Bone Mineral Density in Different Menopause Stages is Associated with Follicle Stimulating Hormone Levels in Healthy Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Body Composition
2.3. Sex Hormones and Inflammatory Marker
2.4. Physical Activity, Energy Intake, and Vascular Function
2.5. Statistical Analysis
3. Results
3.1. Population
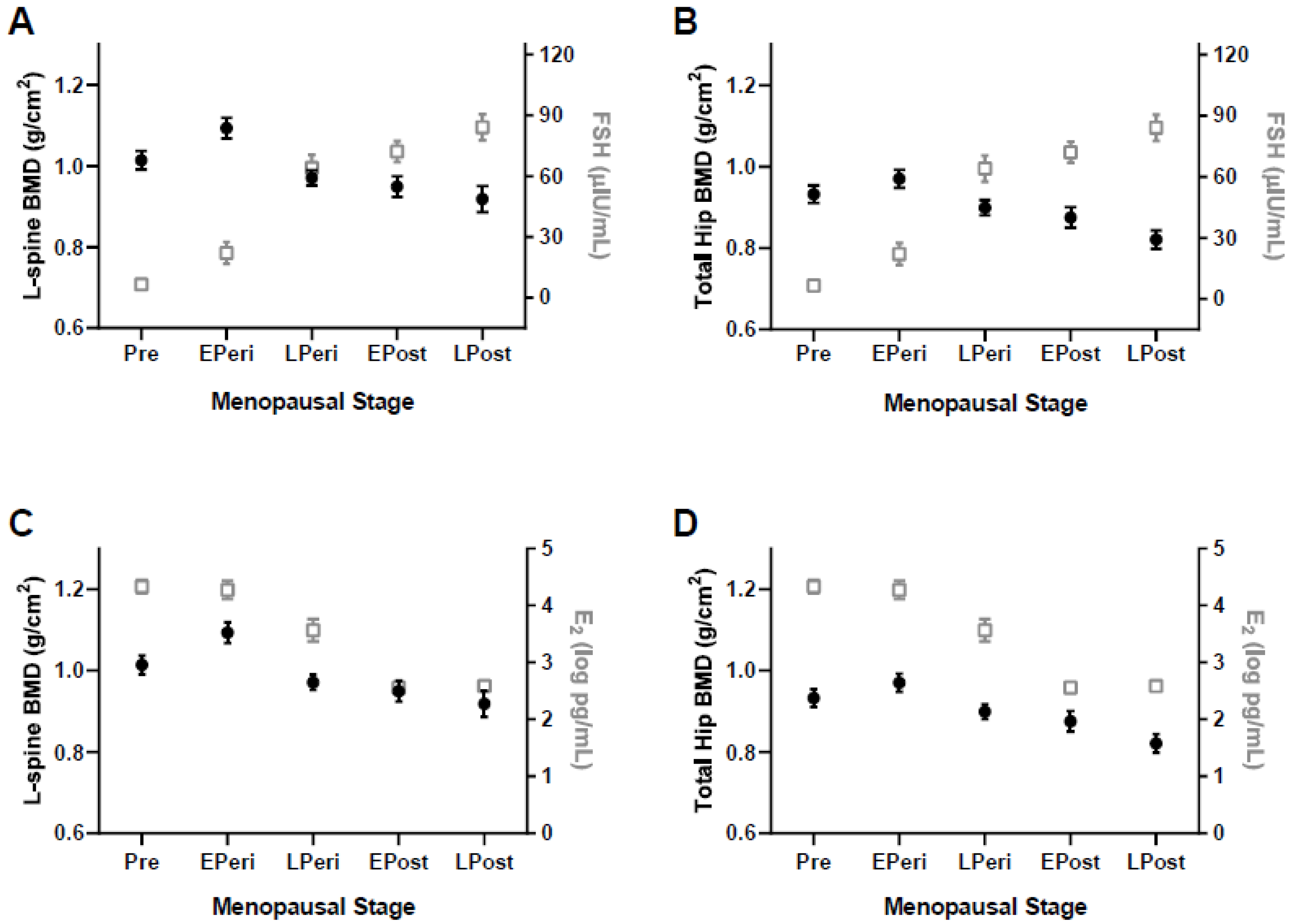
3.2. Spine and Hip BMD Analysis
3.3. Prevalence of LBM and Osteoporosis
3.4. Associations
4. Discussion
4.1. Menopause, BMD, and Physical Activity
4.2. FSH and BMD
4.3. Potential Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar]
- Sowers, M.; Zheng, H.; Jannausch, M.L.; McConnell, D.; Nan, B.; Harlow, S.; Randolph, J.F. Amount of Bone Loss in Relation to Time around the Final Menstrual Period and Follicle-Stimulating Hormone Staging of the Transmenopause. J. Clin. Endocrinol. Metab. 2010, 95, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Sowers, M.; Han, W.; Huang, M.H.; Finkelstein, J.S.; Crandall, C.J.; Lee, J.S.; Karlamangla, A.S. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women’s Health across the Nation (SWAN). J. Bone Miner. Res. 2012, 27, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Brockwell, S.E.; Mehta, V.; Greendale, G.A.; Sowers, M.R.; Ettinger, B.; Lo, J.C.; Johnston, J.M.; Cauley, J.A.; Danielson, M.E.; et al. Bone Mineral Density Changes during the Menopause Transition in a Multiethnic Cohort of Women. J. Clin. Endocrinol. Metab. 2008, 93, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.; Lappe, J.M.; Davies, K.; Recker, R. Transmenopausal changes in the trabecular bone structure. Bone 2007, 41, 111–116. [Google Scholar] [CrossRef]
- Cooper, D.M.; Thomas, C.D.L.; Clement, J.G.; Turinsky, A.L.; Sensen, C.W.; Hallgrímsson, B. Age-dependent change in the 3D structure of cortical porosity at the human femoral midshaft. Bone 2007, 40, 957–965. [Google Scholar] [CrossRef]
- van Staa, T.P.; Dennison, E.M.; Leufkens, H.G.; Cooper, C. Epidemiology of fractures in England and Wales. Bone 2001, 29, 517–522. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Curran, M.P.; Wagstaff, A.J. Estradiol and norgestimate: A review of their combined use as hormone replacement therapy in postmenopausal women. Drugs Aging 2001, 18, 863–885. [Google Scholar] [CrossRef]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.; Zheng, H.; Tomey, K.; A Karvonengutierrez, C.; Jannausch, M.L.; Li, X.; Yosef, M.; Symons, J.P. Changes in Body Composition in Women over Six Years at Midlife: Ovarian and Chronological Aging. J. Clin. Endocrinol. Metab. 2007, 92, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Peng, Y.; Sharrow, A.C.; Iqbal, J.; Zhang, Z.; Papachristou, D.J.; Zaidi, S.; Zhu, L.-L.; Yaroslavskiy, B.B.; Zhou, H.; et al. FSH Directly Regulates Bone Mass. Cell 2006, 125, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Blair, H.; Cao, J.; Yuen, T.; Latif, R.; Guo, L.; Tourkova, I.L.; Li, J.; Davies, T.F.; Sun, L.; et al. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14574–14579. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Tourkova, I.; Yuen, T.; Robinson, L.J.; Bian, Z.; Zaidi, M.; Blair, H.C. Blocking FSH action attenuates osteoclastogenesis. Biochem. Biophys. Res. Commun. 2012, 422, 54–58. [Google Scholar] [CrossRef]
- Shieh, A.; Greendale, G.A.; Cauley, J.A.; Karvonen-Gutierrez, C.; Crandall, C.J.; Karlamangla, A.S. Estradiol and Follicle-Stimulating Hormone as Predictors of Onset of Menopause Transition-Related Bone Loss in Pre- and Perimenopausal Women. J. Bone Miner. Res. 2019, 34, 2246–2253. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Yu, S.-J.; Zhang, H.; Xie, H.; Luo, X.-H.; Peng, Y.-Q.; Yuan, L.-Q.; Dai, R.-C.; Sheng, Z.-F.; Liu, S.-P.; et al. Early bone mineral density decrease is associated with FSH and LH, not estrogen. Clin. Chim. Acta 2013, 415, 69–73. [Google Scholar] [CrossRef]
- Sowers, M.; Jannausch, M.; McConnell, D.; Little, R.; Greendale, G.A.; Finkelstein, J.S.; Neer, R.M.; Johnston, J.; Ettinger, B. Hormone Predictors of Bone Mineral Density Changes during the Menopausal Transition. J. Clin. Endocrinol. Metab. 2006, 91, 1261–1267. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the men-opause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef]
- Soules, M.R.; Sherman, S.; Parrott, E.; Rebar, R.; Santoro, N.; Utian, W.; Woods, N. Stages of Reproductive Aging Workshop (STRAW). J. Women’s Health Gend. Based Med. 2001, 10, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, R.E.; Evans, E.M.; Schechtman, K.B.; Ehsani, A.A.; Kohrt, W.M. Contributions of total and regional fat mass to risk for car-diovascular disease in older women. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1023–E1028. [Google Scholar] [CrossRef] [PubMed]
- WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129. [Google Scholar]
- Park, Y.M.; Jankowski, C.M.; Ozemek, C.; Hildreth, K.L.; Kohrt, W.M.; Moreau, K.L. Appendicular lean mass is lower in late com-pared with early perimenopausal women: Potential role of FSH. J. Appl. Physiol. 2020, 128, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Kriska, A.M.; Caspersen, C.J.; Pereira, M.A.; Fitzgerald, S.J.; Gregg, E.W.; Joswiak, M.L.; Ryan, W.J.; Suminski, R.R.; Utter, A.C.; Zmuda, J.M. A collection of Physical Activity Ques-tionnaires for health-related research. Med. Sci. Sports Exerc. 1997, 29 (Suppl. 6), 1–205. [Google Scholar] [CrossRef]
- Stevenson, E.T.; Davy, K.P.; Seals, D.R. Hemostatic, metabolic, and androgenic risk factors for coronary heart disease in physi-cally active and less active postmenopausal women. Arter. Thromb. Vasc. Biol. 1995, 15, 669–677. [Google Scholar] [CrossRef]
- Moreau, K.L.; Meditz, A.; Deane, K.D.; Kohrt, W.M. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1211–H1218. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Sowers, M.R.; Clark, M.K.; Hollis, B.; Wallace, R.B.; Jannausch, M. Radial bone mineral density in pre- and perimenopausal wom-en: A prospective study of rates and risk factors for loss. J. Bone Miner. Res. 1992, 7, 647–657. [Google Scholar] [CrossRef]
- Recker, R.; Lappe, J.; Davies, K.; Heaney, R. Characterization of Perimenopausal Bone Loss: A Prospective Study. J. Bone Miner. Res. 2000, 15, 1965–1973. [Google Scholar] [CrossRef]
- Oguma, Y.; Sesso, H.D.; Paffenbarger, R.S., Jr.; Lee, I.M. Physical activity and all cause mortality in women: A review of the evi-dence. Br. J. Sports Med. 2002, 36, 162–172. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; De Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.; Kanaley, J.A.; Padilla, J.; Zidon, T.; Welly, R.J.; Will, M.J.; Britton, S.L.; Koch, L.G.; Ruegsegger, G.N.; Booth, F.W.; et al. Effects of intrinsic aerobic capacity and ovariectomy on voluntary wheel running and nucleus accumbens dopamine receptor gene expression. Physiol. Behav. 2016, 164, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Randolph, J.F., Jr.; Sowers, M.; Gold, E.B.; Mohr, B.A.; Luborsky, J.; Santoro, N.; McConnell, D.S.; Finkelstein, J.S.; Korenman, S.G.; Matthews, K.A.; et al. Reproductive hormones in the early menopau-sal transition: Relationship to ethnicity, body size, and menopausal status. J. Clin. Endocrinol. Metab. 2003, 88, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Bianchi, G.; Brandi, M.L.; Giannini, S.; Ortolani, S.; DiMunno, O.; Frediani, B.; Rossini, M. Determinants of Bone Turnover Markers in Healthy Premenopausal Women. Calcif. Tissue Int. 2008, 82, 341–347. [Google Scholar] [CrossRef]
- Devleta, B.; Adem, B.; Senada, S. Hypergonadotropic amenorrhea and bone density: New approach to an old problem. J. Bone Miner. Metab. 2004, 22, 360–364. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Y.; Fan, M.; Chen, D.; Bian, Z. FSH Aggravates Periodontitis-related Bone Loss in Ovariectomized Rats. J. Dent. Res. 2010, 89, 366–371. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Y.; Xu, W.; Bian, Z. Protective Effects of Follicle-stimulating Hormone Inhibitor on Alveolar Bone Loss Resulting from Experimental Periapical Lesions in Ovariectomized Rats. J. Endod. 2010, 36, 658–663. [Google Scholar] [CrossRef]
- Sowers, M.R.; Greendale, G.A.; Bondarenko, I.; Finkelstein, J.S.; Cauley, J.A.; Neer, R.M.; Ettinger, B. Endogenous hormones and bone turn-over markers in pre- and perimenopausal women: SWAN. Osteoporos. Int. 2003, 14, 191–197. [Google Scholar] [CrossRef]

| Variable | Pre n = 30 | EPeri n = 31 | LPeri n = 30 | EPost n = 24 | LPost n = 26 | p-Value |
|---|---|---|---|---|---|---|
| Age, yr | 38 ± 6 | 50 ± 3 | 50 ± 4 | 55 ± 3 | 62 ± 4 | <0.001 |
| Weight, kg | 66.3 ± 10.0 | 71.4 ± 11.0 | 67.4 ± 11.9 | 71.9 ± 13.4 | 66.8 ± 13.4 | 0.25 |
| Height, cm | 163 ± 6 | 165 ± 6 | 166 ± 8 | 164 ± 6 | 161 ± 8 | 0.10 |
| BMI, kg/m2 | 24.7 ± 3.7 | 26.1 ± 3.9 | 24.5 ± 3.9 | 26.7 ± 5.2 | 25.6 ± 4.6 | 0.27 |
| Lean mass, kg | 42.2 ± 3.6 | 44.3 ± 5.7 | 40.5 ± 5.1 | 42.2 ± 6.5 | 38.8 ± 5.5 | <0.01 |
| Fat mass, kg | 21.6 ± 7.7 | 24.7 ± 6.9 | 24.8 ± 8.1 | 27.5 ± 8.2 | 25.3 ± 9.6 | 0.12 |
| BMC, kg | 2.14 ± 0.27 | 2.33 ± 0.34 | 2.08 ± 0.26 | 2.06 ± 0.22 | 1.92 ± 0.37 | <0.001 |
| Estradiol, pg/mL *,† | 79 (64–104) | 70 (37–141) | 34 (10–94) | 11 (10–14) | 10 (10–14) | <0.001 |
| Estrone, ng/dL *,† | 61 (41–70) | 60 (34–88) | 43 (30–69) | 25 (23–32) | 26 (23–37) | <0.001 |
| FSH, μIU/mL † | 6.5 ± 3.4 | 22.0 ± 30.0 | 64.1 ± 35.5 | 73.4 ± 27.1 | 84.1 ± 33.3 | <0.001 |
| Progesterone, ng/dL *,† | 0.4 (0.2–0.6) | 0.5 (0.2–0.8) | 0.3 (0.3–0.5) | 0.3 (0.1–0.4) | 0.2 (0.1–0.4) | <0.01 |
| Testosterone, ng/dL *,† | 24 (17–32) | 22 (17–35) | 20 (17–25) | 17 (17–22) | 17 (17–35) | 0.22 |
| Energy intake, kcal/kg ‡ | 25 ± 10 | 28 ± 8 | 28 ± 7 | 26 ± 7 | 27 ± 7 | 0.79 |
| LTPA, MET h/wk *,§ | 8(4–18) | 14 (8–18) | 7 (5–14) | 10 (5–14) | 10 (6–25) | 0.59 |
| IL6, pg/mL ‡ | 1.28 ± 1.24 | 1.03 ± 0.65 | 1.10 ± 0.74 | 1.13 ± 0.87 | 0.86 ± 0.26 | 0.72 |
| Brachial FMD, % † | 11.0 ± 3.6 | 8.4 ± 2.8 | 6.9 ± 2.1 | 6.1 ± 2.0 | 4.9 ± 1.8 | <0.001 |
| Variable | Pre n = 30 | EPeri n = 31 | LPeri n = 30 | EPost n = 24 | LPost n = 26 | p-Value |
|---|---|---|---|---|---|---|
| Spine | ||||||
| L1-4 BMD, g/cm2 | 1.014 ± 0.126 | 1.093 ± 0.146 | 0.971 ± 0.103 b | 0.949 ± 0.132 b | 0.918 ± 0.167 b | <0.001 |
| L1-4 T-score | −0.3 ± 1.1 | 0.4 ± 1.3 | −0.7 ± 0.9 b | −0.9 ± 1.2 b | −1.2 ± 1.5 b | <0.001 |
| Hip | ||||||
| Neck BMD, g/cm2 | 0.799 ± 0.106 | 0.835 ± 0.119 | 0.767 ± 0.089 | 0.727 ± 0.099 b | 0.684 ± 0.097 a,b,c | <0.001 |
| Troch BMD, g/cm2 | 0.699 ± 0.096 | 0.719 ± 0.105 | 0.670 ± 0.074 | 0.661 ± 0.096 b | 0.617 ± 0.091 a,b | <0.01 |
| Total BMD, g/cm2 | 0.932 ± 0.119 | 0.970 ± 0.124 | 0.899 ± 0.106 | 0.875 ± 0.126 b | 0.821 ± 0.122 a,b | <0.001 |
| Total Hip T-score | −0.1 ± 1.0 | 0.2 ± 1.0 | −0.4 ± 0.9 | −0.5 ± 1.0 b | −1.0 ± 1.0 a,b | <0.001 |
| Prevalence of LBM and Osteoporosis | ||||||
| LBM, n (%) | 11 (36.6) | 9 (29.0) | 15 (50.0) | 13 (54.2) | 14 (53.8) | |
| Osteoporosis, n (%) | 0 (0) | 0 (0) | 1 (3.3) | 3 (12.5) | 7 (26.9) | |
| Spine BMD | Total Hip BMD | |
|---|---|---|
| Pearson Correlations | ||
| Age | −0.147 † | −0.242 ** |
| Estradiol | 0.274 ** | 0.256 ** |
| Estrone | 0.239 * | 0.227 * |
| FSH | −0.341 *** | −0.271 ** |
| Progesterone | 0.155 † | 0.173 † |
| Testosterone | 0.072 | 0.076 |
| Energy intake | −0.015 | −0.063 |
| LTPA | −0.008 | 0.050 |
| Partial Correlations of BMD with FSH and Estradiol | ||
| FSH vs. BMD | −0.341 *** | −0.271 ** |
| Adj for Age | −0.348 *** | −0.174 † |
| Adj for Estradiol | −0.228 * | −0.148 |
| Adj for Estrone | −0.270 ** | −0.195 * |
| Adj for Progesterone | −0.320 *** | −0.244 ** |
| Adj for Testosterone | −0.337 *** | −0.265 ** |
| Adj for energy intake | −0.366 ** | −0.271 * |
| Adj for LTPA | −0.359 *** | −0.307 *** |
| Estradiol vs. BMD | 0.274 ** | 0.256 ** |
| Adj for Age | 0.261 ** | 0.161 † |
| Adj for FSH | 0.086 | 0.118 |
| Adj for Estrone | 0.141 | 0.127 |
| Adj for Progesterone | 0.237 * | 0.212 * |
| Adj for Testosterone | 0.265 ** | 0.246 ** |
| Adj for energy intake | 0.249 * | 0.248 * |
| Adj for LTPA | 0.279 ** | 0.259 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-M.; Jankowski, C.M.; Swanson, C.M.; Hildreth, K.L.; Kohrt, W.M.; Moreau, K.L. Bone Mineral Density in Different Menopause Stages is Associated with Follicle Stimulating Hormone Levels in Healthy Women. Int. J. Environ. Res. Public Health 2021, 18, 1200. https://doi.org/10.3390/ijerph18031200
Park Y-M, Jankowski CM, Swanson CM, Hildreth KL, Kohrt WM, Moreau KL. Bone Mineral Density in Different Menopause Stages is Associated with Follicle Stimulating Hormone Levels in Healthy Women. International Journal of Environmental Research and Public Health. 2021; 18(3):1200. https://doi.org/10.3390/ijerph18031200
Chicago/Turabian StylePark, Young-Min, Catherine M. Jankowski, Christine M. Swanson, Kerry L. Hildreth, Wendy M. Kohrt, and Kerrie L. Moreau. 2021. "Bone Mineral Density in Different Menopause Stages is Associated with Follicle Stimulating Hormone Levels in Healthy Women" International Journal of Environmental Research and Public Health 18, no. 3: 1200. https://doi.org/10.3390/ijerph18031200
APA StylePark, Y.-M., Jankowski, C. M., Swanson, C. M., Hildreth, K. L., Kohrt, W. M., & Moreau, K. L. (2021). Bone Mineral Density in Different Menopause Stages is Associated with Follicle Stimulating Hormone Levels in Healthy Women. International Journal of Environmental Research and Public Health, 18(3), 1200. https://doi.org/10.3390/ijerph18031200







