Mitigation of CyanoHABs Using Phoslock® to Reduce Water Column Phosphorus and Nutrient Release from Sediment
Abstract
:1. Introduction
2. Materials and Methods
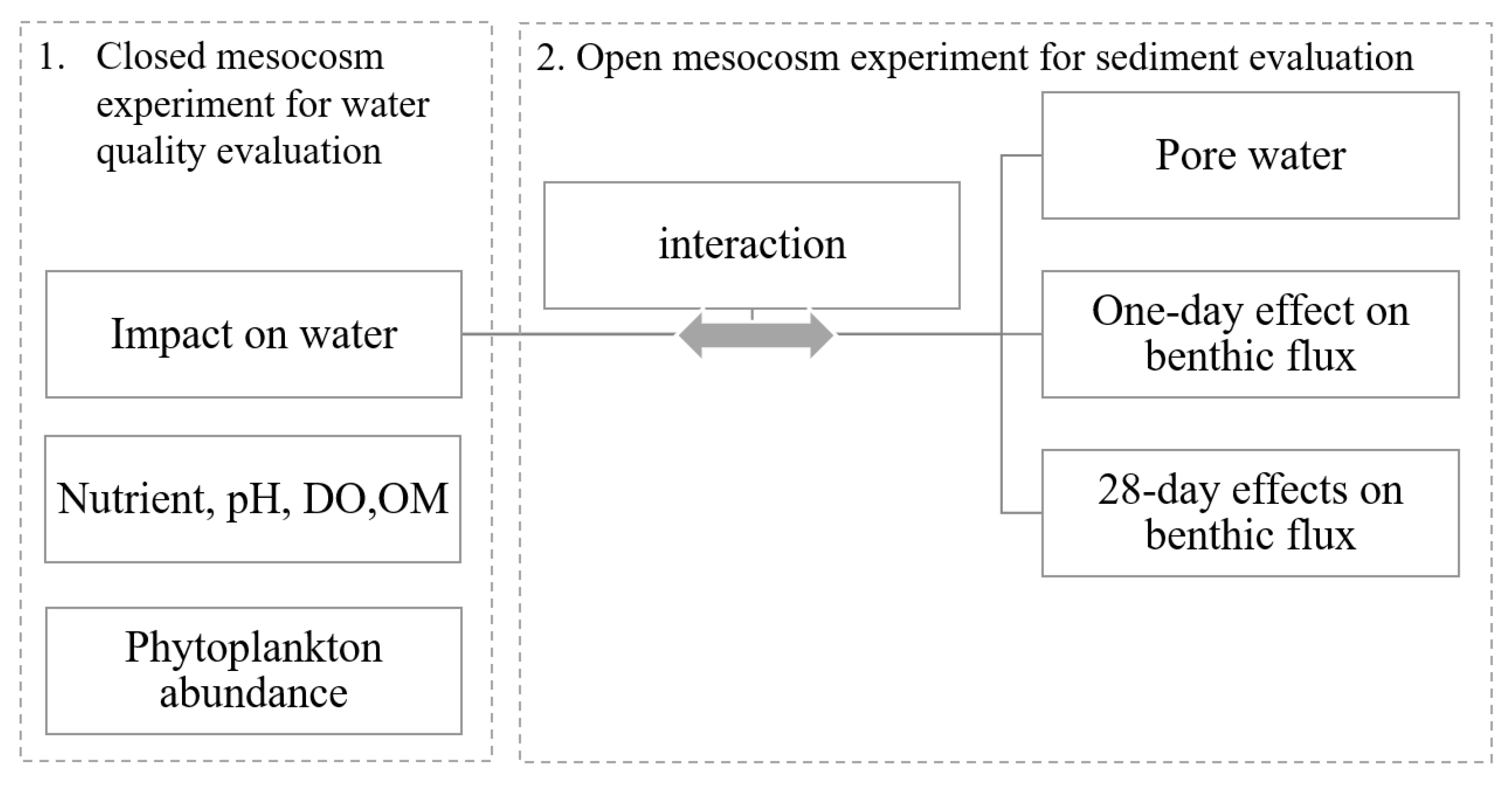
2.1. Experiment Design
2.2. Phoslock® Impacts on the Water Column
2.3. Phoslock® Effects on Sediment Nutrient Processes
2.4. Chemical and Biological Analysis
2.5. Statistical Analysis
3. Results
3.1. Bloom Progression in the Lake
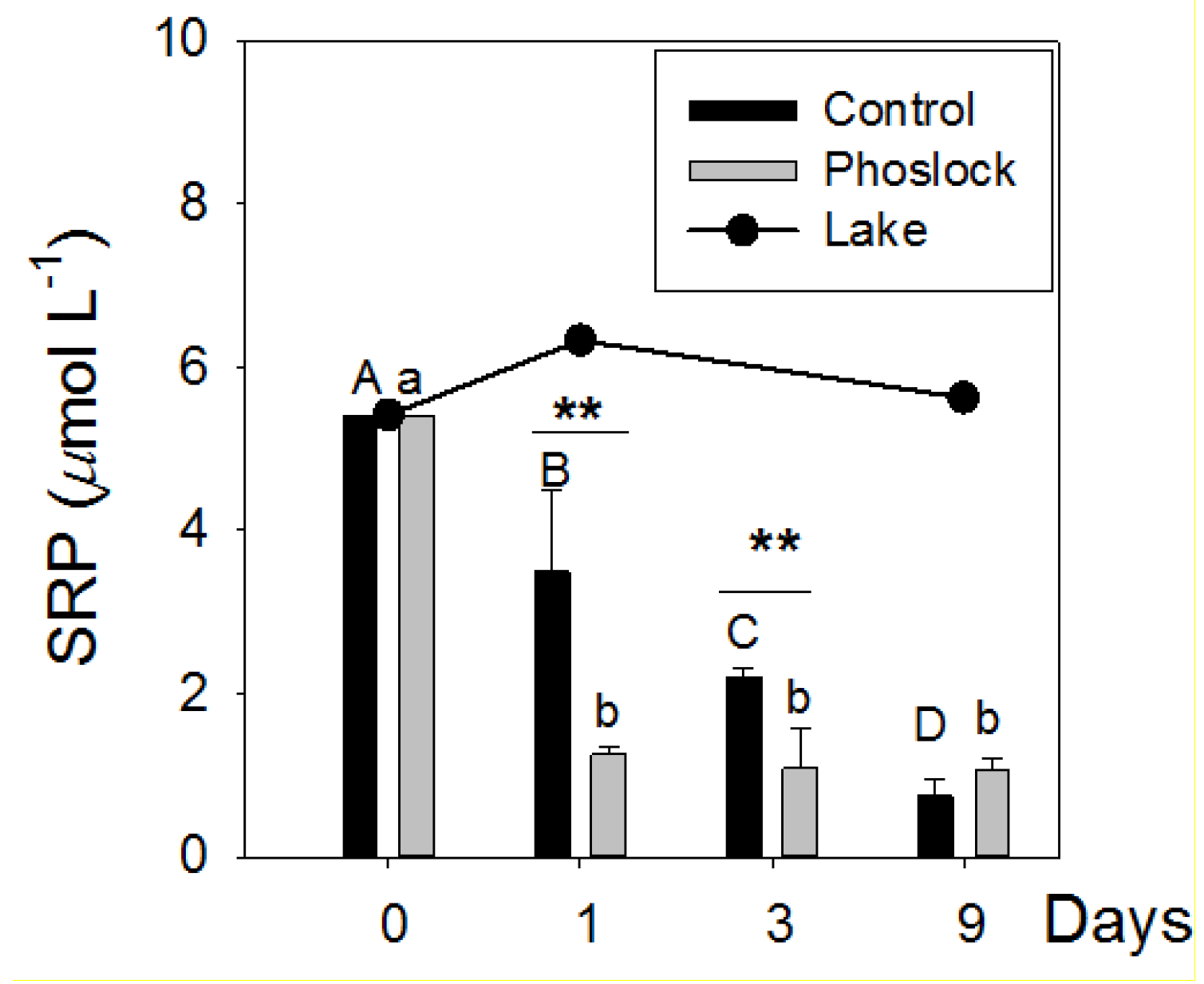
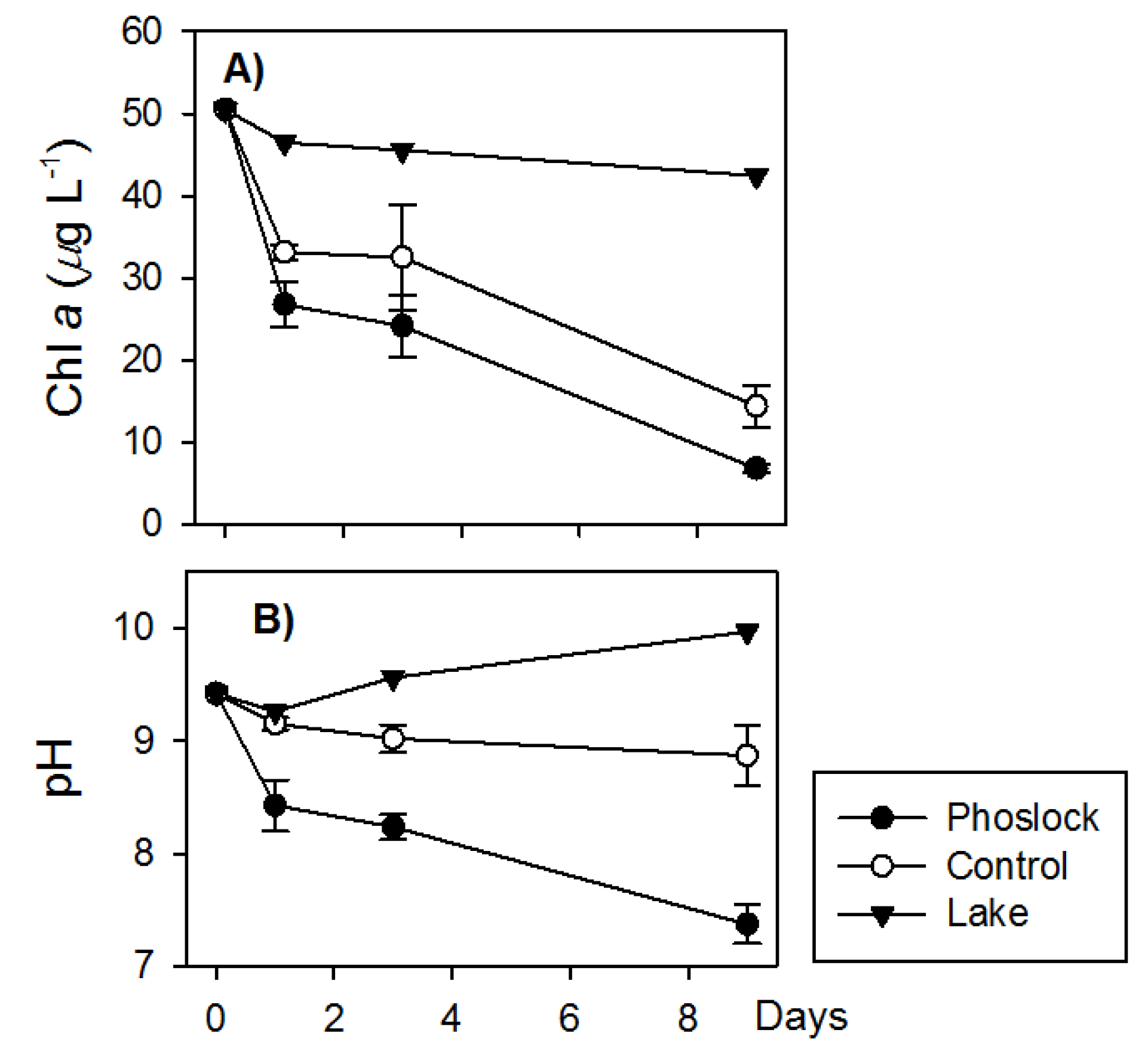
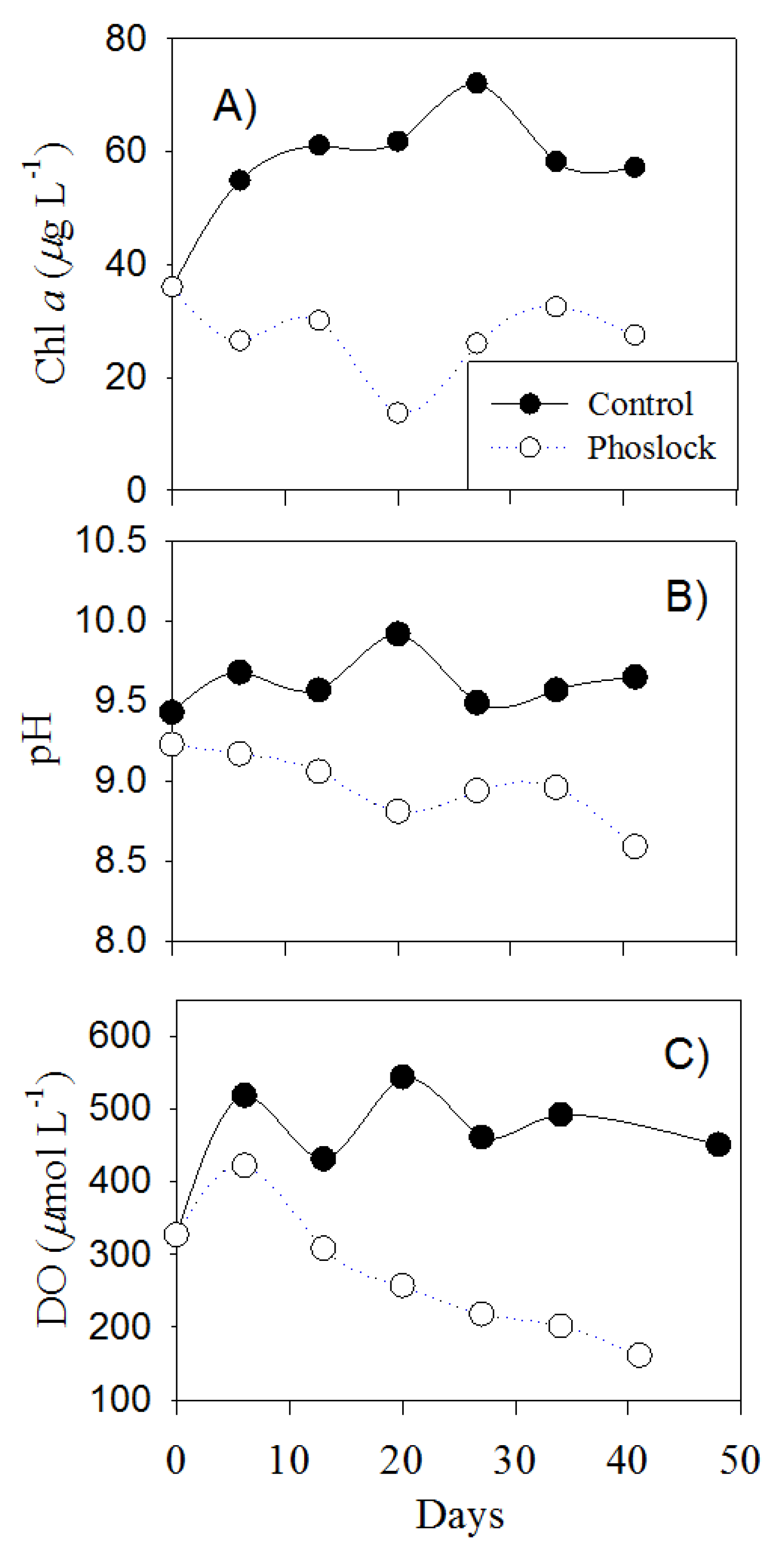
3.2. Lake Water Treated with Phoslock®
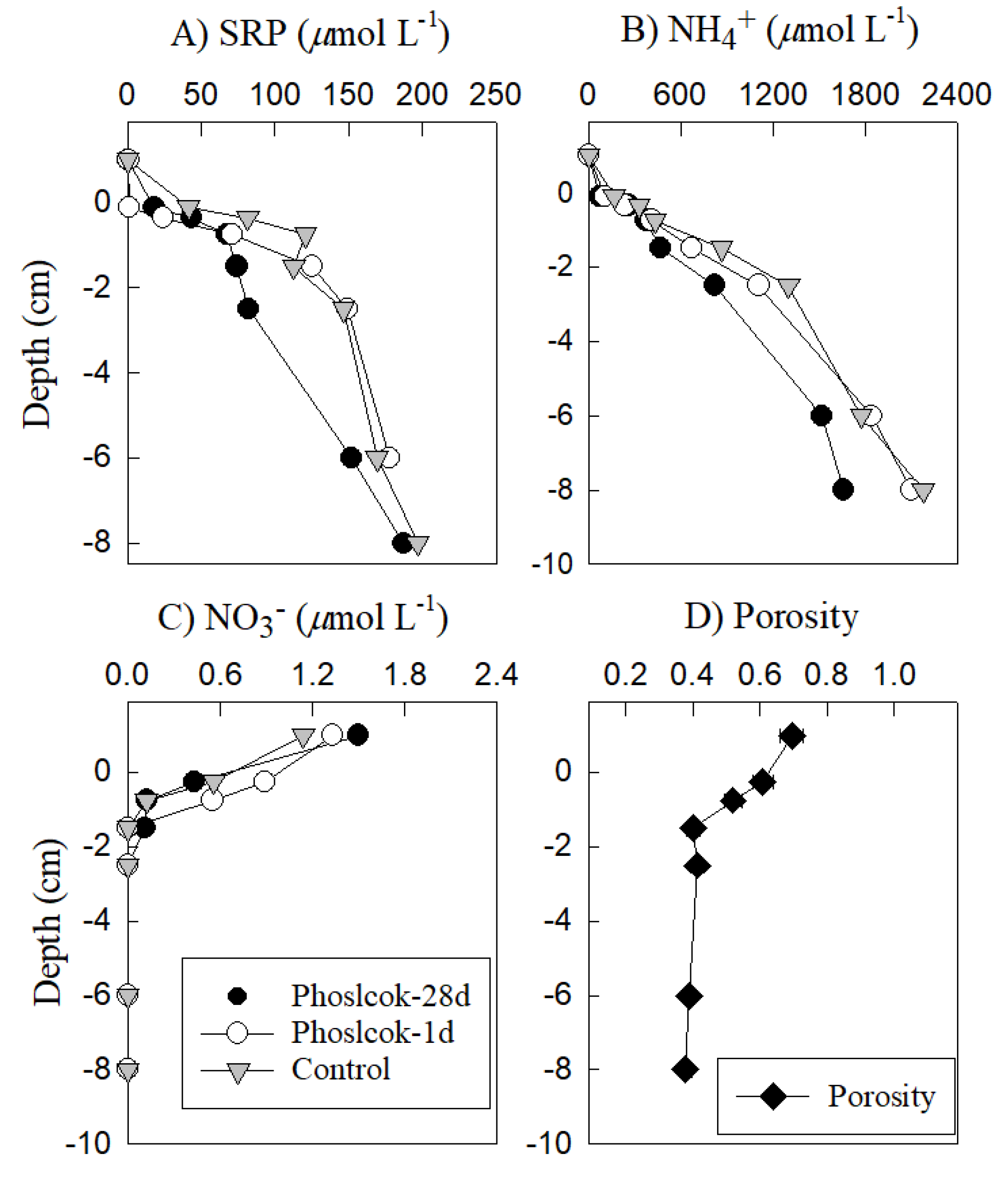
3.3. Sediment Pore Water Changes with Phoslock® Treatment
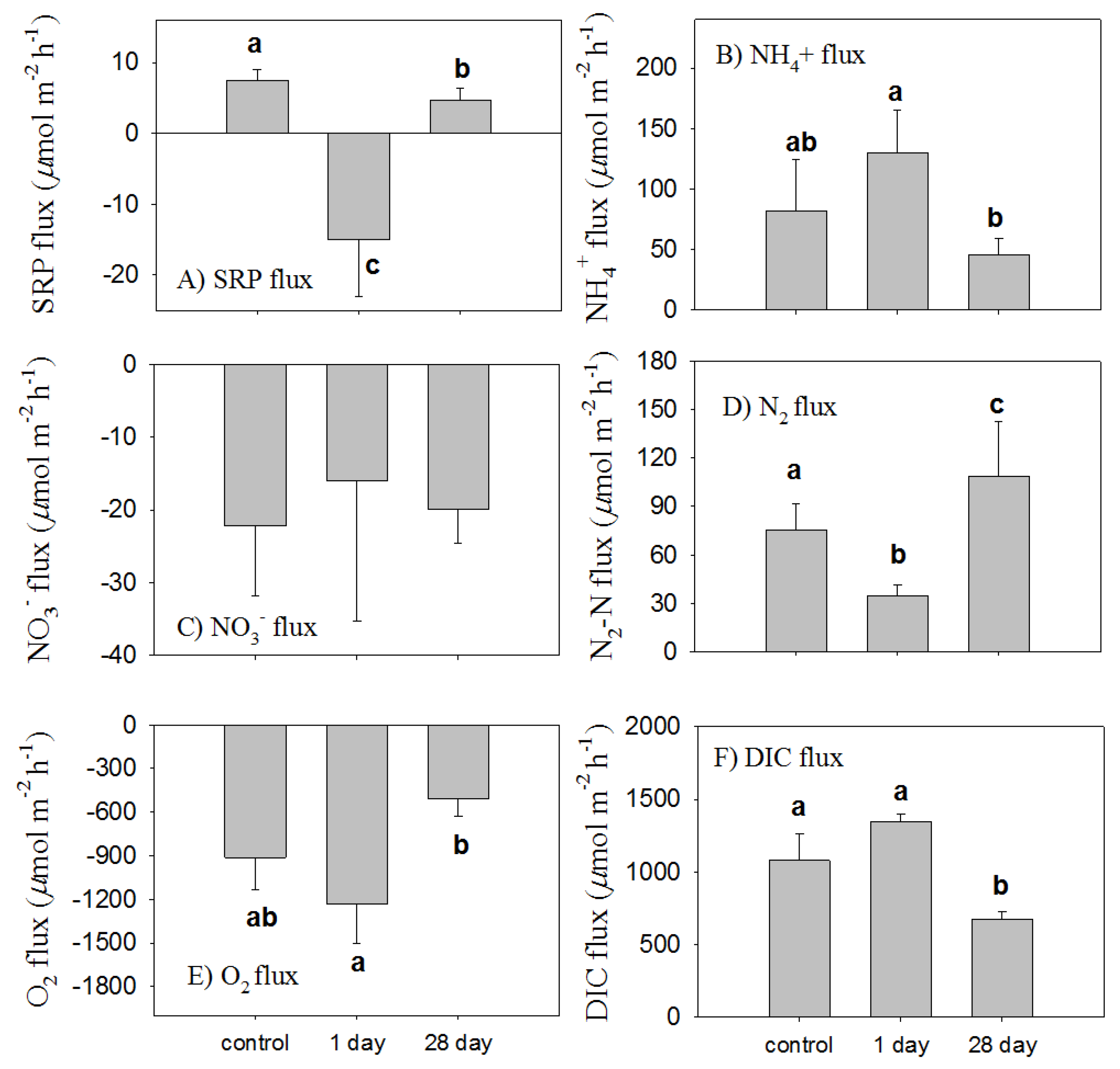
3.4. The Short-Term Effect of Phoslock® on Sediment Flux
3.5. Long-Term Changes in Sediment Fluxes through Phoslock® Capping
3.6. Water Column Changes after Phoslock® Treatment on Sediments
4. Discussion
4.1. Phoslock® Influences on Water Chemistry
4.2. Changes in Sediment Fluxes One Day after Phoslock® Addition
4.3. Long-Term Changes in Sediment Fluxes after Adding Phoslock®
4.4. Ecological Response due to Phoslock®
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Genet. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Gobler, C.J.; Hansen, P.J. Dynamic CO2 and pH levels in coastal, estuarine, and inland waters: Theoretical and observed effects on harmful algal blooms. Harmful Algae 2020, 91, 101594. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloğlu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najjar, R.G.; Pyke, C.R.; Adams, M.B.; Breitburg, D.; Hershner, C.; Kemp, M.; Howarth, R.; Mulholland, M.R.; Paolisso, M.; Secor, D.; et al. Potential climate-change impacts on the Chesapeake Bay. Estuarine Coast. Shelf Sci. 2010, 86, 1–20. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [Green Version]
- Zehr, J.P.; Capone, D.G. Changing perspectives in marine nitrogen fixation. Science 2020, 368, 729. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Biegala, I.C.; Dutrieux, P.; Slemons, L.O.; Capone, D.G. Nitrogen fixation in the western equatorial Pacific: Rates, diazotrophic cyanobacterial size class distribution, and biogeochemical significance. Glob. Biogeochem. Cycles 2009, 23, 23. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total. Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neil, J.; Davis, T.; Burford, M.; Gobler, C. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Z.; Long, Y.; Feng, M. Replenishment of urban landscape ponds with reclaimed water: Spatiotemporal variations of water quality and mechanism of algal inhibition with alum sludge. Sci. Total. Environ. 2021, 790, 148052. [Google Scholar] [CrossRef]
- Hilt, S.; Brothers, S.; Jeppesen, E.; Veraart, A.; Kosten, S. Translating Regime Shifts in Shallow Lakes into Changes in Ecosystem Functions and Services. Bioscience 2017, 67, 928–936. [Google Scholar] [CrossRef]
- Hupfer, M.; Reitzel, K.; Kleeberg, A.; Lewandowski, J. Long-term efficiency of lake restoration by chemical phosphorus precipitation: Scenario analysis with a phosphorus balance model. Water Res. 2016, 97, 153–161. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Yang, B.; Lin, H.; Miller, T.R.; Newton, R.J.; Guo, L. Causal relationship between alkaline phosphatase activities and phosphorus dynamics in a eutrophic coastal lagoon in Lake Michigan. Sci. Total. Environ. 2021, 787, 147681. [Google Scholar] [CrossRef]
- Boynton, W.; Garber, J.H.; Summers, R.; Kemp, W.M. Inputs, Transformations, and Transport of Nitrogen and Phosphorus in Chesapeake Bay and Selected Tributaries. Estuaries 1995, 18, 285–314. [Google Scholar] [CrossRef]
- Brandes, J.A.; Devol, A.A.H.; Deutsch, C. New Developments in the Marine Nitrogen Cycle. Chem. Rev. 2007, 107, 577–589. [Google Scholar] [CrossRef]
- Seitzinger, S.P. The effect of pH on the release of phosphorus from Potomac estuary sediments: Implications for blue-green algal blooms. Estuar. Coast. Shelf Sci. 1991, 33, 409–418. [Google Scholar] [CrossRef]
- Kemp, W.M.; Boynton, W.; Adolf, J.E.; Boesch, D.F.; Boicourt, W.C.; Brush, G.; Cornwell, J.; Fisher, T.R.; Glibert, P.; Hagy, J.D.; et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005, 303, 1–29. [Google Scholar] [CrossRef]
- Roden, E.E.; Edmonds, J.W. Phosphate mobilization in iron-rich anaerobic sediments: Microbial Fe (III) oxide reduction versus iron-sulfide formation. Archiv für Hydrobiologie 1997, 139, 347–378. [Google Scholar] [CrossRef]
- Hickey, C.W.; Gibbs, M.M. Lake sediment phosphorus release management—Decision support and risk assessment framework. New Zealand J. Mar. Freshw. Res. 2009, 43, 819–856. [Google Scholar] [CrossRef] [Green Version]
- Diatloff, E.; Asher, C.J.; Smith, F.W. Use of GEOCHEM-PC to predict rare earth element (REE) species in nutrient solutions. Plant Soil 1993, 155, 251–254. [Google Scholar] [CrossRef]
- Firsching, F.H.; Burne, S.N. Solubility products of the trivalent rare-earth phosphates. J. Chem. Eng. Data 1991, 36, 93–95. [Google Scholar] [CrossRef]
- Ross, G.; Haghseresht, F.; Cloete, T. The effect of pH and anoxia on the performance of Phoslock®, a phosphorus binding clay. Harmful Algae 2008, 7, 545–550. [Google Scholar] [CrossRef]
- Burns, N.; McIntosh, J.; Scholes, P. Managing the lakes of the Rotorua District, New Zealand. Lake Reserv. Manag. 2009, 25, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Sun, Q.; Chen, X.; Liu, Q.; Wang, D.; Lin, J.; Zhang, C.; Tsang, D.C. Synergistic adsorption of phosphorus by iron in lanthanum modified bentonite (Phoslock®): New insight into sediment phosphorus immobilization. Water Res. 2018, 134, 32–43. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Effects of lanthanum and lanthanum-modified clay on growth, survival and reproduction of Daphnia magna. Water Res. 2010, 44, 309–319. [Google Scholar] [CrossRef]
- Gibbs, M.M.; Hickey, C.W.; Özkundakci, D. Sustainability assessment and comparison of efficacy of four P-inactivation agents for managing internal phosphorus loads in lakes: Sediment incubations. Hydrobiology 2010, 658, 253–275. [Google Scholar] [CrossRef]
- Van Oosterhout, F.; Lürling, M. The effect of phosphorus binding clay (Phoslock®) in mitigating cyanobacterial nuisance: A laboratory study on the effects on water quality variables and plankton. Hydrobiology 2012, 710, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Zhong, C.; Gan, L.; He, X.; Yu, J.; Zhang, X.; Liu, Z. Effects of Lanthanum Modified Bentonite and Polyaluminium Chloride on the Environmental Variables in the Water and Sediment Phosphorus Form in Lake Yanglan, China. Water 2021, 13, 1947. [Google Scholar] [CrossRef]
- Gao, Y.; Cornwell, J.C.; Stoecker, D.K.; Owens, M.S. Effects of cyanobacterial-driven pH increases on sediment nutrient fluxes and coupled nitrification-denitrification in a shallow fresh water estuary. Biogeosciences 2012, 9, 2697–2710. [Google Scholar] [CrossRef] [Green Version]
- Kana, T.M.; Sullivan, M.B.; Cornwell, J.C.; Groszkowski, K.M. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol. Oceanogr. 1998, 13, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Kana, T.M.; Darkangelo, C.; Hunt, M.D.; Oldham, J.B.; Bennett, G.E.; Cornwell, J. Membrane Inlet Mass Spectrometer for Rapid High-Precision Determination of N2, O2, and Ar in Environmental Water Samples. Anal. Chem. 1994, 66, 4166–4170. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis, 2nd ed.; Parsons, T.R., Ed.; Pergamon Press: New York, NY, USA, 1984; p. 178. [Google Scholar]
- Kopp, J.F.; McKee, G.D. Methods for Chemical Analysis of Water and Wastes; United States Environmental Protection Angency (EPA): Cincinnati, OH, USA, 1983.
- Folk, R.L. Sediment grain size analysis. In Petrology of Sedimentary Rocks; Folk, R.L., Ed.; Hemphill Publishing Co.: Austin, TX, USA, 1974; pp. 3–5. [Google Scholar]
- Boudreau, B.P. Diagenetic Models and Their Implementation: Modeling Transport and Reactions in Aquatic Sediments; Springer: New York, NY, USA, 1997. [Google Scholar]
- Kaczorkiewicz, M.; Heese, T. Recultivation of Lake Goldap. New application of Phoslock sorbent. Przem. Chem. 2008, 87, 479–481. [Google Scholar]
- Haghseresht, F.; Wang, S.; Do, D. A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl. Clay Sci. 2009, 46, 369–375. [Google Scholar] [CrossRef]
- Vopel, K.; Gibbs, M.; Hickey, C.W.; Quinn, J. Modification of sediment—water solute exchange by sediment-capping materials: Effects on O2 and pH. Mar. Freshw. Res. 2008, 59, 1101–1110. [Google Scholar] [CrossRef]
- Heggie, D.T.; Logan, G.A.; Smith, C.S.; Fredericks, D.J.; Palmer, D. Biogeochemical processes at the sediment–water interface, Bombah Broadwater, Myall Lakes. Hydrobiology 2008, 608, 49–67. [Google Scholar] [CrossRef]
- Lürling, M.; Faassen, E.J. Controlling toxic cyanobacteria: Effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Res. 2012, 46, 1447–1459. [Google Scholar] [CrossRef]
- Robb, M.; Greenop, B.; Goss, Z.; Douglas, G.; Adeney, J. Application of PhoslockTM, an innovative phosphorus binding clay, to two Western Australian waterways: Preliminary findings. Hydrobiology 2003, 494, 237–243. [Google Scholar] [CrossRef]
- Bailey, E.; Owens, M.; Boynton, W.; Cornwell, J. Sediment Phosphorus Flux: pH Interaction in the Tidal Freshwater Potomac River Estuary; UMCES Report TS-505-08-CBL; Interstate Commission on the Potomac River Basin: Rockville, MD, USA, 2006; pp. 1–91. [Google Scholar]
- Petersen, N.R.; Rysgaard, S.; Nielsen, L.P.; Revsbech, N.P. Diurnal variation of denitrification and nitrification in sediments colonized by benthic microphytes. Limnol. Oceanogr. 1994, 39, 573–579. [Google Scholar] [CrossRef]
- Magalhães, C.; Bordalo, A.; Wiebe, W. Temporal and spatial patterns of intertidal sediment-water nutrient and oxygen fluxes in the Douro River estuary, Portugal. Mar. Ecol. Prog. Ser. 2002, 233, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, J.; Kemp, W.M.; Kana, T.M. Denitrification in coastal ecosystems: Methods, environmental controls, and ecosystem level controls, a review. Aquat. Ecol. 1999, 33, 41–54. [Google Scholar] [CrossRef]
- Baumann, B.; Snozzi, M.; Zehnder, A.J.; Van Der Meer, J.R. Dynamics of denitrification activity of Paracoccus denitrificans in continuous culture during aerobic-anaerobic changes. J. Bacteriol. 1996, 178, 4367–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meis, S.; Spears, B.M.; Maberly, S.C.; O’Malley, M.B.; Perkins, R.G. Sediment amendment with Phoslock® in Clatto Reservoir (Dundee, UK): Investigating changes in sediment elemental composition and phosphorus fractionation. J. Environ. Manag. 2012, 93, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.; Pennock, J.; Boynton, W. Seasonal and interannual patterns of sediment-water nutrient and oxygen fluxes in Mobile Bay, Alabama (USA): Regulating factors and ecological significance. Mar. Ecol. Prog. Ser. 1996, 141, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Newell, R.I.E.; Cornwell, J.C.; Owens, M.S. Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: A laboratory study. Limnol. Oceanogr. 2002, 47, 1367–1379. [Google Scholar] [CrossRef]
- Sĭmek, M.; Laughlin, R.J.; Bru, D.; Chèneby, D.; Watson, C.J.; Philippot, L. Insights into the Effect of Soil pH on N2O and N2 Emissions and Denitrifier Community Size and Activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef] [Green Version]
- Isnansetyo, A.; Thien, N.D.; Seguchi, M. Potential of mud sediment of the Ariake Sea tidal flat and the individual effect of temperature, pH, salinity and ammonium concentration on its nitrification rate. Res. J. Environ. Earth Sci. 2011, 3, 587–599. [Google Scholar]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Niriella, D.; Carnahan, R.P. Comparison Study of Zeta Potential Values of Bentonite in Salt Solutions. J. Dispers. Sci. Technol. 2006, 27, 123–131. [Google Scholar] [CrossRef]
- Buragohain, P.; Sredeep, S.; Saiyouri, N. A study on the adsorption of ammonium in Bentonite and Kaolinite. Int. J. Chem. 2013, 1, 2320–4087. [Google Scholar]
- Seitzinger, S.P.; Gardner, W.S.; Spratt, A.K. The Effect of Salinity on Ammonium Sorption in Aquatic Sediments: Implications for Benthic Nutrient Recycling. Estuaries 1991, 14, 167–174. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Simkins, S.; Groffman, P. Perspectives on measurement of denitrification in the field including recommended protocols for acetylene based methods. Plant Soil 1989, 115, 261–284. [Google Scholar] [CrossRef]
- Capone, D.G.; Burns, J.A.; Montoya, J.; Subramaniam, A.; Mahaffey, C.; Gunderson, T.; Michaels, A.F.; Carpenter, E.J. Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Glob. Biogeochem. Cycles 2005, 19, 2024. [Google Scholar] [CrossRef]
- Afsar, A.; Groves, S. Eco-Toxicity Assessment of Phoslock. Available online: http://www.phoslock.eu/media/7407/Eco-toxicity-Assessment-Report-May-2009.pdf (accessed on 12 December 2021).
- Mattheiss, J.; Sellner, K.G.; Ferrier, D. Lake Anita Louise Peroxide Treatment Summary—December 2016; CCWS Contribution #17-01; Center for Coastal and Watershed Studies, Hood College: Frederick, MD, USA, 2017; p. 9. [Google Scholar]
- Pan, G.; Yang, B.; Wang, D.; Chen, H.; Tian, B.-H.; Zhang, M.-L.; Yuan, X.-Z.; Chen, J. In-lake algal bloom removal and submerged vegetation restoration using modified local soils. Ecol. Eng. 2011, 37, 302–308. [Google Scholar] [CrossRef]
- Sellner, K.G.; Place, A.; Williams, E.; Gao, Y.; VanDolah, E.; Paolisso, M.; Bowers, H.; Roche, S. Hydraulics and barley straw (Hordeum vulgare) as effective treatment options for a cyanotoxin-impacted lake. In Proceedings of the 16th International conference on Harmful Algae, Wellington, NZ, USA, 27–31 October 2014; Mackenzie, L., Ed.; The International Society for the Study of Harmful Algae (ISSHA), Cawthron Institute: Nelson, New Zealand, 2015; pp. 218–221. [Google Scholar]
- Anderson, C.R.; Sellner, K.G.; Anderson, D.M. Bloom prevention and control. In Harmful Algal Blooms (HABs) and Desalination: A Guide to Impacts, Monitoring, and Management; Anderson, D.M., Boerlage, S.F.E., Dixon, M.B., Eds.; UNESCO: Paris, France, 2017; pp. 205–222. [Google Scholar]
- Sellner, K.G.; Rensel, J. A review of prevention, control, and mitigation options for harmful algal blooms. In Harmful Algal Blooms: A Compendium Desk Reference; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 433–492. [Google Scholar]

| Experiment Information | Phoslock® Application | ||
|---|---|---|---|
| - | - | Water | Sediment |
| time | 2–11 July | 26 July–12 September | |
| container | closed-bottom | open-bottom | |
| sediment nutrient input | no | yes | |
| Initial conditions in lake water (mean ± standard error) | |||
| temperature (°C) | 32.5 ± 2.77 | 27.6 ± 2.19 | |
| salinity | 0.2 ± 0.01 | 0.2 ± 0.02 | |
| pH | 9.6 ± 0.1 | 9.5 ± 0.13 | |
| microcystin (µg L−1) | 18.0 (n = 1) | 7.8 ± 2.83 | |
| Parameters | Phoslock® | Controls |
|---|---|---|
| Properties in water column— | ||
| temperature (°C) | 25.79 | 26.05 |
| pH | 8.80 | 8.97 |
| DO (µmol L−1) | 97.81 | 149.06 |
| Chl a (ug L−1) | 32.70 | 69.80 |
| SRP (µmol L−1) | 1.47 | 1.13 |
| NO3− (µmol L−1) | - | 0.48 |
| NO2− (µmol L−1) | 0.22 | 0.22 |
| NH4+ (µmol L−1) | 0.06 | 0.05 |
| Sediment— | ||
| sand (%) | 18.52 ± 0.31 | |
| silt (%) | 56.24 ± 0.36 | |
| clay(%) | 25.31 ± 0.43 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sellner, K.; Place, A.; Cornwell, J.; Gao, Y. Mitigation of CyanoHABs Using Phoslock® to Reduce Water Column Phosphorus and Nutrient Release from Sediment. Int. J. Environ. Res. Public Health 2021, 18, 13360. https://doi.org/10.3390/ijerph182413360
Li J, Sellner K, Place A, Cornwell J, Gao Y. Mitigation of CyanoHABs Using Phoslock® to Reduce Water Column Phosphorus and Nutrient Release from Sediment. International Journal of Environmental Research and Public Health. 2021; 18(24):13360. https://doi.org/10.3390/ijerph182413360
Chicago/Turabian StyleLi, Ji, Kevin Sellner, Allen Place, Jeffrey Cornwell, and Yonghui Gao. 2021. "Mitigation of CyanoHABs Using Phoslock® to Reduce Water Column Phosphorus and Nutrient Release from Sediment" International Journal of Environmental Research and Public Health 18, no. 24: 13360. https://doi.org/10.3390/ijerph182413360
APA StyleLi, J., Sellner, K., Place, A., Cornwell, J., & Gao, Y. (2021). Mitigation of CyanoHABs Using Phoslock® to Reduce Water Column Phosphorus and Nutrient Release from Sediment. International Journal of Environmental Research and Public Health, 18(24), 13360. https://doi.org/10.3390/ijerph182413360








