A Preterm Case of Cow’s Milk Allergy Presenting with Recurrent Ascites Treated with Donor Breast Milk
Abstract
1. Introduction
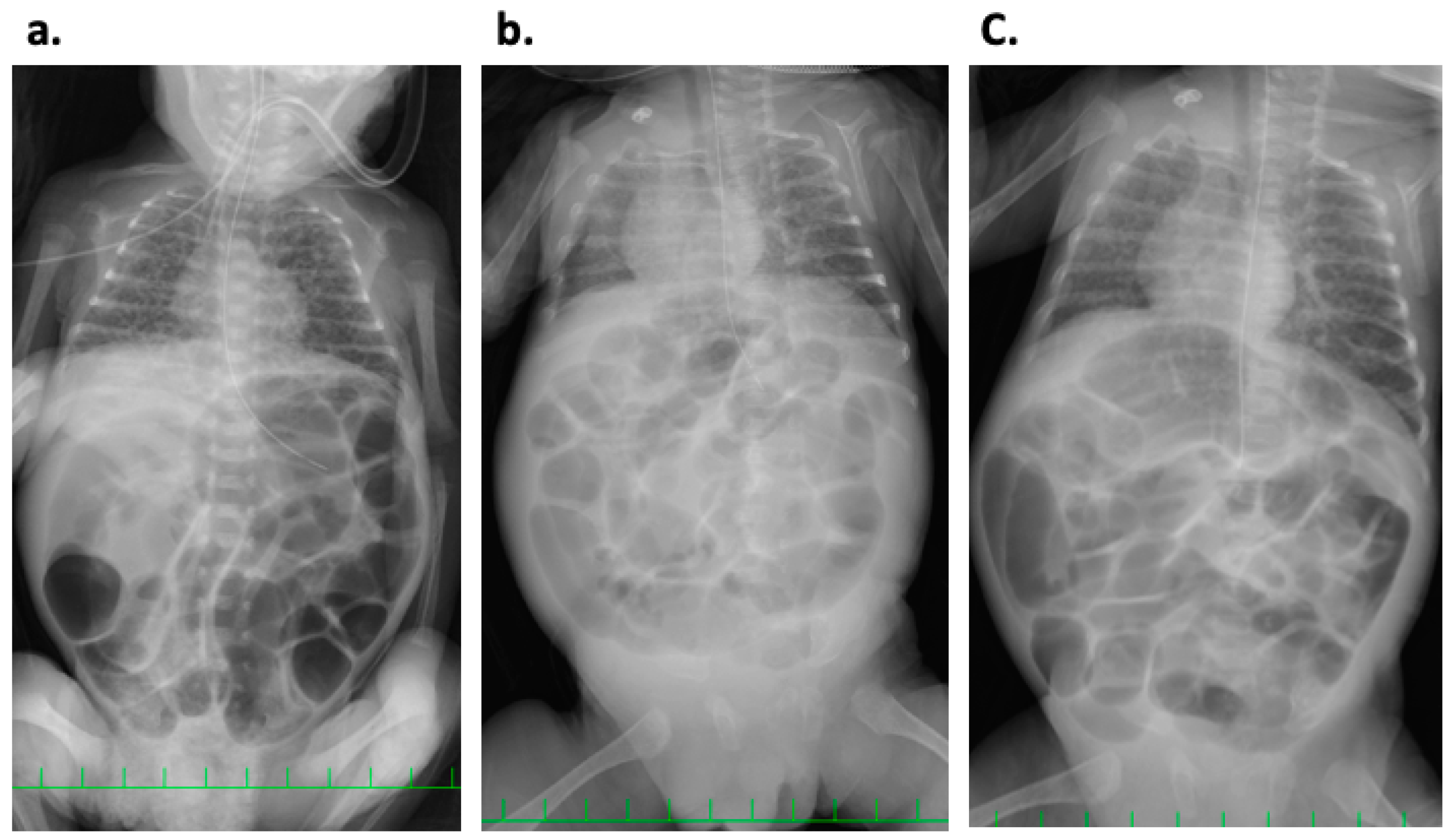
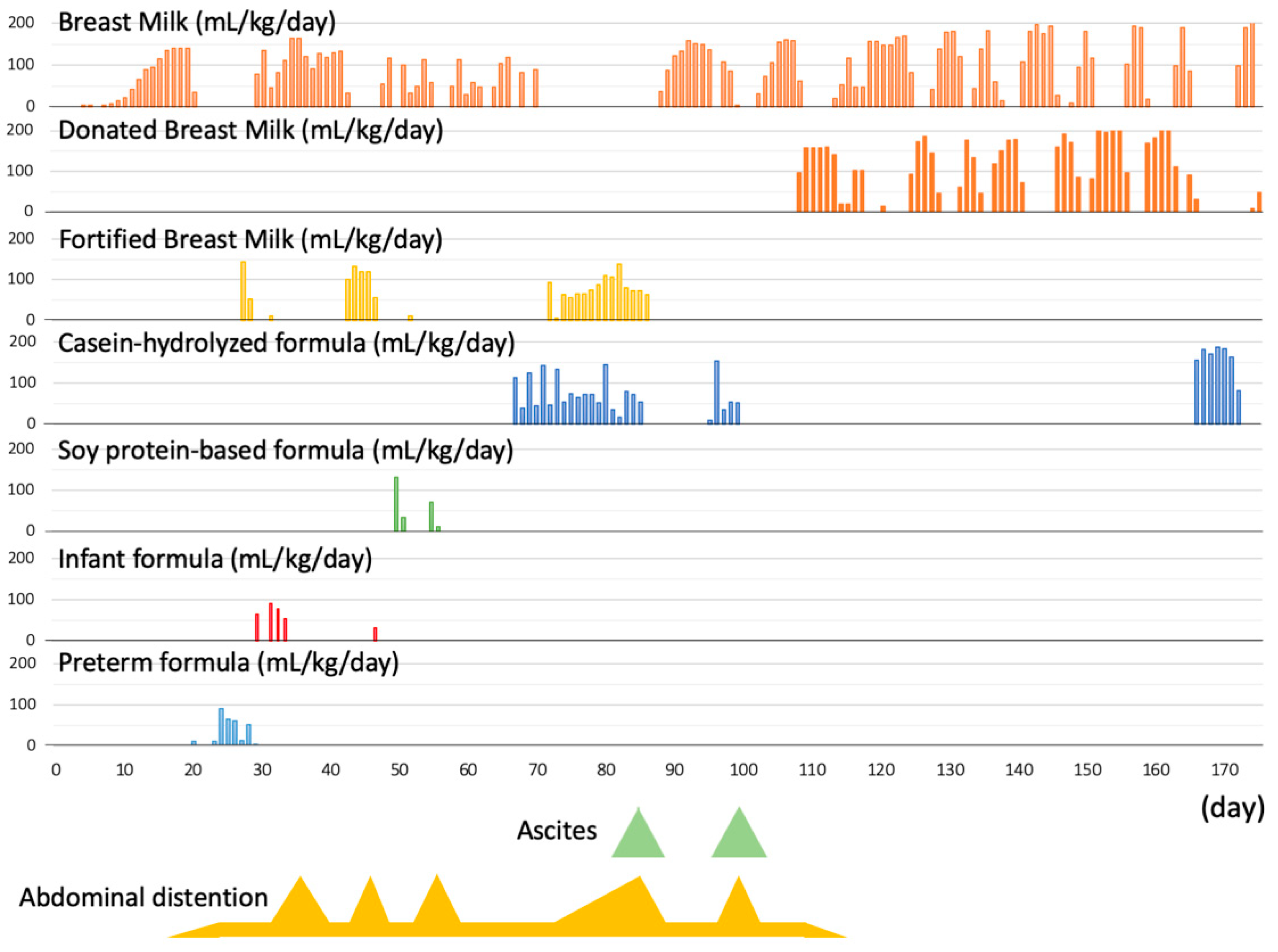
2. Case History
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Heine, R.G. Non-IgE mediated cow’s milk allergy in EuroPrevall. Allergy 2015, 70, 1679–1680. [Google Scholar] [CrossRef] [PubMed]
- Cordova, J.; Sriram, S.; Patton, T.; Jericho, H.; Gokhale, R.; Weinstein, D.; Sentongo, T. Manifestations of Cow’s-Milk Protein Intolerance in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 140–144. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itahashi, K.; Imai, T. Management of neonatal cow’s milk allergy in high-risk neonates. Pediatr. Int. 2009, 51, 544–547. [Google Scholar] [PubMed]
- Miyazawa, T.; Itabashi, K.; Imai, T. Retrospective multicenter survey on food-related symptoms suggestive of cow’s milk allergy in NICU neonates. Allergol. Int. 2013, 62, 85–90. [Google Scholar] [CrossRef]
- Vandenplas, Y. Prevention and Management of Cow’s Milk Allergy in Non-Exclusively Breastfed Infants. Nutrients 2017, 9, 731. [Google Scholar] [CrossRef]
- Morita, Y.; Iwakura, H.; Ohtsuka, H.; Kohno, Y.; Shimojo, N. Milk allergy in the neonatal intensive care unit: Comparison between premature and full-term neonates. Asia Pac. Allergy 2013, 3, 35–41. [Google Scholar]
- De Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; du Toit, G.; et al. Preventing food allergy in infancy and childhood: Systematic review of randomised controlled trials. Pediatr. Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef]
- Tudehope, D.I. Human milk and the nutritional needs of preterm infants. J. Pediatr. 2013, 162 (Suppl. 3), S17–S25. [Google Scholar]
- Section on, B. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the Establishment and Operation of Human Milk Banks in Europe: A Consensus Statement From the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrition; Section on Breastfeeding; Committee on Fetus and Newborn. Newborn, Donor Human Milk for the High-Risk Infant: Preparation, Safety, and Usage Options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Shimizu, T.; Ida, S.; Ito, S.; Inokuchi, M.; Ohura, T.; Okumura, A.; Kawai, M.; Kikuchi, T.; Sakurai, M.; et al. Policy statement of enteral nutrition for preterm and very low birthweight infants. Pediatr. Int. 2020, 62, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Brown, T.; Meyer, R.; Walsh, J.; Shah, N.; Nowak-Wegrzyn, A.; Chen, T.X.; Fleischer, D.M.; Heine, R.G.; Levin, M.; et al. Better recognition, diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy: iMAP-an international interpretation of the MAP (Milk Allergy in Primary Care) guideline. Clin. Transl. Allergy 2017, 7, 26. [Google Scholar] [CrossRef]
- Fox, A.; Brown, T.; Walsh, J.; Venter, C.; Meyer, R.; Nowak-Wegrzyn, A.; Levin, M.; Spawls, H.; Beatson, J.; Lovis, M.T.; et al. An update to the Milk Allergy in Primary Care guideline. Clin. Transl. Allergy 2019, 9, 40. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the Cow’s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carvajal, E.; Peeters, S.; Balduck, N.; Jaddioui, Y.; Ribes-Koninckx, C.; Huysentruyt, K. The Cow’s Milk-Related Symptom Score (CoMiSS(TM)): Health Care Professional and Parent and Day-to-Day Variability. Nutrients 2020, 12, 438. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Steenhout, P.; Jarvi, A.; Garreau, A.S.; Mukherjee, R. Pooled Analysis of the Cow’s Milk-related-Symptom-Score (CoMiSS) as a Predictor for Cow’s Milk Related Symptoms. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 22–26. [Google Scholar] [CrossRef]
- Prasad, R.; Venkata, R.S.A.; Ghokale, P.; Chakravarty, P.; Anwar, F. Cow’s Milk-related Symptom Score as a predictive tool for cow’s milk allergy in Indian children aged 0-24 months. Asia Pac. Allergy 2018, 8, e36. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Qiong, C.; She, T.; Bian, Y.; Lin, S.; Li, H. Component resolved diagnostic study of cow’s milk allergy in infants and young children in northern China. Int. Immunopharmacol. 2018, 61, 126–131. [Google Scholar] [CrossRef]
- Sicherer, S.H. Clinical aspects of gastrointestinal food allergy in childhood. Pediatrics 2003, 111 Pt 3, 1609–1616. [Google Scholar]
- Blackshaw., K.; Valtchev., P.; Koolaji., N.; Berry., N.; Schindeler., A.; Dehghani., F.; Banati., R.B. The risk of infectious pathogens in breast-feeding, donated human milk and breast milk substitutes. Public Health Nutr. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Adhisivam, B.; Kohat, D.; Tanigasalam, V.; Bhat, V.; Plakkal, N.; Palanivel, C. Does fortification of pasteurized donor human milk increase the incidence of necrotizing enterocolitis among preterm neonates? A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2019, 32, 3232–3237. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients 2016, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Haiden, N.; Ziegler, E.E. Human Milk Banking. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Cho, J.Y.; Kim, M.J.; Kim, E.J.; Park, E.Y.; Park, S.A.; Kim, I.Y.; Choi, Y.S.; Bae, C.W.; Chung, S.H. The Experience of Human Milk Banking for 8 Years: Korean Perspective. J. Korean Med. Sci. 2016, 31, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Han, S.P.; Wei, Q.F.; Zheng, F.Y.; Zhang, T.; Chen, H.M.; Mao, M. The data and characteristics of the human milk banks in mainland China. World J. Pediatr. 2019, 15, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.Y.; Cheng, S.W.; Wu, T.Z.; Fang, L.J. Characteristics of the first human milk bank in Taiwan. Pediatr. Neonatol. 2013, 54, 28–33. [Google Scholar] [CrossRef]
- Caubet, J.C.; Szajewska, H.; Shamir, R.; Nowak-Wegrzyn, A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr. Allergy Immunol. 2017, 28, 6–17. [Google Scholar] [CrossRef]
- Cong, Y.; Li, Y.; Li, L. Immunoglobulin E and immunoglobulin G cross-reactive allergens and epitopes between cow milk alphaS1-casein and soybean proteins. J. Dairy Sci. 2020, 103, 9815–9824. [Google Scholar] [CrossRef]
- Nutrition, E.C.; Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; et al. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar]

| Days | 0 | 30 | 60 | 90 | 120 | 150 | 173 | 197 | 253 |
|---|---|---|---|---|---|---|---|---|---|
| Bodyweight (g) | 506 | 802 | 946 | 1222 | 1486 | 1954 | 2490 | 3221 | 4713 |
| Height (cm) | N/A | 31.4 | 35.2 | 36.4 | 38.4 | 43.0 | 44.0 | 49.4 | 56.9 |
| Head circumference (cm) | N/A | 24.0 | 26.0 | 28.0 | 30.2 | 32.4 | 33.0 | 35.0 | 38.2 |
| Chest circumference (cm) | N/A | 20.0 | 24.0 | 23.2 | 26.4 | 28.4 | 29.0 | 36.0 | 39.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakasone, R.; Fujioka, K.; Suga, S.; Abe, S.; Ashina, M.; Nishida, K.; Sakurai, M.; Mizuno, K.; Nozu, K.; Iijima, K. A Preterm Case of Cow’s Milk Allergy Presenting with Recurrent Ascites Treated with Donor Breast Milk. Int. J. Environ. Res. Public Health 2021, 18, 1187. https://doi.org/10.3390/ijerph18031187
Nakasone R, Fujioka K, Suga S, Abe S, Ashina M, Nishida K, Sakurai M, Mizuno K, Nozu K, Iijima K. A Preterm Case of Cow’s Milk Allergy Presenting with Recurrent Ascites Treated with Donor Breast Milk. International Journal of Environmental Research and Public Health. 2021; 18(3):1187. https://doi.org/10.3390/ijerph18031187
Chicago/Turabian StyleNakasone, Ruka, Kazumichi Fujioka, Shutaro Suga, Shinya Abe, Mariko Ashina, Kosuke Nishida, Motoichiro Sakurai, Katsumi Mizuno, Kandai Nozu, and Kazumoto Iijima. 2021. "A Preterm Case of Cow’s Milk Allergy Presenting with Recurrent Ascites Treated with Donor Breast Milk" International Journal of Environmental Research and Public Health 18, no. 3: 1187. https://doi.org/10.3390/ijerph18031187
APA StyleNakasone, R., Fujioka, K., Suga, S., Abe, S., Ashina, M., Nishida, K., Sakurai, M., Mizuno, K., Nozu, K., & Iijima, K. (2021). A Preterm Case of Cow’s Milk Allergy Presenting with Recurrent Ascites Treated with Donor Breast Milk. International Journal of Environmental Research and Public Health, 18(3), 1187. https://doi.org/10.3390/ijerph18031187







