Effects of Acute Visual Stimulation Exercise on Attention Processes: An ERP Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
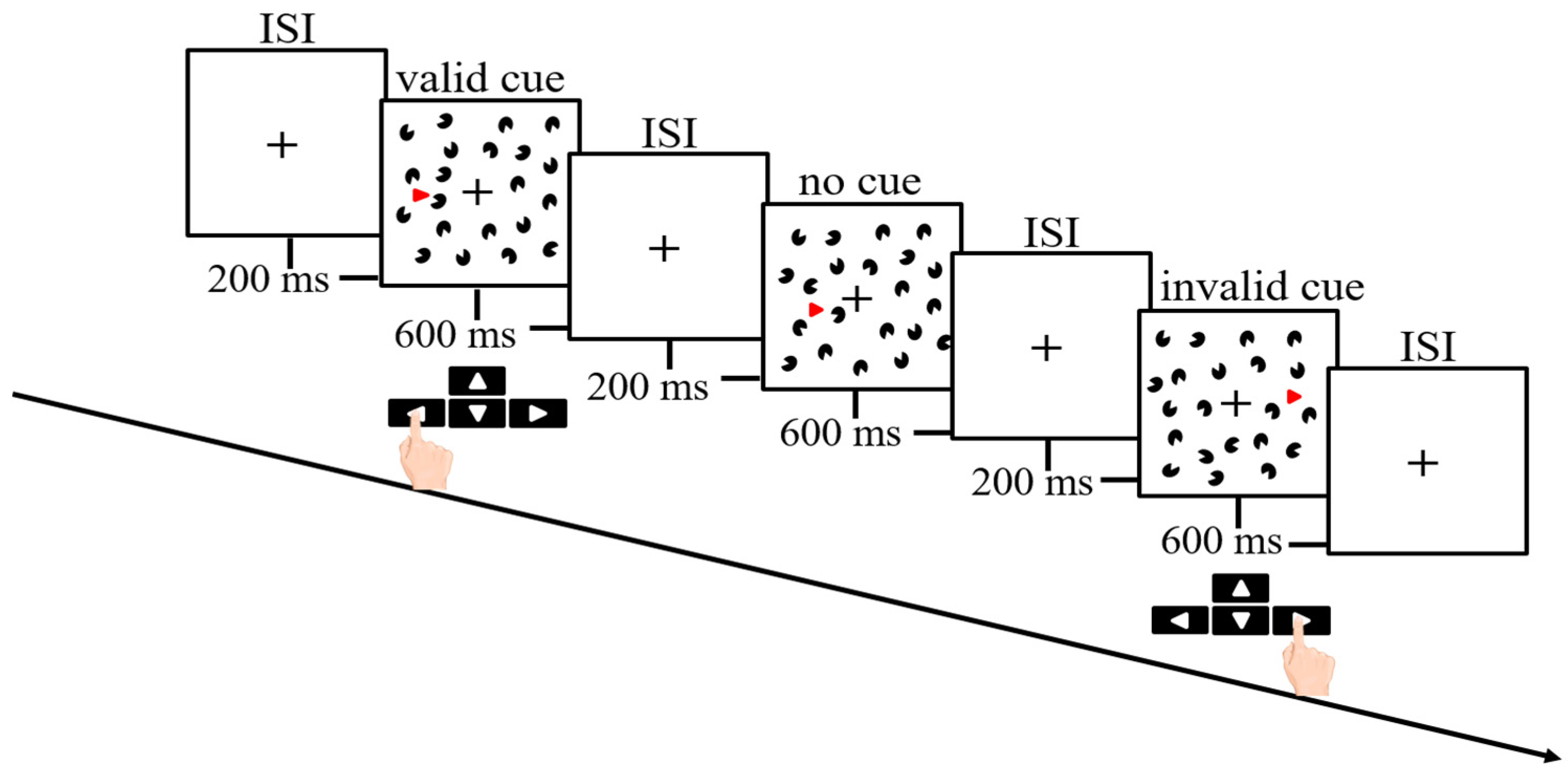
2.3. Kanizsa Triangle Task
2.4. Electroencephalography Measurements
2.5. Statistical Analysis
3. Results
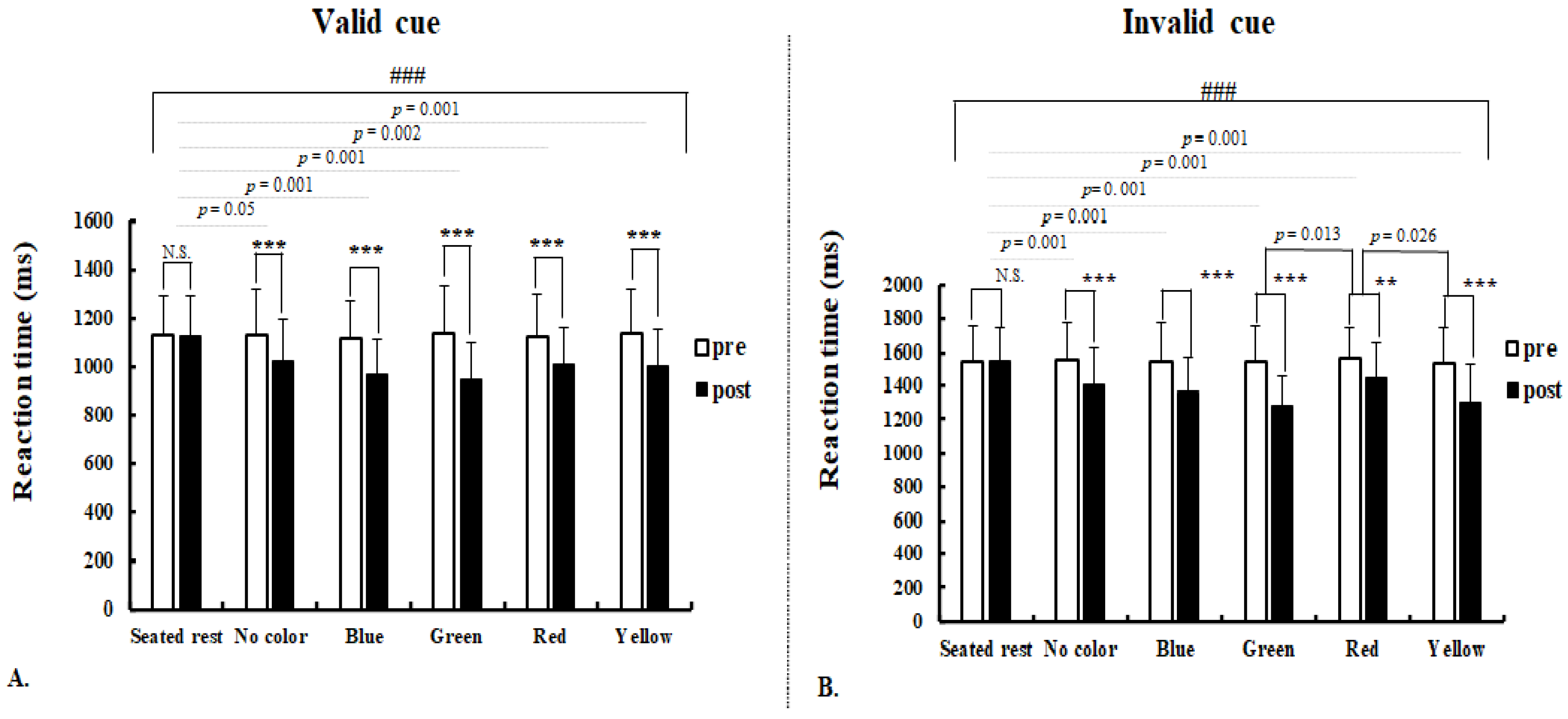
3.1. Behavioral Indices
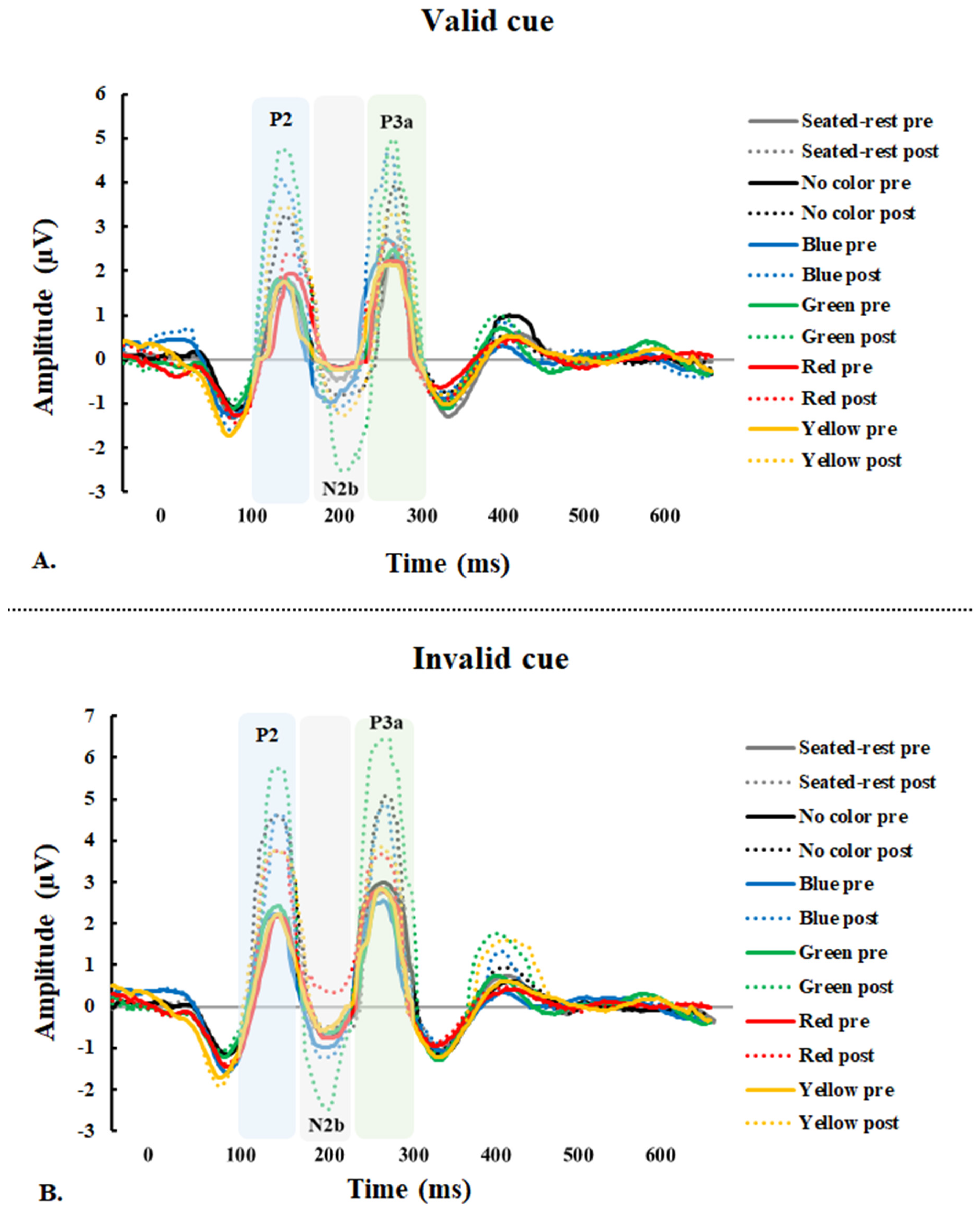
3.2. Event-related Potential Data
3.2.1. P2 Amplitude
3.2.2. N2b Amplitude
3.2.3. P3a Amplitude
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, S.E.; Posner, M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Snowden, R.J. Visual attention to color: Parvocellular guidance of attentional resources? Psychol. Sci. 2002, 13, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Worschech, F.; Ansorge, U. Top-down search for color prevents voluntary directing of attention to informative singleton cues. Exp. Psychol. 2012, 59, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Eimer, M.; Grubert, A. The gradual emergence of spatially selective target processing in visual search: From feature-specific to object-based attentional control. J. Exp. Psychol. Hum. Percept. Perform. 2014, 40, 1819–1831. [Google Scholar] [CrossRef]
- Eimer, M.; Kiss, M. Top-down search strategies determine attentional capture in visual search: Behavioral and electrophysiological evidence. Atten Percept. Psychophys. 2010, 72, 951–962. [Google Scholar] [CrossRef]
- Grubert, A.; Eimer, M. Rapid parallel attentional target selection in single-color and multiple-color visual search. J. Exp. Psychol. Hum. Percept. Perform. 2015, 41, 86–101. [Google Scholar] [CrossRef]
- Brosch, T.; Sharma, D. The role of fear-relevant stimuli in visual search: A comparison of phylogenetic and ontogenetic stimuli. Emotion 2005, 5, 360–364. [Google Scholar] [CrossRef]
- Hodsoll, S.; Viding, E.; Lavie, N. Attentional capture by irrelevant emotional distractor faces. Emotion 2011, 11, 346–353. [Google Scholar] [CrossRef]
- Huang, Y.M.; Baddeley, A.; Young, A.W. Attentional capture by emotional stimuli is modulated by semantic processing. J. Exp. Psychol. Hum. Percept. Perform. 2008, 34, 328–339. [Google Scholar] [CrossRef]
- Clarke, T.; Costall, A.J. The emotional connotations of color: A qualitative investigation. Color Res. Appl. 2008, 33, 406–410. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, J.; Kim, K.S.; Pak, C.H.J.C.R. Application: Human brain activity and emotional responses to plant color stimuli. Color Res. Appl. 2014, 39, 307–316. [Google Scholar] [CrossRef]
- Bekdash, M.; Asirvadam, V.S.; Kamel, N.; Hutapea, D.K. Identifying the human attention to different colors and intensities using P300. In Proceedings of the 2015 IEEE International Conference on Signal and Image Processing Applications (ICSIPA), Kuala Lumpur, Malaysia, 19–21 October 2015; pp. 538–541. [Google Scholar]
- Sur, S.; Sinha, V.K. Event-related potential: An overview. Ind. Psychiatry J. 2009, 18, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Rushby, J.A.; De Blasio, F.M. Neurophysiological correlates of visuospatial attention and the social dynamics of gaze processing. Cogn. Affect. Behav. Neurosci. 2019, 19, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Cid-Fernández, S.; Lindín, M.; Díaz, F. Effects of aging and involuntary capture of attention on event-related potentials associated with the processing of and the response to a target stimulus. Front. Hum. Neurosci. 2014, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Tatler, B.W.; Land, M.F. Vision and the representation of the surroundings in spatial memory. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-K.; Alderman, B.L.; Chu, C.-H.; Wang, C.-C.; Song, T.-F.; Chen, F.-T. Acute exercise has a general facilitative effect on cognitive function: A combined ERP temporal dynamics and BDNF study. Psychophysiology 2017, 54, 289–300. [Google Scholar] [CrossRef]
- Dilley, E.K.; Zou, L.; Loprinzi, P.D. The effects of acute exercise intensity on episodic and false memory among young adult college students. Health Promot. Perspect. 2019, 9, 143–149. [Google Scholar] [CrossRef]
- Du Rietz, E.; Barker, A.R.; Michelini, G.; Rommel, A.S.; Vainieri, I.; Asherson, P.; Kuntsi, J. Beneficial effects of acute high-intensity exercise on electrophysiological indices of attention processes in young adult men. Behav. Brain Res. 2019, 359, 474–484. [Google Scholar] [CrossRef]
- Hsieh, S.-S.; Huang, C.-J.; Wu, C.-T.; Chang, Y.-K.; Hung, T.-M. Acute Exercise Facilitates the N450 Inhibition Marker and P3 Attention Marker during Stroop Test in Young and Older Adults. J. Clin. Med. 2018, 7, 391. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Harris, F.; McRaney, K.; Chism, M.; Deming, R.; Jones, T.; Zou, L.; Tan, M. Effects of Acute Exercise and Learning Strategy Implementation on Memory Function. Medicina 2019, 55, 568. [Google Scholar] [CrossRef]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Callow, D.D.; Smith, J.C. Brain activation during executive control after acute exercise in older adults. Int. J. Psychophysiol. 2019, 146, 240–248. [Google Scholar] [CrossRef]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Michelson, C.S.; Callow, D.D.; Ranadive, S.M.; Gentili, R.J.; Smith, J.C. Semantic Memory Activation After Acute Exercise in Healthy Older Adults. J. Int. Neuropsychol. Soc. 2019, 25, 557–568. [Google Scholar] [CrossRef]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Nyhuis, C.C.; Spira, A.P.; Callow, D.D.; Smith, J.C. Caudate Volume Mediates the Interaction between Total Sleep Time and Executive Function after Acute Exercise in Healthy Older Adults. Brain Plast. 2019, 5, 69–82. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K.C.; Pontifex, M.B.; Scudder, M.R.; Brown, M.L.; Hillman, C.H. The effects of single bouts of aerobic exercise, exergaming, and videogame play on cognitive control. Clin. Neurophysiol. 2011, 122, 1518–1525. [Google Scholar] [CrossRef]
- Ligeza, T.S.; Maciejczyk, M.; Kalamala, P.; Szygula, Z.; Wyczesany, M. Moderate-intensity exercise boosts the N2 neural inhibition marker: A randomized and counterbalanced ERP study with precisely controlled exercise intensity. Biol. Psychol. 2018, 135, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Albinet, C.; Canivet, A.; Gierski, F.; Morrone, I.; Besche-Richard, C. Brief aerobic exercise immediately enhances visual attentional control and perceptual speed. Testing the mediating role of feelings of energy. Acta Psychol. 2018, 191, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Wang, C.-H.; Pan, C.-Y.; Chen, F.-C.; Huang, S.-Y.; Tseng, Y.-T. The effects of different exercise types on visuospatial attention in the elderly. Psychol. Sport Exerc. 2016, 26, 130–138. [Google Scholar] [CrossRef]
- Llorens, F.; Sanabria, D.; Huertas, F. The Influence of Acute Intense Exercise on Exogenous Spatial Attention Depends on Physical Fitness Level. Exp. Psychol. 2015, 62, 20–29. [Google Scholar] [CrossRef]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Boil. Fenn. 1957, 35, 307–315. [Google Scholar]
- Senkowski, D.; Röttger, S.; Grimm, S.; Foxe, J.J.; Herrmann, C.S. Kanizsa subjective figures capture visual spatial attention: Evidence from electrophysiological and behavioral data. Neuropsychologia 2005, 43, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Wu, S.; Ji, H.; Smith, J.C.; Park, J.-J.; Ji, H. Executive Function and the P300 after Treadmill Exercise and Futsal in College Soccer Players. Sports 2017, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.; Kojima, H. Acute moderate-intensity exercise generally enhances neural resources related to perceptual and cognitive processes: A randomized controlled ERP study. Ment. Health Phys. Act. 2020, 19, 100363. [Google Scholar] [CrossRef]
- Aley, L.; Miller, E.W.; Bode, S.; Hall, L.; Markusic, J.; Nicholson, M.; Winegardner, M. Effects of Age, Task Complexity, and Exercise on Reaction Time of Women During Ambulation Tasks. J. Geriatr. Phys. Ther. 2007, 30, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Davranche, K.; Hall, B.; McMorris, T. Effect of Acute Exercise on Cognitive Control Required during an Eriksen Flanker Task. J. Sport Exerc. Psychol. 2009, 31, 628–639. [Google Scholar] [CrossRef]
- Dux, P.E.; Tombu, M.N.; Harrison, S.; Rogers, B.P.; Tong, F.; Marois, R. Training Improves Multitasking Performance by Increasing the Speed of Information Processing in Human Prefrontal Cortex. Neuron 2009, 63, 127–138. [Google Scholar] [CrossRef]
- Zhou, F.; Qin, C. Acute Moderate-Intensity Exercise Generally Enhances Attentional Resources Related to Perceptual Processing. Front. Psychol. 2019, 10, 2547. [Google Scholar] [CrossRef]
- Spreckelmeyer, K.N.; Kutas, M.; Urbach, T.P.; Altenmüller, E.; Münte, T.F. Combined perception of emotion in pictures and musical sounds. Brain Res. 2006, 1070, 160–170. [Google Scholar] [CrossRef]
- Akers, A.; Barton, J.; Cossey, R.; Gainsford, P.; Griffin, M.; Micklewright, D. Visual color perception in green exercise: Positive effects on mood and perceived exertion. Environ. Sci. Technol. 2012, 46, 8661–8666. [Google Scholar] [CrossRef]
- Rogerson, M.; Barton, J. Effects of the Visual Exercise Environments on Cognitive Directed Attention, Energy Expenditure and Perceived Exertion. Int. J. Environ. Res. Public Health 2015, 12, 7321–7336. [Google Scholar] [CrossRef]
- Briki, W.; Majed, L. Adaptive Effects of Seeing Green Environment on Psychophysiological Parameters When Walking or Running. Front. Psychol. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Qi, Z.; Shi, J.; Zhang, M.; Luo, W. The Early Facilitative and Late Contextual Specific Effect of the Color Red on Attentional Processing. Front. Hum. Neurosci. 2018, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Cho, K.-I.; Ji, H.; Kim, K.-H.; Park, J.-J.; Kim, B.H.; Cho, K.-I.; Skjaeret, N.; Rosly, M. Exergaming Improves Executive Functions in Patients With Metabolic Syndrome: Randomized Controlled Trial. JMIR Serious Games 2019, 7, e13575. [Google Scholar] [CrossRef] [PubMed]
- Drollette, E.S.; Scudder, M.R.; Raine, L.B.; Moore, R.D.; Saliba, B.J.; Pontifex, M.B.; Hillman, C.H. Acute exercise facilitates brain function and cognition in children who need it most: An ERP study of individual differences in inhibitory control capacity. Dev. Cogn. Neurosci. 2014, 7, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sood, S.; Singh, M.; Beena; Sakshi. Effect of acute moderate exercise on cognitive event-related potentials n100, p200, n200, and interpeak latencies. Indian J. Psychol. Med. 2010, 32, 131–135. [Google Scholar] [CrossRef]
- To, W.T.; De Ridder, D.; Menovsky, T.; Hart, J.; Vanneste, S. The role of the dorsal Anterior Cingulate Cortex (dACC) in a cognitive and emotional counting Stroop task: Two cases. Restor. Neurol. Neurosci. 2017, 35, 333–345. [Google Scholar] [CrossRef]
- Balconi, M.; Bortolotti, A. Emotional face recognition, empathic trait (BEES), and cortical contribution in response to positive and negative cues. The effect of rTMS on dorsal medial prefrontal cortex. Cogn. Neurodyn. 2012, 7, 13–21. [Google Scholar] [CrossRef]
- Buzzell, G.A.; Fedota, J.R.; Roberts, D.M.; McDonald, C.G. The N2 ERP component as an index of impaired cognitive control in smokers. Neurosci. Lett. 2014, 563, 61–65. [Google Scholar] [CrossRef]
- Yeung, N.; Botvinick, M.M.; Cohen, J.D. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol. Rev. 2004, 111, 931–959. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Hillman, C.H.; Polich, J. Age, physical fitness, and attention: P3a and P3b. Psychophysiology 2009, 46, 379–387. [Google Scholar] [CrossRef]
- Bullock, T.; Cecotti, H.; Giesbrecht, B. Multiple stages of information processing are modulated during acute bouts of exercise. Neuroscience 2015, 307, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Takano, K.; Wada, M.; Saeki, N.; Kansaku, K. Effect of the Green/Blue Flicker Matrix for P300-Based Brain-Computer Interface: An EEG-fMRI Study. Front. Neurol. 2012, 3, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Bieger, J.; Garcia Molina, G.; Aarts, R.M. A survey of stimulation methods used in SSVEP-based BCIs. Comput. Intell. Neurosci. 2010, 2010, 702357. [Google Scholar] [CrossRef]
- Okuno, T.; Sugiyama, T.; Kohyama, M.; Kojima, S.; Oku, H.; Ikeda, T. Ocular blood flow changes after dynamic exercise in humans. Eye 2005, 20, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Secher, N.H. Cerebral blood flow and metabolism during exercise. Prog. Neurobiol. 2000, 61, 397–414. [Google Scholar] [CrossRef]
- MacIntosh, B.J.; Crane, D.E.; Sage, M.D.; Rajab, A.S.; Donahue, M.J.; McIlroy, W.E.; Middleton, L.E. Impact of a Single Bout of Aerobic Exercise on Regional Brain Perfusion and Activation Responses in Healthy Young Adults. PLoS ONE 2014, 9, e85163. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Paulson, E.S.; Cook, D.B.; Verber, M.D.; Tian, Q. Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: Implications for fMRI. J. Neurosci. Methods 2010, 191, 258–262. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Gwizdala, K.L.; Weng, T.B.; Zhu, D.C.; Voss, M.W. Cerebral blood flow is not modulated following acute aerobic exercise in preadolescent children. Int. J. Psychophysiol. 2018, 134, 44–51. [Google Scholar] [CrossRef]

| Variable | Values Are Mean ± Standard Deviation |
|---|---|
| Sample Size (n) | 22 (10 Females) |
| Age (years) | 24.0 ± 3.0 |
| Height (cm) | 169.91 ± 7.39 |
| Weight (kg) | 61.06 ± 9.42 |
| Valid Cue | Invalid Cue | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Pre | Post | Session p (η2p) | Session × Intervention p (η2p) | Session Effect p (η2p) | Pre-Post Difference Comparison p (η2p) | Pre | Post | Session p (η2p) | Session × Intervention p (η2p) | Session Effect p (η2p) | Pre-Post Difference Comparison p (η2p) |
| P2 Amplitude (μV) | ||||||||||||
| Seated rest | 1.80 ± 1.83 | 1.75 ± 1.63 | 0.670 (0.508) | 0.001 (0.326) | 0.001 (0.507) | 0.001 (0.653) G > S, R, N, Y; S, N, B, Y > R | 2.21 ± 1.46 | 2.24 ± 1.62 | 0.790 (0.505) | 0.041 (0.228) | 0.001 (0.465) | 0.050 (0.454) S < N, B, G G > R, Y |
| No color | 1.67 ± 2.00 | 3.25 ± 1.72 | 0.001 (0.725) | 2.18 ± 1.92 | 4.59 ± 1.80 | 0.001 (0.820) | ||||||
| Blue | 1.71 ± 1.55 | 4.06 ± 1.53 | 0.001 (0.860) | 2.26 ± 1.73 | 4.37 ± 1.68 | 0.001 (0.809) | ||||||
| Green | 1.82 ± 1.78 | 4.72 ± 1.87 | 0.001 (0.387) | 2.41 ± 1.60 | 5.72 ± 1.71 | 0.001 (1.000) | ||||||
| Red | 1.93 ± 1.82 | 2.37 ± 1.71 | 0.090 (0.570) | 2.14 ± 1.58 | 3.74 ± 2.74 | 0.023 (0.694) | ||||||
| Yellow | 1.73 ± 1.85 | 3.43 ± 1.83 | 0.001 (0.743) | 2.21 ± 1.53 | 3.75 ± 1.70 | 0.002 (0.750) | ||||||
| N2b Amplitude (μV) | ||||||||||||
| Seated rest | −0.45 ± 2.10 | −0.49 ± 2.09 | 0.714 (0.505) | 0.001 (0.165) | 0.001 (0.125) | 0.004 (0.582) S < N, B, G, R, Y; G > N, B, R, Y | −0.70 ± 2.1.9 | −0.70 ± 1.99 | 0.992 (0.500) | 0.002 (0.139) | 0.492 (0.004) | 0.003 (0.621) G > N, B, R, Y; S > R |
| No color | −0.17 ± 2.47 | −0.80 ± 2.32 | 0.189 (0.574) | −0.74 ± 2.16 | −0.49 ± 2.67 | 0.638 (0.524) | ||||||
| Blue | −0.98 ± 2.11 | −1.13 ± 2.00 | 0.704 (0.521) | −1.00 ± 2.16 | −1.22 ± 2.05 | 0.624 (0.529) | ||||||
| Green | −0.23 ± 2.14 | −2.50 ± 2.10 | 0.001 (0.776) | −0.61 ± 2.20 | −2.46 ± 3.08 | 0.001 (0.687) | ||||||
| Red | −0.21 ± 2.13 | −0.17 ± 1.71 | 0.935 (0.623) | −0.76 ± 2.28 | 0.34 ± 2.76 | 0.112 (0.621) | ||||||
| Yellow | −0.24 ± 2.37 | −1.28 ± 2.34 | 0.074 (0.047) | −0.50 ± 2.19 | −0.57 ± 2.69 | 0.907 (0.508) | ||||||
| P3a Amplitude (μV) in Fz Region | ||||||||||||
| Seated rest | 2.35 ± 0.96 | 2.27 ± 0.96 | 0.128 (0.521) | 0.001 (0.334) | 0.017 (0.548) | 0.001 (0.588) S < N, B, G, Y; G > N, B, R, Y; N, B > R N, B > Y B > R, Y | 2.81 ± 1.27 | 2.80 ± 1.41 | 0.951 (0.502) | 0.001 (0.284) | 0.001 (0.505) | 0.001 (0.653) S < N, B, G, Y; G > N, B, R, Y |
| No color | 2.22 ± 1.31 | 4.05 ± 1.49 | 0.001 (0.822) | 2.83 ± 1.46 | 5.20 ± 1.49 | 0.001 (0.872) | ||||||
| Blue | 2.48 ± 1.05 | 4.68 ± 1.47 | 0.001 (0.888) | 2.66 ± 1.08 | 5.08 ± 1.28 | 0.001 (0.926) | ||||||
| Green | 2.23 ± 1.23 | 5.00 ± 1.22 | 0.001 (0.945) | 2.94 ± 1.45 | 6.83 ± 1.45 | 0.001 (0.971) | ||||||
| Red | 2.11 ± 1.09 | 2.84 ± 1.41 | 0.033 (0.659) | 2.73 ± 1.51 | 3.85 ± 2.13 | 0.047 (0.666) | ||||||
| Yellow | 2.34 ± 0.99 | 3.66 ± 1.88 | 0.006 (0.733) | 2.73 ± 1.25 | 4.50 ± 2.62 | 0.020 (0.729) | ||||||
| P3a Amplitude (μV) in Cz Region | ||||||||||||
| Seated rest | 2.21 ± 1.16 | 2.42 ± 1.00 | 0.125 (0.555) | 0.001 (0.236) | 0.001 (0.448) | 0.001 (0.560) S < N, B, G; G > R, Y: N, B > R | 2.77 ± 1.08 | 2.88 ± 1.35 | 0.205 (0.525) | 0.001 (0.299) | 0.001 (0.474) | 0.001 (0.730) S < N, B, G; G > N, B, R, Y |
| No color | 2.29 ± 1.27 | 3.91 ± 1.46 | 0.001 (0.799) | 2.97 ± 1.25 | 5.05 ± 1.35 | 0.001 (0.871) | ||||||
| Blue | 2.69 ± 1.06 | 4.64 ± 1.77 | 0.001 (0.828) | 2.53 ± 1.10 | 4.87 ± 1.23 | 0.001 (0.922) | ||||||
| Green | 2.46 ± 1.22 | 4.97 ± 1.31 | 0.001 (0.920) | 2.83 ± 1.47 | 6.43 ± 1.35 | 0.001 (0.964) | ||||||
| Red | 2.22 ± 1.27 | 2.64 ± 1.32 | 0.257 (0.591) | 2.85 ± 1.58 | 3.68 ± 2.15 | 0.097 (0.622) | ||||||
| Yellow | 2.13 ± 1.13 | 3.30 ± 1.76 | 0.012 (0.712) | 2.81 ± 1.28 | 3.83 ± 2.35 | 0.105 (0.468) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Ji, H.; Won, J.; Liu, X.; Park, J.-J. Effects of Acute Visual Stimulation Exercise on Attention Processes: An ERP Study. Int. J. Environ. Res. Public Health 2021, 18, 1107. https://doi.org/10.3390/ijerph18031107
Wu S, Ji H, Won J, Liu X, Park J-J. Effects of Acute Visual Stimulation Exercise on Attention Processes: An ERP Study. International Journal of Environmental Research and Public Health. 2021; 18(3):1107. https://doi.org/10.3390/ijerph18031107
Chicago/Turabian StyleWu, Shanshan, Hongqing Ji, Junyeon Won, Xiaolong Liu, and Jung-Jun Park. 2021. "Effects of Acute Visual Stimulation Exercise on Attention Processes: An ERP Study" International Journal of Environmental Research and Public Health 18, no. 3: 1107. https://doi.org/10.3390/ijerph18031107
APA StyleWu, S., Ji, H., Won, J., Liu, X., & Park, J.-J. (2021). Effects of Acute Visual Stimulation Exercise on Attention Processes: An ERP Study. International Journal of Environmental Research and Public Health, 18(3), 1107. https://doi.org/10.3390/ijerph18031107







