Implementation of Physical Activity Programs for Rural Cancer Survivors: Challenges and Opportunities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Physical Activity Program
2.3. Quality of Life Assessment
2.4. Implementation Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics at Baseline
3.2. Program Implementation
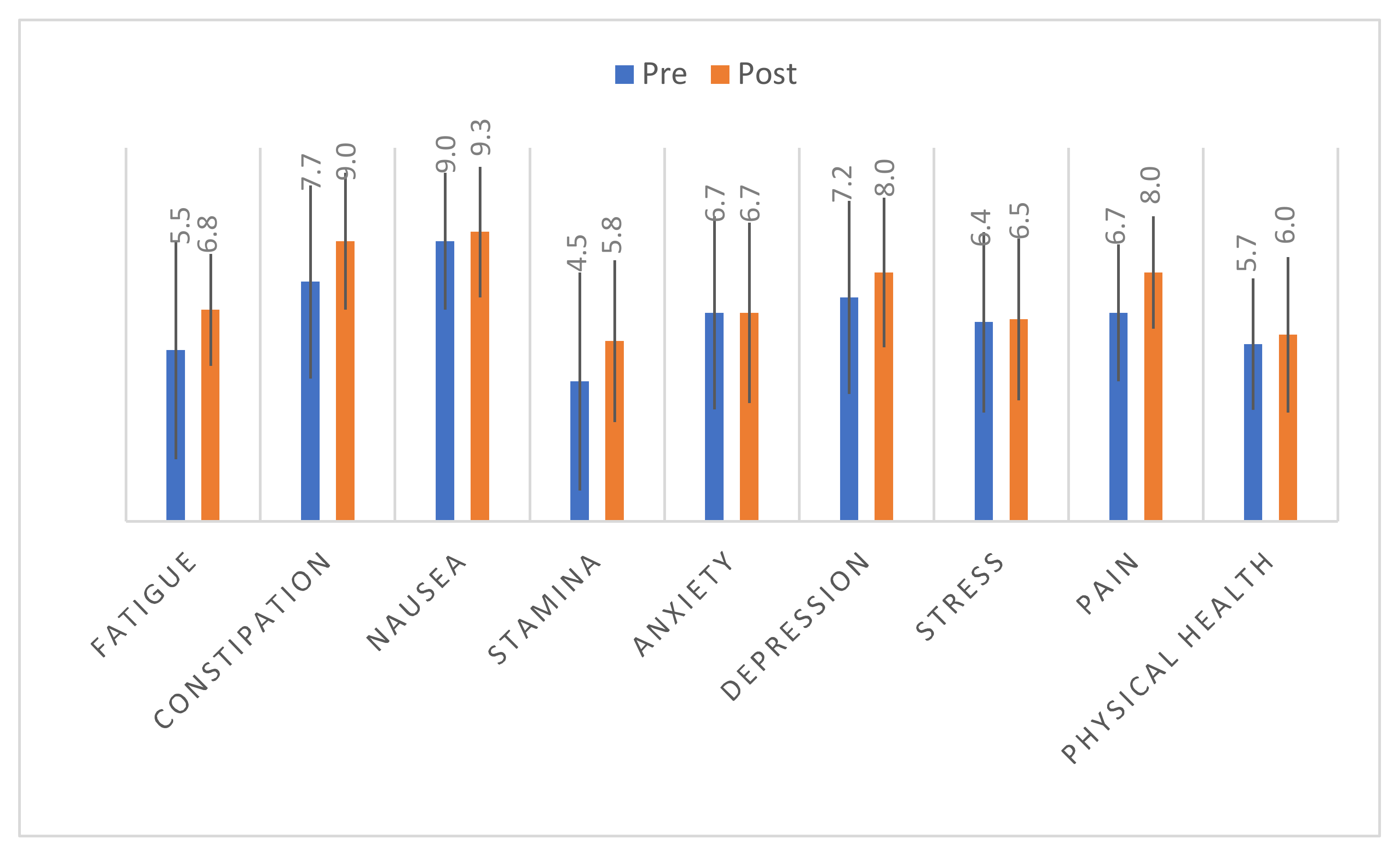
3.3. Changes in Sleep
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise guidelines for cancer survivors: Consensus statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedenreich, C.M.; Neilson, H.K.; Farris, M.S.; Courneya, K.S. Physical activity and cancer outcomes: A precision medicine approach. Clin. Cancer Res. 2016, 22, 4766–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligibel, J.A.; Denlinger, C.S. New NCCN guidelines for survivorship care. J. Natl. Compr. Cancer Netw. 2013, 11, 640–644. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Olson, E.A.; Mullen, S.P.; Rogers, L.Q.; Courneya, K.S.; Verhulst, S.; McAuley, E. Meeting physical activity guidelines in rural breast cancer survivors. Am. J. Health Behav. 2014, 38, 890–899. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.Q.; Markwell, S.J.; Courneya, K.S.; McAuley, E.; Verhulst, S. Physical activity type and intensity among rural breast cancer survivors: Patterns and associations with fatigue and depressive symptoms. J. Cancer Surviv. Res. Pract. 2011, 5, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Weaver, K.E.; Geiger, A.M.; Lu, L.; Case, L.D. Rural-urban disparities in health status among US cancer survivors. Cancer 2013, 119, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Blake, K.D.; Moss, J.L.; Gaysynsky, A.; Srinivasan, S.; Croyle, R.T. Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol. Biomark. 2017, 26, 992–997. [Google Scholar] [CrossRef] [Green Version]
- Bettencourt, B.A.; Schlegel, R.J.; Talley, A.E.; Molix, L.A. The breast cancer experience of rural women: A literature review. Psychooncology 2007, 16, 875–887. [Google Scholar] [CrossRef]
- Reis, J.P.; Bowles, H.R.; Ainsworth, B.E.; Dubose, K.D.; Smith, S.; Laditka, J.N. Nonoccupational physical activity by degree of urbanization and U.S. geographic region. Med. Sci. Sports Exerc. 2004, 36, 2093–2098. [Google Scholar] [CrossRef]
- Befort, C.A.; Nazir, N.; Perri, M.G. Prevalence of obesity among adults from rural and urban areas of the United States: Findings from NHANES (2005–2008). J. Rural Health 2012, 28, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.X.; Wen, M.; Kowaleski-Jones, L. Rural-urban differences in objective and subjective measures of physical activity: Findings from the National Health and Nutrition Examination Survey (NHANES) 2003-2006. Prev. Chronic. Dis. 2014, 11, E141. [Google Scholar] [CrossRef] [Green Version]
- Henley, S.J.; Anderson, R.N.; Thomas, C.C.; Massetti, G.M.; Peaker, B.; Richardson, L.C. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties—United States. MMWR Surveill. Summ. 2017, 66, 1–13. [Google Scholar] [CrossRef]
- Charlton, M.; Schlichting, J.; Chioreso, C.; Ward, M.; Vikas, P. Challenges of rural cancer care in the United States. Oncology 2015, 29, 633–640. [Google Scholar]
- Kraemer, W.J.; Adams, K.; Cafarelli, E.; Dudley, G.A.; Dooly, C.; Feigenbaum, M.S.; Fleck, S.J.; Franklin, B.; Fry, A.C.; Hoffman, J.R.; et al. American College of Sports Medicine Position Stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2002, 34, 364–380. [Google Scholar]
- Ferrell, B.R.; Hassey-Dow, K.; Grant, M. Quality of Life Patient/Cancer Survivor Version (QOL-CSV). Measurement Instrument Database for the Social Science. 2012. Available online: https://www.cityofhope.org/doc/1431763601572-qol-cs.pdf (accessed on 5 October 2021).
- Ferrell, B.R.; Dow, K.H.; Grant, M. Measurement of the quality of life in cancer survivors. Qual. Life Res. 1995, 4, 523–531. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Dow, K.H.; Leigh, S.; Ly, J.; Gulasekaram, P. Quality of life in long-term cancer survivors. Oncol. Nurs. Forum 1995, 22, 915–922. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, R.E.; Harden, S.M.; Gaglio, B.; Rabin, B.; Smith, M.L.; Porter, G.C.; Ory, M.G.; Estabrooks, P.A. RE-AIM Planning and evaluation framework: Adapting to new science and practice with a 20-year review. Front. Public Health 2019, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Carroll, C.; Patterson, M.; Wood, S.; Booth, A.; Rick, J.; Balain, S. A Conceptual framework for implementation fidelity. Implement. Sci. 2007, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Youngstedt, S.D.; Kline, C.E. Epidemiology of exercise and sleep. Sleep Biol. Rhythm. 2006, 4, 215–221. [Google Scholar] [CrossRef]
- Kline, C.E.; Crowley, E.P.; Ewing, G.B.; Burch, J.B.; Blair, S.N.; Durstine, J.L.; Davis, J.M.; Youngstedt, S.D. The effect of exercise training on obstructive sleep apnea and sleep quality: A randomized controlled trial. Sleep 2011, 34, 1631–1640. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Wang, Y.; Xiong, H.; Zhao, Y.; Sun, F. Effects of exercise intervention in breast cancer survivors: A meta-analysis of 33 randomized controlled trails. Onco Targets Ther. 2016, 9, 2153–2168. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.Q.; Courneya, K.S.; Oster, R.A.; Anton, P.M.; Robbs, R.S.; Forero, A.; McAuley, E. Physical activity and sleep quality in breast cancer survivors: A randomized trial. Med. Sci. Sports Exerc. 2017, 49, 2009–2015. [Google Scholar] [CrossRef]
- Hollen, P.J.; Msaouel, P.; Gralla, R.J. Determining issues of importance for the evaluation of quality of life and patient-reported outcomes in breast cancer: Results of a survey of 1072 patients. Breast Cancer Res. Treat. 2015, 151, 679–686. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Moe, E.L.; Perry, C.K.; Medysky, M.; Pommier, R.; Vetto, J.; Naik, A. Enhancing an oncologist’s recommendation to exercise to manage fatigue levels in breast cancer patients: A randomized controlled trial. Supportive Care Cancer 2018, 26, 905–912. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.; Oh, M.; Park, H.; Chae, J.; Kim, D.-I.; Lee, M.K.; Yoon, Y.J.; Lee, C.W.; Park, S.; et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: A randomized controlled trial. Cancer 2015, 121, 2740–2748. [Google Scholar] [CrossRef]
- Møller, T.; Lillelund, C.; Andersen, C.; Ejlertsen, B.; Nørgaard, L.; Christensen, K.B.; Vadstrup, E.; Diderichsen, F.; Hendriksen, C.; Bloomquist, K.; et al. At cancer diagnosis: A “window of opportunity” for behavioural change towards physical activity. A randomised feasibility study in patients with colon and breast cancer. BMJ Open 2013, 3, e003556. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.W.; Courneya, K.S.; Fairey, A.S.; Mackey, J.R. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: A single-blind, randomized controlled trial. Ann. Behav. Med. 2004, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligibel, J.A.; Jones, L.W.; Brewster, A.M.; Clinton, S.K.; Korde, L.A.; Oeffinger, K.C.; Bender, C.M.; Tan, W.; Merrill, J.K.; Katta, S.; et al. Oncologists’ attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: Findings of an ASCO survey of the oncology workforce. J. Oncol. Pract. 2019, 15, e520–e528. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.; Bainbridge, D.; Tomasone, J.; Cheifetz, O.; Juergens, R.A.; Sussman, J. Oncology care provider perspectives on exercise promotion in people with cancer: An examination of knowledge, practices, barriers, and facilitators. Supportive Care Cancer 2017, 25, 2297–2304. [Google Scholar] [CrossRef]
- Ulrich, C.M.; Himbert, C.; Boucher, K.; Wetter, D.W.; Hess, R.; Kim, J.; Lundberg, K.; Ligibel, J.A.; Barnes, C.A.; Rushton, B.; et al. Precision-exercise-prescription in patients with lung cancer undergoing surgery: Rationale and design of the PEP study trial. BMJ Open 2018, 8, e024672. [Google Scholar] [CrossRef]
- Courneya, K.S.; Rogers, L.Q.; Campbell, K.L.; Vallance, J.K.; Friedenreich, C.M. Top 10 research questions related to physical activity and cancer survivorship. Res. Q. Exerc. Sport 2015, 86, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Demark-Wahnefried, W.; Schmitz, K.H.; Alfano, C.M.; Bail, J.R.; Goodwin, P.J.; Thomson, C.A.; Bradley, D.W.; Courneya, K.S.; Befort, C.A.; Denlinger, C.S.; et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J. Clin. 2018, 68, 64–89. [Google Scholar] [CrossRef]
- Sallis, J.F. Needs and challenges related to multilevel interventions: Physical activity examples. Health Educ. Behav. 2018, 45, 661–667. [Google Scholar] [CrossRef]
- Breslow, L. Social ecological strategies for promoting healthy lifestyles. Am. J. Health Promot. 1996, 10, 253–257. [Google Scholar] [CrossRef]
- Sallis, J.F.; Owen, N.; Fisher, E.B. Ecological models of health behavior. In Health Behavior and Health Education: Theory, Research, and Practice; Jossey-Bass Inc.: San Francisco, CA, USA, 2015; pp. 465–486. [Google Scholar]
| Characteristic | Median (IQR) or N (%) |
|---|---|
| Age | 66.0 (12.5) |
| Female | 18 (75.0) |
| Cancer type | |
| Breast | 8 (33.3) |
| Gastrointestinal | 3 (12.5) |
| Gynecologic | 3 (12.5) |
| Prostate | 3 (12.5) |
| Lung | 1 (4.2) |
| Kidney | 1 (4.2) |
| Skin | 2 (8.3) |
| Multiple myeloma | 1 (4.2) |
| Sarcoma | 1 (4.2) |
| Missing | 1 (3.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirko, K.A.; Dorn, J.M.; Dearing, J.W.; Alfano, C.M.; Wigton, A.; Schmitz, K.H. Implementation of Physical Activity Programs for Rural Cancer Survivors: Challenges and Opportunities. Int. J. Environ. Res. Public Health 2021, 18, 12909. https://doi.org/10.3390/ijerph182412909
Hirko KA, Dorn JM, Dearing JW, Alfano CM, Wigton A, Schmitz KH. Implementation of Physical Activity Programs for Rural Cancer Survivors: Challenges and Opportunities. International Journal of Environmental Research and Public Health. 2021; 18(24):12909. https://doi.org/10.3390/ijerph182412909
Chicago/Turabian StyleHirko, Kelly A., Joan M. Dorn, James W. Dearing, Catherine M. Alfano, Annemarie Wigton, and Kathryn H. Schmitz. 2021. "Implementation of Physical Activity Programs for Rural Cancer Survivors: Challenges and Opportunities" International Journal of Environmental Research and Public Health 18, no. 24: 12909. https://doi.org/10.3390/ijerph182412909
APA StyleHirko, K. A., Dorn, J. M., Dearing, J. W., Alfano, C. M., Wigton, A., & Schmitz, K. H. (2021). Implementation of Physical Activity Programs for Rural Cancer Survivors: Challenges and Opportunities. International Journal of Environmental Research and Public Health, 18(24), 12909. https://doi.org/10.3390/ijerph182412909








