Long-Term Water Quality Patterns in an Estuarine Reservoir and the Functional Changes in Relations of Trophic State Variables Depending on the Construction of Serial Weirs in Upstream Reaches
Abstract
1. Introduction
2. Materials and Methods
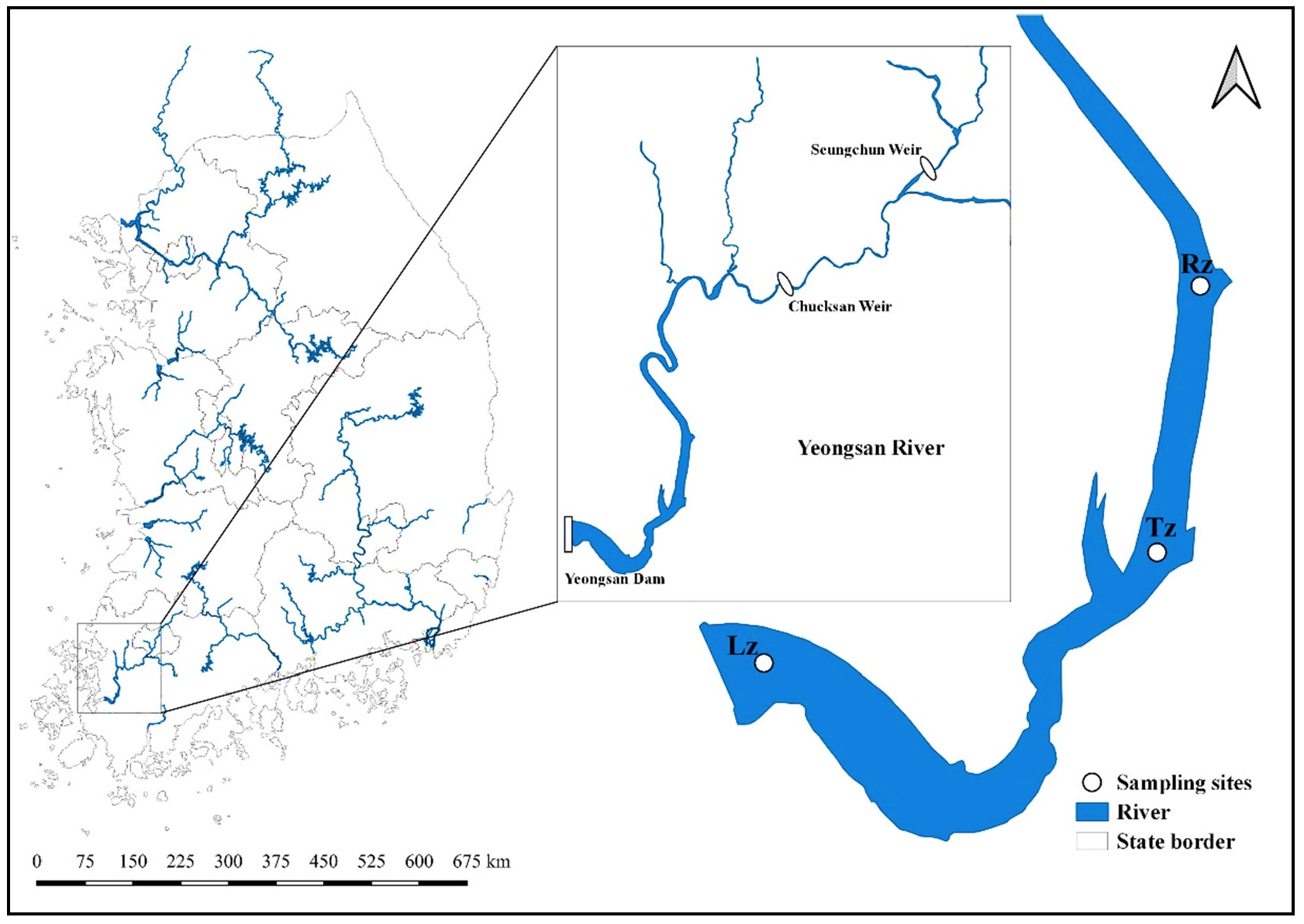
2.1. Study Area and Sites
2.2. Long-Term Time Series Data
2.3. Trophic State Index Deviation and Non-Algal Turbidity
2.4. Statistical Approaches
3. Results and Discussion
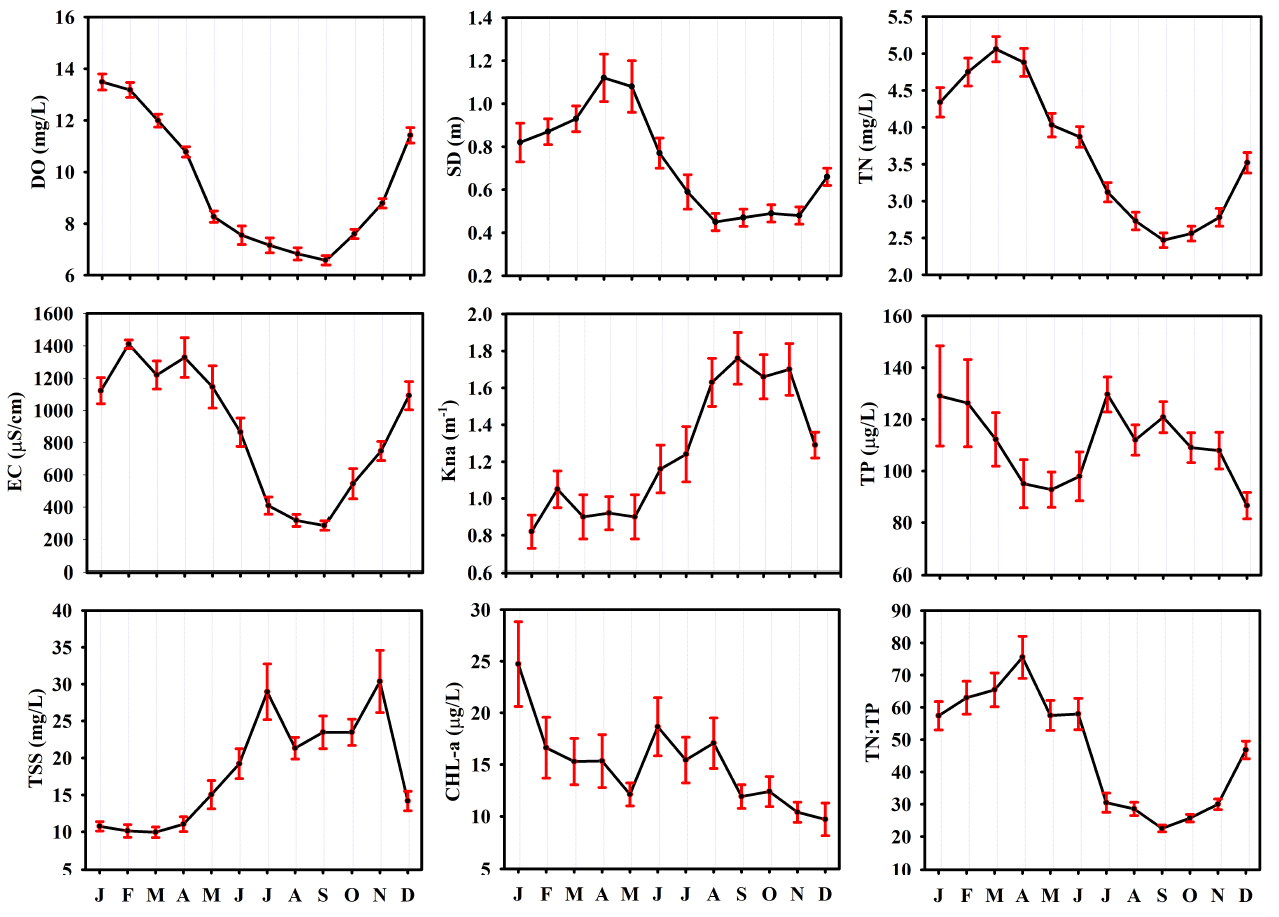
3.1. Seasonal Heterogeneities of Water Quality Parameters on the Longitudinal Zones in the Reservoir
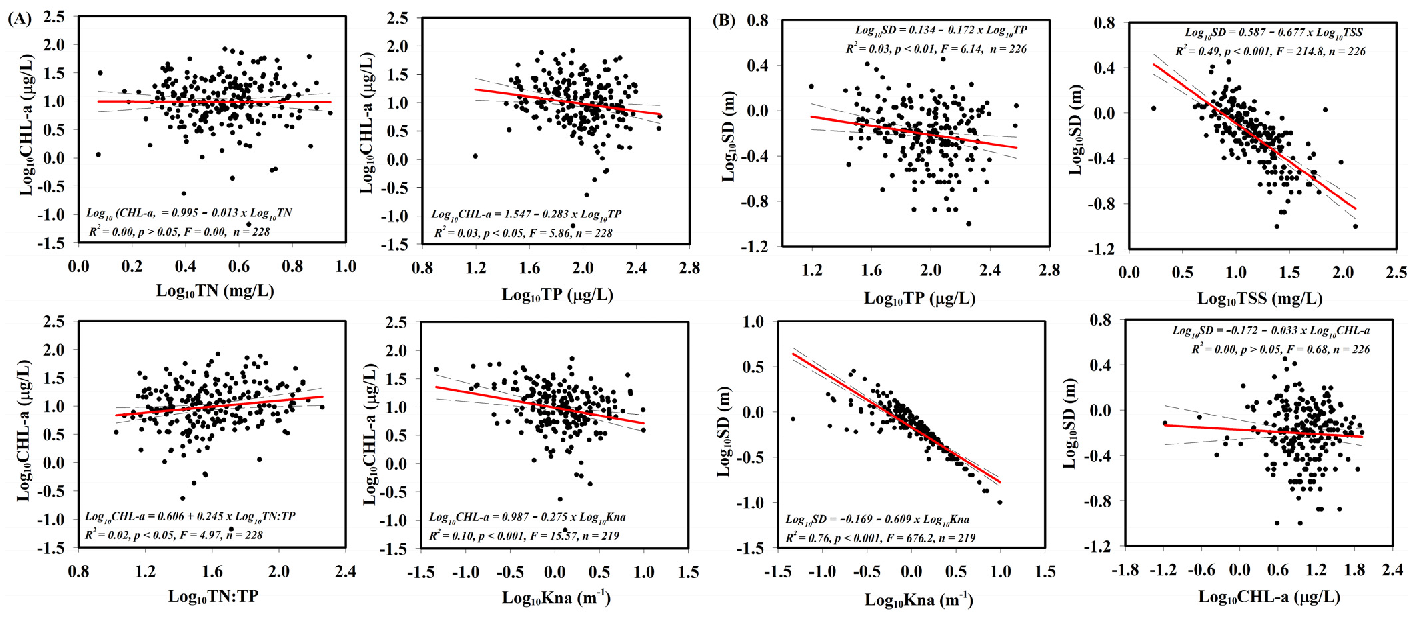
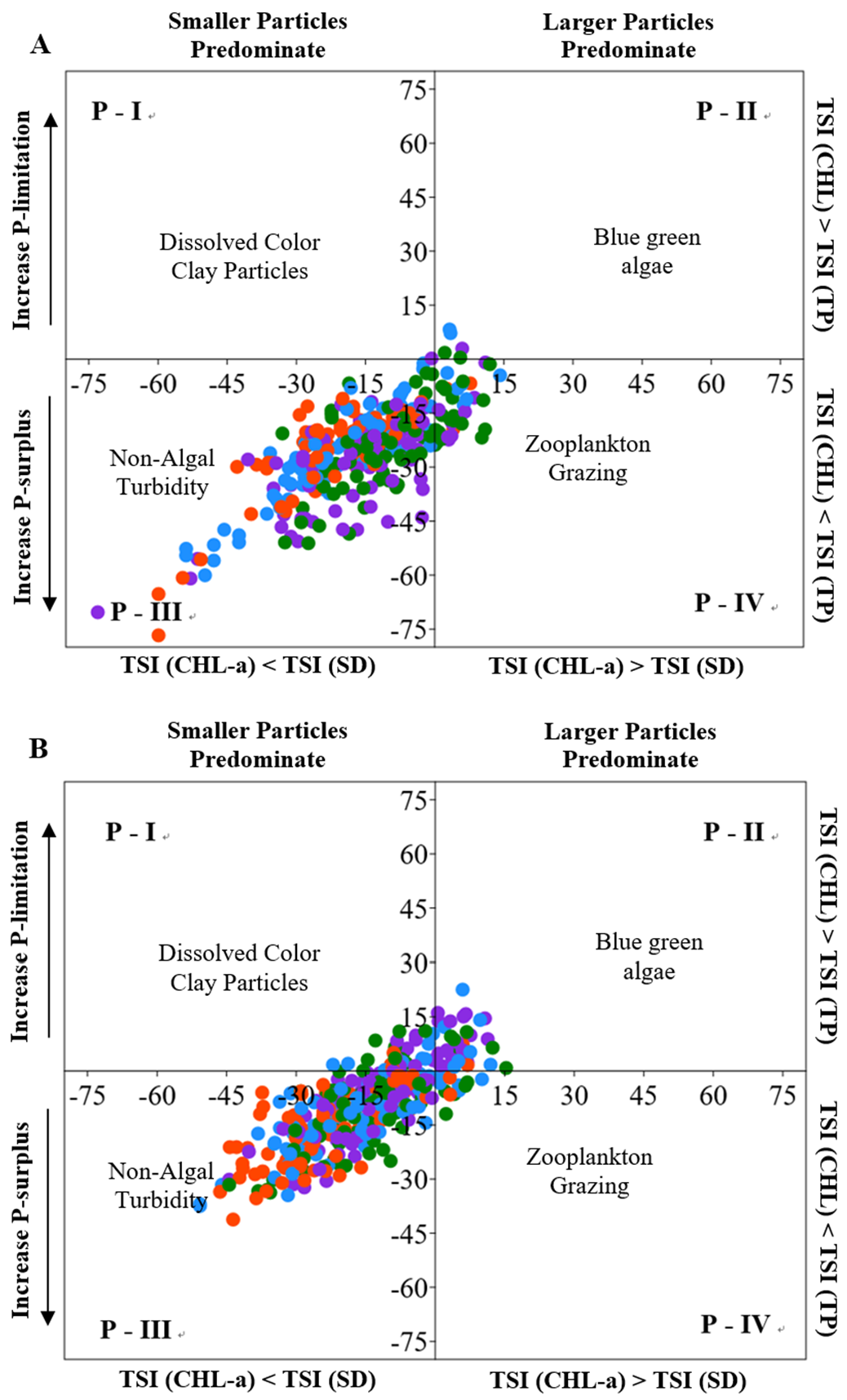
3.2. Influences of Nutrients, Non-Algal Turbidity, and Total Suspended Solids on the Reservoir Water Clarity and Algal Biomass
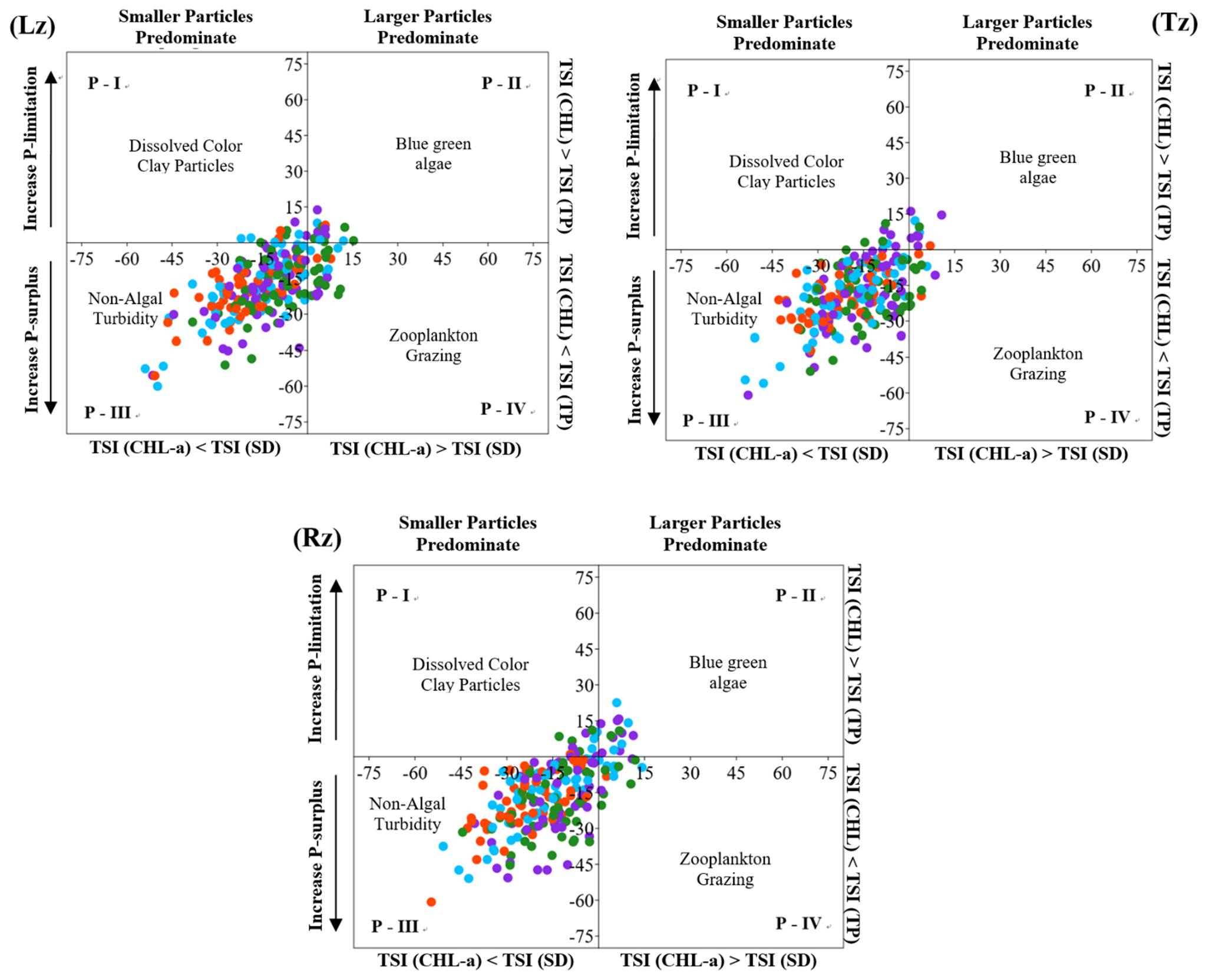
3.3. Impact of Serial Weir Constructions on the Nutrient Regimes, CHL-a, and TSID within the Estuarine Reservoir, along with Monsoonal Rainfall Intensity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrick, D.E.; Hall, J.W.; Dobson, A.; Damania, R.; Grafton, R.Q.; Hope, R.; Hepburn, C.; Bark, R.; Boltz, F.; De Stefano, L.; et al. Valuing water for sustainable development. Science 2017, 358, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Strobl, R.O.; Robillard, P.D. Network design for water quality monitoring of surface freshwaters: A review. J. Environ. Manag. 2008, 87, 639–648. [Google Scholar] [CrossRef]
- Dobson, M.; Frid, C. Ecology of Aquatic Systems, 2nd ed.; Dobson, M., Frid, C., Eds.; Oxford University Press: Oxford, UK, 2008; ISBN 9780199297542. [Google Scholar]
- Costa, C.R.; Costa, M.F.; Dantas, D.V.; Barletta, M. Interannual and Seasonal Variations in Estuarine Water Quality. Front. Mar. Sci. 2018, 5, 301. [Google Scholar] [CrossRef]
- Guan, Y.F.; Wang, J.Z.; Ni, H.G.; Zeng, E.Y. Organochlorine pesticides and polychlorinated biphenyls in riverine runoff of the Pearl River Delta, China: Assessment of mass loading, input source and environmental fate. Environ. Pollut. 2009, 157, 618–624. [Google Scholar] [CrossRef]
- Rodrigues, E.T.; Pardal, M.Â. Primary Productivity Temporal Fluctuations in a Nutrient-Rich Estuary due to Climate-Driven Events. Estuaries Coasts 2015, 38, 1–12. [Google Scholar] [CrossRef]
- Lee, Y.G.; An, K.G.; Ha, P.T.; Lee, K.Y.; Kang, J.H.; Cha, S.M.; Cho, K.H.; Lee, Y.S.; Chang, I.S.; Kim, K.W.; et al. Decadal and seasonal scale changes of an artificial lake environment after blocking tidal flows in the Yeongsan Estuary region, Korea. Sci. Total Environ. 2009, 407, 6063–6072. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Yang, J.S.; Park, K. Changes in water quality after the construction of an estuary dam in the Geum river estuary dam system, Korea. J. Coast. Res. 2014, 30, 1278–1286. [Google Scholar] [CrossRef]
- Ralston, D.K.; Brosnahan, M.L.; Fox, S.E.; Lee, K.D.; Anderson, D.M. Temperature and residence time controls on an estuarine harmful algal bloom: Modeling hydrodynamics and Alexandrium fundyense in Nauset estuary. Estuaries Coasts 2015, 38, 2240–2258. [Google Scholar] [CrossRef] [PubMed]
- UNESCO/WHO/UNEP. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring, 1st ed.; Chapman, D.V., Ed.; CRC Press: Cambridge, UK, 1992; ISBN 0412448408. [Google Scholar]
- An, K.G.; Kim, D.S. Response of reservoir water quality to nutrient inputs from streams and in-lake fishfarms. Water Air Soil Pollut. 2003, 149, 27–49. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; ISBN 9780127447605. [Google Scholar]
- Jones, J.R.; Knowlton, M.F.; An, K.G. Trophic state, seasonal patterns and empirical models in South Korean reservoirs. Lake Reserv. Manag. 2003, 19, 64–78. [Google Scholar] [CrossRef]
- Choi, I.C.; Shin, H.J.; Nguyen, T.T.; Tenhunen, J. Water policy reforms in South Korea: A historical review and ongoing challenges for sustainable water governance and management. Water 2017, 9, 717. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kang, J.H.; Ki, S.J.; Cha, S.M.; Cho, K.H.; Lee, Y.S.; Park, Y.; Lee, S.W.; Kim, J.H. Factors dominating stratification cycle and seasonal water quality variation in a Korean estuarine reservoir. J. Environ. Monit. 2010, 12, 1072–1081. [Google Scholar] [CrossRef]
- Atique, U.; An, K.-G. Landscape heterogeneity impacts water chemistry, nutrient regime, organic matter and chlorophyll dynamics in agricultural reservoirs. Ecol. Indic. 2020, 110, 105813. [Google Scholar] [CrossRef]
- MOE. Water Environment. In White Paper of ECOREA: Environmental Review, 2015; Korea Ministry of Environment (MOE): Sejong, Korea, 2015; Volume 2, pp. 2–24. [Google Scholar]
- Ansari, A.A.; Singh Gill, S.; Lanza, G.R.; Rast, W. Eutrophication: Causes, Consequences and Control; Springer: New York, NY, USA, 2011; ISBN 9789048196241. [Google Scholar]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the expansion of harmful algal blooms across the freshwater-to-marine continuum. Environ. Sci. Technol. 2018, 52, 5519–5529. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, C.; Zheng, G.; Xue, J.; Zhang, L. The establishment of season-specific eutrophication assessment standards for a water-supply reservoir located in Northeast China based on chlorophyll-a levels. Ecol. Indic. 2018, 85, 11–20. [Google Scholar] [CrossRef]
- Huszar, V.L.; Caraco, N.F.; Roland, F.; Cole, J. Nutrient-chlorophyll relationships in tropical-subtropical lakes: Do temperate models fit? Biogeochemistry 2006, 79, 239–250. [Google Scholar] [CrossRef]
- Phillips, G.; Pietiläinen, O.P.; Carvalho, L.; Solimini, A.; Solheim, A.L.; Cardoso, A.C. Chlorophyll–nutrient relationships of different lake types using a large European dataset. Aquatic Ecol. 2008, 42, 213–226. [Google Scholar] [CrossRef]
- Bachmann, R.W.; Bigham, D.L.; Hoyer, M.V.; Canfield, D.E., Jr. Factors determining the distributions of total phosphorus, total nitrogen, and chlorophyll a in Florida lakes. Lake Reserv. Manag. 2012, 28, 10–26. [Google Scholar] [CrossRef]
- Kärcher, O.; Filstrup, C.T.; Brauns, M.; Tasevska, O.; Patceva, S.; Hellwig, N.; Walz, A.; Frank, K.; Markovic, D. Chlorophyll a relationships with nutrients and temperature, and predictions for lakes across perialpine and Balkan mountain regions. Inland Waters 2020, 10, 29–41. [Google Scholar] [CrossRef]
- Smith, V.H.; Shapiro, J. Chlorophyll-phosphorus relations in individual lakes. Their importance to lake restoration strategies. Environ. Sci. Technol. 1981, 15, 444–451. [Google Scholar] [CrossRef]
- Abell, J.M.; Özkundakci, D.; Hamilton, D.P.; Jones, J.R. Latitudinal variation in nutrient stoichiometry and chlorophyll-nutrient relationships in lakes: A global study. Fundam. Appl. Limnol. 2012, 181, 1–14. [Google Scholar] [CrossRef]
- Zou, W.; Zhu, G.; Cai, Y.; Vilmi, A.; Xu, H.; Zhu, M.; Gong, Z.; Zhang, Y.; Qin, B. Relationships between nutrient, chlorophyll a and Secchi depth in lakes of the Chinese Eastern Plains ecoregion: Implications for eutrophication management. J. Environ. Manag. 2020, 260, 109923. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.E.; Simpson, J. A Coordinator’s Guide to Volunteer Lake Monitoring Methods; North American Lake Management Society: Madison, WI, USA, 1996. [Google Scholar]
- Walter, K.; Dodds, W.; Matt, R. Trophic State and Eutrophication. In Freshwater Ecology: Concepts and Environmental Applications of Limnology, 3rd ed.; Walter, K., Dodds, W., Matt, R., Eds.; Elsevier Academic Press: London, UK, 2020; pp. 539–581. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Carlson, R.E.; Havens, K.E. Simple graphical methods for the interpretation of relationships between trophic state variables. Lake Reserv. Manag. 2005, 21, 107–118. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, C.; Zhang, F.Y. Development of water quality model in the Satilla River Estuary, Georgia. Ecol. Model. 2004, 178, 457–482. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Song, E.S.; Cho, K.A.; Shin, Y.S. Exploring the dynamics of dissolved oxygen and vertical density structure of water column in the Youngsan Lake. J. Environ. Sci. Int. 2015, 24, 163–174. [Google Scholar] [CrossRef][Green Version]
- Cho, K.H.; Park, Y.; Kang, J.H.; Ki, S.J.; Cha, S.; Lee, S.W.; Kim, J.H. Interpretation of seasonal water quality variation in the Yeongsan Reservoir, Korea using multivariate statistical analyses. Water Sci. Technol. 2009, 59, 2219–2226. [Google Scholar] [CrossRef]
- Sin, Y.; Lee, E.; Lee, Y.; Shin, K. The river-estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuar. Coast. Shelf Sci. 2015, 163, 279–289. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hong, S.; Song, Y.S.; Lee, H.; Kim, H.C.; Ryu, J.; Park, J.; Kwon, B.O.; Lee, C.H.; Khim, J.S. Seasonal variability of estuarine dynamics due to freshwater discharge and its influence on biological productivity in Yeongsan River Estuary, Korea. Chemosphere 2017, 181, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Sin, Y.; Lee, H. Changes in hydrology, water quality, and algal blooms in a freshwater system impounded with engineered structures in a temperate monsoon river estuary. J. Hydrol. Reg. Stud. 2020, 32, 100744. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J. The serial discontinuity concept of lotic ecosystems. In Dynamics of Lotic Ecosystems; Fontane, T.D., Bartell, S.M., Eds.; Ann Arbor Scientific Publications: Ann Arbor, MI, USA, 1983; pp. 29–42. [Google Scholar]
- MOE. Standard Methods for the Examination of Water Quality Contamination, 7th ed.; Ministry of Environemnt (MOE): Gwacheon, Korea, 2000; p. 435. (In Korean) [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: New York, NY, USA, 2005. [Google Scholar]
- Walker, W.W., Jr. An empirical analysis of phosphorus, nitrogen, and turbidity effects on reservoir chlorophyll-a levels. Can. Water Resour. J. 1982, 7, 88–107. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Zhang, D.; Tang, Q.; Xu, H.; Huang, S.; Shang, D.; Liu, R. Water quality assessment and source identification of the Shuangji River (China) using multivariate statistical methods. PLoS ONE 2021, 16, e0245525. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Atique, U.; An, K.-G. Reservoir Water Quality Assessment Based on Chemical Parameters and the Chlorophyll Dynamics in Relation to Nutrient Regime. Pol. J. Environ. Stud. 2019, 28, 1043–1061. [Google Scholar] [CrossRef]
- Hayes, N.M.; Deemer, B.R.; Corman, J.R.; Razavi, N.R.; Strock, K.E. Key differences between lakes and reservoirs modify climate signals: A case for a new conceptual model. Limnol. Oceanogr. Lett. 2017, 2, 47–62. [Google Scholar] [CrossRef]
- Walter, K.; Dodds, W.; Matt, R. Lakes and Reservoirs: Physiography. In Freshwater Ecology: Concepts and Environmental Applications of Limnology, 3rd ed.; Walter, K., Dodds, W., Matt, R., Eds.; Elsevier Academic Press: London, UK, 2020; pp. 156–186. [Google Scholar]
- Hara, J.; Atique, U.; An, K.G. Multiyear links between water chemistry, algal chlorophyll, drought-flood regime, and nutrient enrichment in a morphologically complex reservoir. Int. J. Environ. Res. Public Health 2020, 17, 3139. [Google Scholar] [CrossRef]
- Mamun, M.; An, K.G. Development of Reservoir Water Quality Index (WQI) Based on Long-term Physicochemical Parameters and Their Spatio-temporal Variations. Asian J. Water Environ. Pollut. 2020, 17, 55–63. [Google Scholar] [CrossRef]
- Jargal, N.; Atique, U.; Mamun, M.; An, K.-G. Seasonal and Long-Term Connections between Trophic Status, Sestonic Chlorophyll, Nutrients, Organic Matter, and Monsoon Rainfall in a Multipurpose Reservoir. Water 2021, 13, 1720. [Google Scholar] [CrossRef]
- Montagna, P.A.; Palmer, T.A.; Beseres Pollack, J. Hydrological Changes and Estuarine Dynamics; Springer Briefs in Environmental Science; Springer: New York, NY, USA, 2013; Volume 8, ISBN 978-1-4614-5832-6. [Google Scholar]
- Telesh, I.V.; Khlebovich, V.V. Principal Processes within the estuarine salinity gradient: A review. Mar. Pollut. Bull. 2010, 61, 149–155. [Google Scholar] [CrossRef]
- Effler, S.W.; Matthews, D.A. Sediment resuspension and drawdown in a water supply reservoir. J. Am. Water Resour. Assoc. 2004, 40, 251–264. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.; Lee, J.E.; Chung, M.S.; Ko, G.P. Identification of human and animal fecal contamination after rainfall in the Han River, Korea. Microbes Environ. 2013, 28, 187–194. [Google Scholar] [CrossRef]
- Paule-Mercado, M.A.; Ventura, J.S.; Memon, S.A.; Jahng, D.; Kang, J.H.; Lee, C.H. Monitoring and predicting the fecal indicator bacteria concentrations from agricultural, mixed land use and urban stormwater runoff. Sci. Total Environ. 2016, 550, 1171–1181. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Y.; Cui, J.; Gan, S.; Yang, X.; Wu, R.; Guo, P. Assessment of Water Quality Evolution in the Pearl River Estuary (South Guangzhou) from 2008 to 2017. Water 2020, 12, 59. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.M.; Kim, D.S.; Hwang, S.J.; An, K.G. Nutrients and chlorophyll-a dynamics in a temperate reservoir influenced by Asian monsoon along with in situ nutrient enrichment bioassays. Limnology 2010, 11, 49–62. [Google Scholar] [CrossRef]
- An, K.G.; Park, S.S.; Ahn, K.H.; Urchin, C.G. Dynamics of nitrogen, phosphorus, algal biomass, and suspended solids in an artificial lentic ecosystem and significant implications of regional hydrology on trophic status. J. Environ. Biol. 2003, 24, 29–38. [Google Scholar] [PubMed]
- Smith, V.H. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in Lake Phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef]
- Song, E.S.; Shin, Y.S.; Jang, N.I.; Lee, J.B. Assessment of nutrient and light limitation of phytoplankton in the Youngsan Lake. Korean J. Environ. Ecol. 2010, 43, 35–43. [Google Scholar]
- Rosenberg, D.M.; McCully, P.; Pringle, C.M. Global-scale environmental effects of hydrological alterations: Introduction. Bioscience 2000, 50, 746–751. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, H.K.; Cheon, S.U. Effects of weir construction on phytoplankton assemblages and water quality in a large river system. Int. J. Environ. Res. Public Health 2018, 15, 2348. [Google Scholar] [CrossRef]
- Atique, U.; Kwon, S.; An, K.G. Linking weir imprints with riverine water chemistry, microhabitat alterations, fish assemblages, chlorophyll-nutrient dynamics, and ecological health assessments. Ecol. Indic. 2020, 117, 106652. [Google Scholar] [CrossRef]
- Mbaka, J.G.; Wanjiru Mwaniki, M. A global review of the downstream effects of small impoundments on stream habitat conditions and macroinvertebrates. Environ. Rev. 2015, 23, 257–262. [Google Scholar] [CrossRef]
- Gippel, C.J.; Blackham, D. Executive Summary. In Review of Environmental Impacts of Flow Regulation and Other Water Resource Developments in the River Murray and Lower Darling River System: Includes Glossary of Terms: Final Report to Murray Darling Basin Commission; Murray Darling Basin Commission: Canberra, Australia, 2002. Available online: https://www.mdba.gov.au/sites/default/files/archived/mdbc-tlm-reports/2105_Review_of_env_impacts_of_flow_regulation_2002.pdf (accessed on 28 September 2021).


| Parameter | PC 1 | PC 2 | PC 3 | PC 4 |
|---|---|---|---|---|
| WT | −0.73 | 0.06 | −0.44 | 0.50 |
| EC | 0.91 | −0.32 | −0.02 | −0.17 |
| TSS | −0.91 | −0.08 | −0.06 | 0.16 |
| Kna | −0.93 | −0.07 | −0.08 | −0.22 |
| SD | 0.95 | −0.02 | −0.14 | 0.22 |
| TN | 0.99 | 0.08 | 0.00 | 0.07 |
| TDN | 0.98 | 0.11 | −0.02 | 0.10 |
| NH4-N | 0.92 | 0.20 | 0.01 | 0.09 |
| NO3-N | 0.98 | 0.01 | −0.09 | −0.05 |
| TP | −0.17 | 0.64 | 0.72 | −0.08 |
| TDP | 0.11 | 0.98 | 0.09 | −0.13 |
| PP | −0.46 | −0.33 | 0.79 | 0.14 |
| PO4-P | −0.23 | 0.87 | 0.11 | −0.09 |
| PP:TP | −0.43 | −0.64 | 0.57 | 0.19 |
| TN:TP | 0.97 | −0.10 | −0.07 | 0.14 |
| FCB | −0.81 | 0.25 | −0.27 | −0.29 |
| CHL-a | 0.30 | 0.31 | 0.66 | 0.40 |
| PREC | −0.49 | 0.42 | −0.37 | 0.65 |
| Eigenvalue | 10.12 | 3.17 | 2.36 | 1.21 |
| % variance | 56.21 | 17.60 | 13.09 | 6.73 |
| Category | Parameter | Mean | ANOVA on Ranks | ||
|---|---|---|---|---|---|
| BWCs | AWCs | H Value | p-Value | ||
| Nutrient | TN (mg/L) | 3.8 | 3.2 | 18.48 | * |
| TP (µg/L) | 125 | 67 | 90.88 | * | |
| TDP (µg/L) | 96 | 38 | 92.22 | * | |
| PP (µg/L) | 28 | 28 | 0.04 | ||
| Ratios of Nutrients | TDP:TP | 0.79 | 0.57 | 39.42 | * |
| PP:TP | 0.21 | 0.43 | 39.42 | * | |
| TN:TP | 30.3 | 51.9 | 32.06 | * | |
| Algal biomass indicator | CHL-a (µg/L) | 7.7 | 12.7 | 18.70 | * |
| Ratios of CHL-a and Nutrients | CHL:TP | 0.059 | 0.2 | 63.50 | * |
| CHL:TN | 0.002 | 0.003 | 11.90 | * | |
| Suspended Solids/inorganic turbidity | TSS (mg/L) | 12.65 | 16.9 | 7.08 | * |
| Kna (m−1) | 1.48 | 1.41 | 1.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jargal, N.; Lee, H.-S.; An, K.-G. Long-Term Water Quality Patterns in an Estuarine Reservoir and the Functional Changes in Relations of Trophic State Variables Depending on the Construction of Serial Weirs in Upstream Reaches. Int. J. Environ. Res. Public Health 2021, 18, 12568. https://doi.org/10.3390/ijerph182312568
Jargal N, Lee H-S, An K-G. Long-Term Water Quality Patterns in an Estuarine Reservoir and the Functional Changes in Relations of Trophic State Variables Depending on the Construction of Serial Weirs in Upstream Reaches. International Journal of Environmental Research and Public Health. 2021; 18(23):12568. https://doi.org/10.3390/ijerph182312568
Chicago/Turabian StyleJargal, Namsrai, Ho-Seong Lee, and Kwang-Guk An. 2021. "Long-Term Water Quality Patterns in an Estuarine Reservoir and the Functional Changes in Relations of Trophic State Variables Depending on the Construction of Serial Weirs in Upstream Reaches" International Journal of Environmental Research and Public Health 18, no. 23: 12568. https://doi.org/10.3390/ijerph182312568
APA StyleJargal, N., Lee, H.-S., & An, K.-G. (2021). Long-Term Water Quality Patterns in an Estuarine Reservoir and the Functional Changes in Relations of Trophic State Variables Depending on the Construction of Serial Weirs in Upstream Reaches. International Journal of Environmental Research and Public Health, 18(23), 12568. https://doi.org/10.3390/ijerph182312568







