Comparative Effect of Antihypertensive Drugs in Improving Arterial Stiffness in Hypertensive Adults (RIGIPREV Study). A Protocol for Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Register
2.2. Ethics
2.3. Inclusion/Exclusion Criteria
2.3.1. Type of Studies
2.3.2. Type of Participants
2.3.3. Intervention Types
2.3.4. Outcome Assessment Type
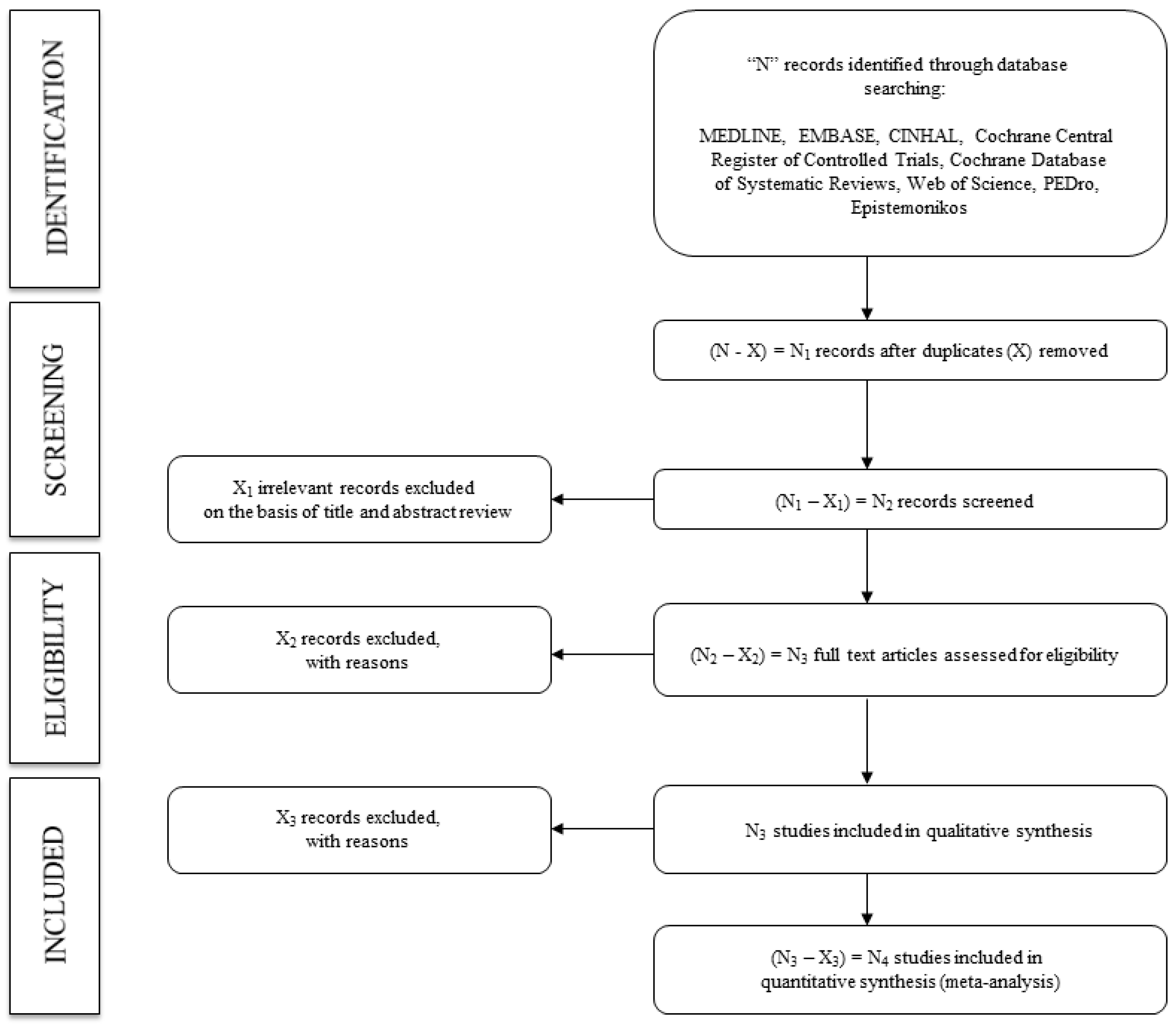
2.4. Search Methods for Study Identification
Electronic Search
2.5. Data Collection and Analysis
2.5.1. Study Selection
2.5.2. Assessing the Risk of Bias in Included Studies
2.5.3. Grading the Quality of Evidence
2.6. Synthesis of Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
List of Antihypertensive Drugs
- −
- Beta-blockers (acebutolol, atenolol, atenolol, betaxolol, bisoprolol, carteolol, esmolol, metoprolol, nadolol, oxprenolol, penbutolol, propranolol, timolol, celiprolol, carvedilol, labetalol, nebivolol, pindololol).
- −
- Diuretics (furosemide, bumetanide, torsemide, bendroflumethiazide, chlorothiazide, chlorthalidone, hydrochlorothiazide, indapamide, polythiazide, trichlormethiazide, amiloride, eplerenone, spironolactone, triamterene).
- −
- Angiotensin-converting enzyme inhibitors (benazepril, captopril, cilazapril, enalapril, fosinopril, imidapril, lisinopril, moexipril, perindopril, quinapril, ramipril, trandolapril, zofenopril).
- −
- Angiotensin II receptor antagonists (candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, valsartan).
- −
- Calcium channel blockers (diltiazem, verapamil, amlodipine, felodipine, isradipine, lacidipine, lercanidipine, manidipine, nicardipine).
- −
- Renin inhibitors (aliskiren).
- −
- Alpha-adrenergic receptor antagonists (doxazosin, prazosin, terazosin).
- −
- Centrally acting agents (clonidine, methyl-dopa, rilmenidine).
- −
- Direct-acting vasodilators (hydralazine, minoxidine).
References
- Banegas, J.R.; Gijón-Conde, T. Epidemiology of hypertension. Hipertens. Riesgo Vasc. 2017, 34, 2–4. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.M.; Duranti, E.; Ippolito, C.; Segnani, C.; Bernardini, N.; Di Candio, G.; Chiarugi, M.; Taddei, S.; Virdis, A. Different impact of essential hypertension on structural and functional age-related vascular changes. Hypertension 2017, 69, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Arterial stiffness and hypertension: Chicken or egg? Hypertension 2014, 64, 210–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikael, L.D.R.; de Paiva, A.M.G.; Gomes, M.M.; Sousa, A.L.L.; Jardim, P.C.B.V.; Vitorino, P.V.; Euzébio, M.B.; Sousa, W.D.M.; Barroso, W.K.S. Vascular aging and arterial stiffness. Arq. Bras. Cardiol. 2017, 109, 253–258. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert Consensus Document on arterial stiffness: Methodological aspects and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [Green Version]
- Najjar, S.S.; Scuteri, A.; Shetty, V.; Wright, J.G.; Muller, D.C.; Fleg, J.L.; Spurgeon, H.P.; Ferrucci, L.; Lakatta, E. Pulse Wave Velocity Is an Independent Predictor of the Longitudinal Increase in Systolic Blood Pressure and of Incident Hypertension in the Baltimore Longitudinal Study of Aging. JACC 2008, 51, 1377–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, G.F. Arterial Stiffness and Wave Reflection in Hypertension: Pathophysiologic and Therapeutic Implications. Curr. Hypertens. Rep. 2004, 6, 436–441. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, C.; Liu, Y.; Zhang, J.; Zhu, Y.; Kan, S.; Wu, Y.; Ruan, C.; Lin, L.; Yang, X.; et al. Arterial Stiffness as a Predictor of Clinical Hypertension. J. Clin. Hypertens. 2015, 17, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Levy, B.I.; Struijker-Boudier, H. Current Perspectives on Arterial Stiffness and Pulse Pressure in Hypertension and Cardiovascular Diseases. Circulation 2003, 107, 2864–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, J. Epidemiology of hypertension. Clinical. Queries Nephrol. 2013, 2, 56–61. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Isnard, R.; Pannier, B.M.; Laurent, S.; London, G.M.; Diebold, B.; Safar, M.E. Pulsatile diameter and elastic modulus of the aortic arch in essential hypertension: A noninvasive study. J. Am. Coll. Cardiol. 1989, 13, 399–405. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, M. Mechanical principles in arterial disease. Hypertension 1995, 26, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; London, G.M. Therapeutic studies and arterial stiffness in hypertension: Recommendations of the European Society of Hypertension. J. Hypertens. 2000, 18, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; PettiCrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Hoboken, NJ, USA, 2008; Updated March 2011. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norrise, S.; Falck-Ytterf, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence pro les and summary of ndings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Goldet, G.; Howick, J. Understanding GRADE: An introduction. JBEM 2013, 6, 50–54. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a metaanalysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- White, I.R. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef] [Green Version]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic Stiffness, Blood Pressure Progression, and Incident Hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.; Montezano, A.C.; Lopez, R.A.; Rios, F.; Touyz, R.M. Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.; Montezano, A.C.; Touyz, R.M. Vascular biology of ageing-Implications in hypertension. J. Mol. Cell. Cardiol. 2015, 83, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Safar, M.E. Mechanism(s) of Systolic Blood Pressure Reduction and Drug Therapy in Hypertension. Hypertension 2007, 50, 167–171. [Google Scholar] [CrossRef] [Green Version]
| “Hypertensive adults”. OR “Hypertensive population” OR “Hypertensive subjects” OR “Arterial hypertension” | AND | “Antihypertensive treatment” OR “Antihypertensive drugs” OR “Beta-blockers” OR acebutolol OR atenolol OR atenolol OR betaxolol OR bisoprolol OR carteolol OR esmolol OR metoprolol OR nadolol OR oxprenolol OR penbutolol OR propranolol OR timolol OR celiprolol OR carvedilol OR labetalol OR nebivolol OR pindololol OR Diuretics OR furosemide OR bumetanide OR torsemide OR bendroflumethiazide OR chlorothiazide OR chlorthalidone OR hydrochlorothiazide OR indapamide OR polythiazide OR trichlormethiazide OR amiloride OR eplerenone OR spironolactone OR triamterene OR “Angiotensin-converting enzyme inhibitors” OR benazepril OR captopril OR cilazapril OR enalapril OR fosinopril OR imidapril OR lisinopril OR moexipril OR perindopril OR quinapril OR ramipril OR trandolapril OR zofenopril OR “Angiotensin II receptor antagonists” OR candesartan OR eprosartan OR irbesartan OR losartan OR olmesartan OR telmisartan OR valsartan OR “Calcium channel blockers” OR diltiazem OR verapamil OR amlodipine OR felodipine OR isradipine OR lacidipine OR lercanidipine OR manidipine OR nicardipine OR “Renin inhibitors” OR aliskiren OR “Alpha-adrenergic receptor antagonists” OR doxazosin OR prazosin OR terazosin OR “Centrally acting agents” OR clonidine OR methyl-dopa OR rilmenidine OR “Direct acting vasodilators” OR hydralazine OR minoxidine | AND | “Arterial stiffness” OR “Pulse wave velocity” OR PWV OR “Augmentation index” OR Aix OR “Ambulatory arterial stiffness index” OR AASI OR “Cardio-ankle vascular index” OR CAVI | AND | “Randomised controlled trial” OR “Randomized clinical trial” OR RCT |
| Population Characteristics | Intervention Characteristics | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Sample Size | Mean Age | Status | Type of Antihypertensive Drugs | Dose | Length | Arterial Stiffness Parameter | Measurement Device | Baseline Levels |
| First author and year of publication | Country in which the study data were collected | Number of participants and percentage of female | Age (years) of the participants range or mean ± SD | Hypertension or Uncontrolled hypertension | Antihypertensive drugs included in the list of drugs in Appendix A | Dose administered and frequency | Length (months) of treatment | Type of arterial stiffness parameter (PWV, Aix, AASI, CAVI) | Arterial stiffness parameter measuring device | Baseline levels of the measured arterial stiffness parameter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavero-Redondo, I.; Saz-Lara, A.; García-Ortiz, L.; Lugones-Sánchez, C.; Notario-Pacheco, B.; Gómez-Sánchez, L.; Martínez-Vizcaíno, V.; Gómez-Marcos, M.Á. Comparative Effect of Antihypertensive Drugs in Improving Arterial Stiffness in Hypertensive Adults (RIGIPREV Study). A Protocol for Network Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 13353. https://doi.org/10.3390/ijerph182413353
Cavero-Redondo I, Saz-Lara A, García-Ortiz L, Lugones-Sánchez C, Notario-Pacheco B, Gómez-Sánchez L, Martínez-Vizcaíno V, Gómez-Marcos MÁ. Comparative Effect of Antihypertensive Drugs in Improving Arterial Stiffness in Hypertensive Adults (RIGIPREV Study). A Protocol for Network Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(24):13353. https://doi.org/10.3390/ijerph182413353
Chicago/Turabian StyleCavero-Redondo, Iván, Alicia Saz-Lara, Luis García-Ortiz, Cristina Lugones-Sánchez, Blanca Notario-Pacheco, Leticia Gómez-Sánchez, Vicente Martínez-Vizcaíno, and Manuel Ángel Gómez-Marcos. 2021. "Comparative Effect of Antihypertensive Drugs in Improving Arterial Stiffness in Hypertensive Adults (RIGIPREV Study). A Protocol for Network Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 24: 13353. https://doi.org/10.3390/ijerph182413353
APA StyleCavero-Redondo, I., Saz-Lara, A., García-Ortiz, L., Lugones-Sánchez, C., Notario-Pacheco, B., Gómez-Sánchez, L., Martínez-Vizcaíno, V., & Gómez-Marcos, M. Á. (2021). Comparative Effect of Antihypertensive Drugs in Improving Arterial Stiffness in Hypertensive Adults (RIGIPREV Study). A Protocol for Network Meta-Analysis. International Journal of Environmental Research and Public Health, 18(24), 13353. https://doi.org/10.3390/ijerph182413353








