Exposure Characteristics of Asbestos Victims; Ministry of Environment: Sejong, Korea, 2019. (In Korean) [Google Scholar]
- Kim, J.S. Imaging diagnosis of asbestosis. J. Korean Med. Assoc. 2009, 52, 465–471. [Google Scholar] [CrossRef][Green Version]
- Reid, A.; Franklin, P.; Olsen, N.; Sleith, J.; Samuel, L.; Aboagye-Sarfo, P.; de Klerk, N.; Musk, A.W. All-cause mortality and cancer incidence among adults exposed to blue asbestos during childhood. Am. J. Ind. Med. 2013, 56, 133–145. [Google Scholar] [CrossRef]
- Reid, A.; Heyworth, J.; de Klerk, N.H. Cancer incidence among women and girls environmentally and occupationally exposed to blue asbestos at Wittenoom, Western Australia. Int. J. Cancer 2008, 122, 2337–2344. [Google Scholar] [CrossRef]
- Gustavsson, P.; Nyberg, F.; Pershagen, G.; Schéele, P.; Jakobsson, R.; Plato, N. Low-dose exposure to asbestos and lung cancer: Dose-response relations and interaction with smoking in a population-based case-referent study in Stockholm, Sweden. Am. J. Epidemiol. 2002, 155, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. A Review of Human Carcinogens. Part C: Arsenic, Metals, Fibres, and Dust; IARC Monographs Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2012; Volume 100, pp. 219–309. [Google Scholar]
- Kwak, K.M.; Paek, D.; Hwang, S.S.; Ju, Y.S. Estimated future incidence of malignant mesothelioma in South Korea: Projection from 2014 to 2033. PLoS ONE 2017, 12, e0183404. [Google Scholar] [CrossRef] [PubMed]
- Varga, C. Asbestos fibres in drinking water: Are they carcinogenic or not? Med. Hypotheses 2000, 55, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A. Asbestos ingestion and gastrointestinal cancer: A possible underestimated hazard. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 419–425. [Google Scholar] [CrossRef]
- Kjærheim, K.; Ulvestad, B.; Martinsen, J.I.; Andersen, A. Cancer of the gastrointestinal tract and exposure to asbestos in drinking water among lighthouse keepers (Norway). Cancer Causes Control 2005, 16, 593–598. [Google Scholar] [CrossRef]
- Hayward, S.B. Field monitoring of chrysotile asbestos in California waters. J. Am. Water Works Assoc. 1984, 76, 66–73. [Google Scholar] [CrossRef]
- Ministry of Environment. Survey of Environmental Pollution Effects in the Vicinity of Abandoned Asbestos Mine in 2018; Ministry of Environment: Sejong, Korea, 2018. (In Korean) [Google Scholar]
- Korea Resources Corporation. Korea Mineral Resources Geographic Information System. Available online: https://www.kmrgis.net/kmrgis/Main/Main/aspx (accessed on 30 May 2020).
- Kim, J.; Yoon, S.; Kim, L.Y.; Kim, D.S. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef]
| Characteristics | Exposed Area (Hongseong) N = 104,198 | Control Area (Buyeo) N = 90,640 | p-Value |
|---|---|---|---|
| Mean age (years) | 40.9 ± 22.5 | 43.5 ± 22.9 | <0.0001 |
| Age (years) | <0.0001 | ||
| <20 | 23,335 (22.4) | 17,844 (19.7) | |
| 20–39 | 26,100 (25.1) | 21,216 (23.4) | |
| 40–64 | 35,361 (33.9) | 31,034 (34.2) | |
| ≥65 | 19,402 (18.6) | 20,546 (22.7) | |
| Sex | 0.1857 | ||
| Men | 51,764 (49.7) | 45,301 (50.0) | |
| Women | 52,434 (50.3) | 45,339 (50.0) | |
| Household income | <0.0001 | ||
| Q1 (lowest) | 22,107 (21.5) | 22,137 (24.8) | |
| Q2 | 21,180 (20.6) | 17,943 (20.1) | |
| Q3 | 28,566 (27.7) | 23,328 (26.1) | |
| Q4 (highest) | 30,938 (30.1) | 25,965 (29.0) | |
| Smoking | <0.0001 | ||
| Never smoker | 48,314 (46.4) | 41,712 (46.0) | |
| Ex-smoker | 7835 (7.5) | 8763 (9.7) | |
| Current smoker | 14,530 (13.9) | 12,405 (13.7) | |
| Missing * | 33,500 (32.2) | 27,758 (30.6) | |
| Alcohol | <0.0001 | ||
| Non-drinking | 41,075 (39.4) | 36,763 (40.6) | |
| Normal drinking | 16,395 (15.7) | 14,406 (15.9) | |
| Heavy drinking ** | 9137 (8.8) | 7524 (8.3) | |
| Missing * | 37,572 (36.1) | 31,945 (35.2) | |
| Average follow-up years | 9.7 ± 3.5 | 10.03 ± 3.3 | <0.0001 |
| Person-year | 1,011,874.6 | 909,433.5 |
| Diseases | No. of Cases | Person-Years | Incidence * | HR ** (95% CI) | |
|---|---|---|---|---|---|
| Crude | Adjusted | ||||
| Asbestosis (J61) | |||||
| Control area | 10 | 909,433.5 | 1.10 | 1 | 1 |
| Exposed area | 668 | 1,011,874.6 | 66.02 | 60.82 (32.57–113.55) | 65.40 (35.02–122.12) |
| Pneumoconiosis (except asbestosis; J60, J62–J65) | |||||
| Control area | 133 | 907,699.4 | 14.65 | 1 | 1 |
| Exposed area | 77 | 1,014,133.8 | 7.59 | 0.52 (0.39–0.69) | 0.57 (0.43–0.75) |
| Pleural effusion (J90–J91) | |||||
| Control area | 490 | 906,782.6 | 54.04 | 1 | 1 |
| Exposed area | 454 | 1,012,520.8 | 44.84 | 0.83 (0.73–0.95) | 0.94 (0.82–1.06) |
| Pleural plaques (J92) | |||||
| Control area | 14 | 909,311.8 | 1.54 | 1 | 1 |
| Exposed area | 54 | 1,014,573.7 | 5.32 | 3.47 (1.93–6.25) | 3.55 (1.96–6.41) |
| COPD (J40-J44) | |||||
| Control area | 18184 | 631,752.2 | 2878.34 | 1 | 1 |
| Exposed area | 17355 | 777,513.3 | 2232.12 | 0.78 (0.76–0.79) | 0.80 (0.79–0.82) |
| Pleurisy (R09.1) | |||||
| Control area | 18 | 909,302.8 | 1.98 | 1 | 1 |
| Exposed area | 15 | 1,014,747.1 | 1.48 | 0.74 (0.37–1.47) | 0.81 (0.41–1.60) |
| Benign lung mass (D02.2, D14.3, D38.1) | |||||
| Control area | 1018 | 900,904.0 | 113.00 | 1 | 1 |
| Exposed area | 617 | 1,009,735.8 | 61.11 | 0.54 (0.49–0.60) | 0.57 (0.52–0.63) |
| Pharyngeal cancer (C10–C13) | |||||
| Control area | 37 | 909,241.8 | 4.07 | 1 | 1 |
| Exposed area | 48 | 1,014,660.4 | 4.73 | 1.17 (0.76–1.79) | 1.29 (0.84–1.99) |
| Esophageal cancer (C15) | |||||
| Control area | 101 | 909,043.7 | 11.11 | 1 | 1 |
| Exposed area | 105 | 1,014,472.6 | 10.35 | 0.93 (0.71–1.22) | 1.04 (0.79–1.38) |
| Stomach cancer (C16) | |||||
| Control area | 1018 | 901,648.6 | 112.90 | 1 | 1 |
| Exposed area | 1100 | 1,006,940.7 | 109.24 | 0.97 (0.89–1.05) | 1.04 (0.98–1.16) |
| Colon cancer (C18) | |||||
| Control area | 598 | 905,936.9 | 66.01 | 1 | 1 |
| Exposed area | 681 | 1,010,434.7 | 67.40 | 1.02 (0.92–1.14) | 1.13 (1.01–1.26) |
| Rectal cancer (C19–C20) | |||||
| Control area | 496 | 905,724.1 | 54.76 | 1 | 1 |
| Exposed area | 458 | 1,011,198.6 | 45.29 | 0.83 (0.73–0.94) | 0.90 (0.79–1.02) |
| Laryngeal cancer (C32) | |||||
| Control area | 53 | 909,148.1 | 5.83 | 1 | 1 |
| Exposed area | 63 | 1,014,448.5 | 6.21 | 1.07 (0.74–1.54) | 1.14 (0.79–1.64) |
| Lung cancer (C33–C34) | |||||
| Control area | 866 | 907,004.2 | 95.48 | 1 | 1 |
| Exposed area | 871 | 1,012,289.2 | 86.04 | 0.90 (0.82–0.99) | 1.02 (0.93–1.12) |
| Mesothelioma (C45) | |||||
| Control area | 5 | 909,463.6 | 0.55 | 1 | 1 |
| Exposed area | 9 | 1,014,844.6 | 0.89 | 1.62 (0.54–4.83) | 1.83 (0.61–5.47) |
| Ovarian cancer (C56) | |||||
| Control area | 77 | 908,952.0 | 8.47 | 1 | 1 |
| Exposed area | 64 | 1,014,298.7 | 6.31 | 0.75 (0.54–1.04) | 0.79 (0.56–1.10) |
| Renal cancer (C56) | |||||
| Control area | 102 | 908,774.9 | 11.22 | 1 | 1 |
| Exposed area | 104 | 1,014,271.5 | 10.25 | 0.92 (0.70–1.21) | 1.03 (0.78–1.35) |
| Variables | Exposed Area | Control Area | HR ** (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Cases | Person-Years | Incidence * | No. of Cases | Person-Years | Incidence * | Unadjusted | Adjusted | |
| Sex | ||||||||
| Men | 8 | 501,194.0 | 1.60 | 1 | 452,184.3 | 0.22 | 7.20 (0.90–57.56) | 8.30 (1.04–66.63) |
| Women | 1 | 513,650.6 | 0.19 | 4 | 457,279.2 | 0.87 | 0.23 (0.03–2.01) | 0.25 (0.03–2.23) |
| Age (years) | ||||||||
| <20 | 0 | 218,039.2 | 0.00 | 0 | 176,845.9 | 0.00 | ‒ | ‒ |
| 20–39 | 0 | 266,223.8 | 0.00 | 0 | 224,046.5 | 0.00 | ‒ | ‒ |
| 40–64 | 4 | 355,563.6 | 1.12 | 1 | 323,485.6 | 0.31 | 3.69 (0.41–33.04) | 4.03 (0.45–36.21) |
| ≥65 | 5 | 174,917.9 | 2.86 | 4 | 185,085.6 | 2.16 | 1.32 (0.36–4.93) | 1.21 (0.33–4.53) |
| Household income | ||||||||
| Q1 (lowest) | 3 | 208,783.9 | 1.44 | 1 | 216,561.5 | 0.46 | 3.06 (0.32–29.40) | 3.31 (0.34–31.84) |
| Q2 | 0 | 206,732.4 | 0.00 | 0 | 177,763.9 | 0.00 | ‒ | ‒ |
| Q3 | 2 | 281,235.1 | 0.71 | 1 | 237,052.2 | 0.42 | 1.66 (0.15–18.29) | 1.82 (0.16–20.12) |
| Q4 (highest) | 4 | 305,751.3 | 1.31 | 3 | 266,423.6 | 1.13 | 1.18 (0.27–5.28) | 1.34 (0.30–6.03 |
| Variables | Exposed Area | Control Area | HR * (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Cases | Person-Years | Incidence ** | No. of Cases | Person-Years | Incidence ** | Unadjusted | Adjusted 1 | |
| Sex | ||||||||
| Men | 391 | 499,450.4 | 78.29 | 7 | 452,155.3 | 1.55 | 51.03 (24.19–107.66) | 53.30 (25.24–112.55) |
| Women | 277 | 512,424.2 | 54.06 | 3 | 457,278.2 | 0.66 | 83.57 (26.79–260.69) | 89.75 (28.77–280.04) |
| Age (years) | ||||||||
| <20 | 1 | 218,032.7 | 0.46 | 0 | 176,845.9 | 0.00 | Infinite | Infinite |
| 20–39 | 4 | 266,211.7 | 1.50 | 0 | 224,046.5 | 0.00 | Infinite | Infinite |
| 40–64 | 294 | 354,525.7 | 82.93 | 5 | 323,475.8 | 1.55 | 54.73 (22.61–132.46) | 51.95 (21.46–125.79) |
| ≥65 | 369 | 173,104.5 | 213.17 | 5 | 185,065.4 | 2.70 | 79.09 (32.73–191.14) | 76.20 (31.51–184.23) |
| Household income | ||||||||
| Q1 (lowest) | 88 | 208,406.6 | 42.23 | 0 | 216,562.6 | 0.00 | Infinite | Infinite |
| Q2 | 126 | 206,140.4 | 61.12 | 4 | 177,742.5 | 2.25 | 27.37 (10.12–74.06) | 29.42 (10.87–79.66) |
| Q3 | 224 | 280,268.1 | 79.92 | 3 | 237,049.4 | 1.27 | 64.20 (20.55–200.55) | 71.54 (22.89–223.60) |
| Q4 (highest) | 218 | 304,760.5 | 71.53 | 3 | 266,416.6 | 1.13 | 64.34 (20.59–201.05) | 73.75 (23.59–230.53) |
| Variables | Exposed Area | Control Area | HR * (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Cases | Person-Years | Incidence ** | No. of Cases | Person-Years | Incidence ** | Unadjusted | Adjusted | |
| Sex | ||||||||
| Men | 28 | 501,068.6 | 5.59 | 9 | 452,065.8 | 1.99 | 2.83 (1.34–6.00) | 2.94 (1.37–6.32) |
| Women | 26 | 513,505.1 | 5.06 | 5 | 457,245.9 | 1.09 | 4.65 (1.79–12.10) | 4.80 (1.83–12.54) |
| Age (years) | ||||||||
| <20 | 0 | 218,039.2 | 0.00 | 0 | 176,845.9 | 0.00 | – | – |
| 20–39 | 0 | 266,203.8 | 0.00 | 1 | 224,045.0 | 0.45 | – | – |
| 40–64 | 26 | 35,548.6 | 73.14 | 5 | 323,537.9 | 1.55 | 4.78 (1.83–12.44) | 4.21 (1.60–11.08) |
| ≥65 | 28 | 174,782.0 | 16.02 | 8 | 184,983.0 | 4.32 | 3.70 (1.69–8.13) | 3.57 (1.62–7.85) |
| Household income | ||||||||
| Q1 (lowest) | 2 | 208,775.6 | 0.96 | 1 | 216,525.9 | 0.46 | 2.07 (0.19–22.78) | 2.14 (0.19–23.61) |
| Q2 | 7 | 206,694.0 | 3.39 | 4 | 177,721.2 | 2.25 | 1.51 (0.44–5.16) | 1.69 (0.49–5.82) |
| Q3 | 21 | 281,120.7 | 7.47 | 2 | 237,034.9 | 0.84 | 8.88 (2.08–37.86) | 9.77 (2.29–41.76) |
| Q4 (highest) | 21 | 305,652.7 | 6.87 | 7 | 266,367.3 | 2.63 | 2.64 (1.12–6.21) | 3.00 (1.27–7.09) |
| Cancer | Exposed Area | Control Area | ||||
|---|---|---|---|---|---|---|
| Obs * | Exp * | SIR * | Obs * | Exp * | SIR * | |
| Pharyngeal cancer (C10–C13) | ||||||
| Men | 39 | 21.8 | 1.79 (1.27–2.45) | 26 | 21.8 | 1.20 (0.78–1.75) |
| Women | 5 | 3.5 | 1.44 (0.47–3.37) | 9 | 3.3 | 2.73 (1.25–5.17) |
| Esophageal cancer (C15) | ||||||
| Men | 93 | 66.4 | 1.40 (1.13–1.72) | 90 | 68.3 | 1.32 (1.06–1.62) |
| Women | 7 | 6.6 | 1.07 (0.43–2.20) | 8 | 6.8 | 1.17 (0.51–2.31) |
| Stomach cancer (C16) | ||||||
| Men | 716 | 580.0 | 1.23 (1.15–1.33) | 622 | 582.2 | 1.07 (0.99–1.16) |
| Women | 299 | 288.3 | 1.04 (0.92–1.16) | 312 | 290.5 | 1.07 (0.96–1.20) |
| Colon cancer (C18) | ||||||
| Men | 389 | 258.8 | 1.50 (1.36–1.66) | 329 | 262.9 | 1.25 (1.12–1.39) |
| Women | 246 | 193.2 | 1.27 (1.12–1.44) | 220 | 198.2 | 1.11 (0.97–1.27) |
| Rectal cancer (C19–C20) | ||||||
| Men | 278 | 208.5 | 1.33 (1.18–1.50) | 304 | 208.5 | 1.46 (1.30–1.63) |
| Women | 150 | 126.8 | 1.18 (1.00–1.39) | 156 | 128.2 | 1.22 (1.03–1.42) |
| Laryngeal cancer (C32) | ||||||
| Men | 60 | 33.3 | 1.80 (1.37–2.32) | 49 | 34.1 | 1.44 (1.06–1.90) |
| Women | 6 | 2.25 | 2.66 (0.98–5.80) | 4 | 2.3 | 1.71 (0.47–4.37) |
| Lung cancer (C33–C34) ** | ||||||
| Men | 562 | 511.1 | 1.10 (1.01–1.19) | 553 | 531.7 | 1.04 (0.96–1.13) |
| Women | 234 | 212.1 | 1.10 (0.97–1.25) | 222 | 220.4 | 1.01 (0.88–1.15) |
| Mesothelioma (C45) ** | ||||||
| Men | 8 | 2.3 | 3.48 (1.50–6.85) | 1 | 2.3 | 0.43 (0.01–2.39) |
| Women | 1 | 1.17 | 0.85 (0.02–4.76) | 3 | 1.2 | 2.55 (0.53–7.45) |
| Ovarian cancer (C56) ** | ||||||
| Women | 58 | 55.1 | 1.05 (0.80–1.36) | 62 | 51.8 | 1.20 (0.92–1.54) |
| Renal cancer (C56) ** | ||||||
| Men | 60 | 75.4 | 0.80 (0.61–1.02) | 55 | 73.4 | 0.75 (0.56–0.97) |
| Women | 34 | 35.4 | 0.96 (0.67–1.34) | 34 | 34.9 | 0.97 (0.67–1.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, K.; Zoh, K.E.; Paek, D. Incidence of Cancer and Asbestos-Related Diseases among Residents Living near Abandoned Asbestos Mines in South Korea: A Retrospective Cohort Study Using National Health Insurance Database. Int. J. Environ. Res. Public Health 2021, 18, 875. https://doi.org/10.3390/ijerph18030875
Kwak K, Zoh KE, Paek D. Incidence of Cancer and Asbestos-Related Diseases among Residents Living near Abandoned Asbestos Mines in South Korea: A Retrospective Cohort Study Using National Health Insurance Database. International Journal of Environmental Research and Public Health. 2021; 18(3):875. https://doi.org/10.3390/ijerph18030875
Chicago/Turabian StyleKwak, Kyeongmin, Kyung Ehi Zoh, and Domyung Paek. 2021. "Incidence of Cancer and Asbestos-Related Diseases among Residents Living near Abandoned Asbestos Mines in South Korea: A Retrospective Cohort Study Using National Health Insurance Database" International Journal of Environmental Research and Public Health 18, no. 3: 875. https://doi.org/10.3390/ijerph18030875
APA StyleKwak, K., Zoh, K. E., & Paek, D. (2021). Incidence of Cancer and Asbestos-Related Diseases among Residents Living near Abandoned Asbestos Mines in South Korea: A Retrospective Cohort Study Using National Health Insurance Database. International Journal of Environmental Research and Public Health, 18(3), 875. https://doi.org/10.3390/ijerph18030875





