Childhood Rotavirus Infection Associated with Temperature and Particulate Matter 2.5 µm: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Ethics Statement
2.3. Statistical Analysis
3. Results
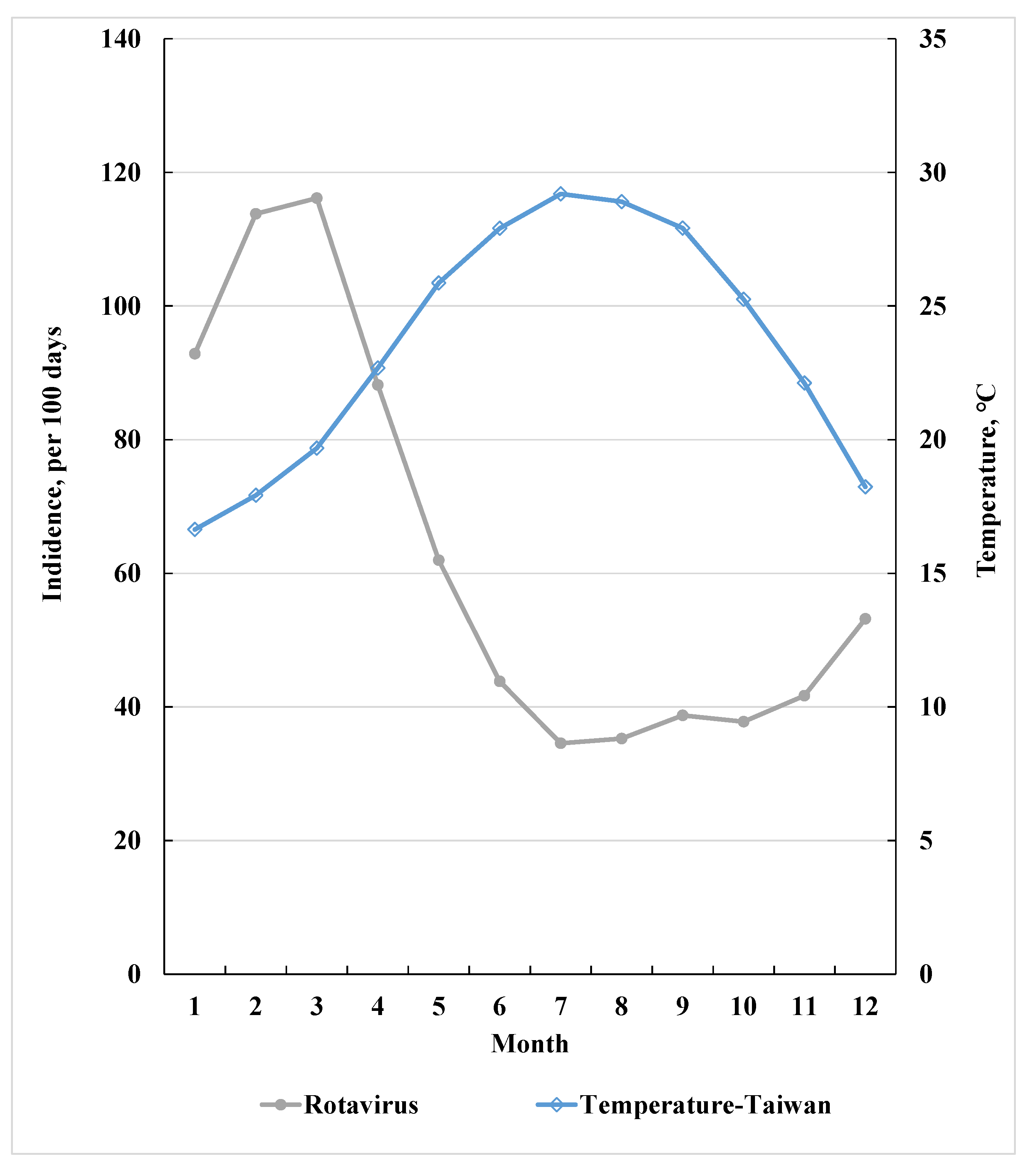
3.1. RvI Incidence Inversely Related to Average Temperature
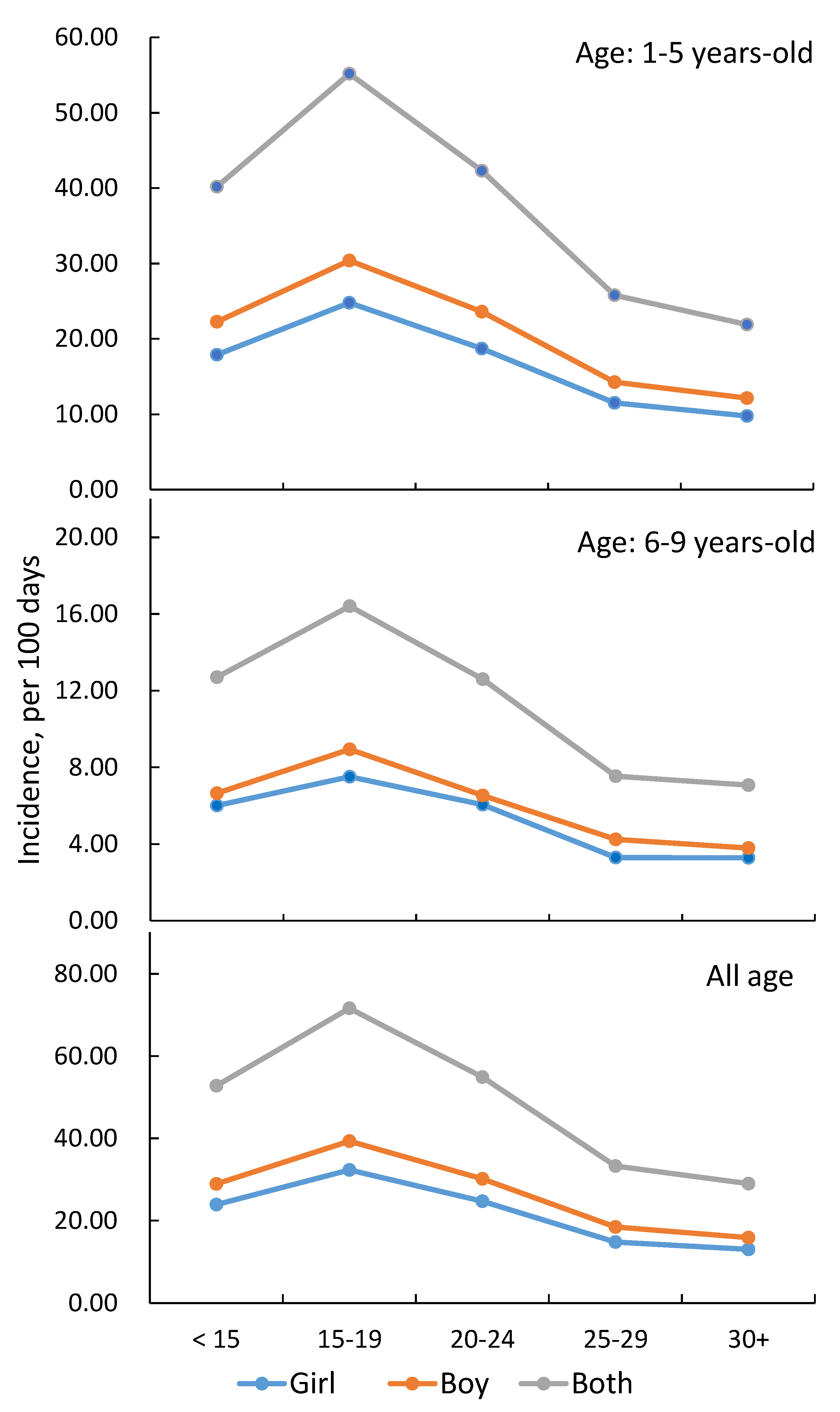
3.2. Incidence and Relative Risk of RvI by Temperature, PM2.5, and Parental Income
3.3. RvI Associated with Interaction between Temperature and PM2.5
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parashar, U.D.; Gibson, C.J.; Bresee, J.S.; Glass, R.I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 2006, 12, 304. [Google Scholar] [CrossRef] [Green Version]
- Dennehy, P.H. Rotavirus infection: A disease of the past? Infect. Dis. Clin. 2015, 29, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, N.; Tate, J.E.; Haynes, A.K.; Parashar, U.D. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination—United States, 2000–2014. Morb. Mortal. Wkly. Rep. 2015, 64, 337. [Google Scholar]
- Hallowell, B.D.; Parashar, U.D.; Curns, A.; DeGroote, N.P.; Tate, J.E. Trends in the laboratory detection of rotavirus before and after implementation of routine rotavirus vaccination—United States, 2000–2018. Morb. Mortal. Wkly. Rep. 2019, 68, 539. [Google Scholar] [CrossRef] [Green Version]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Rotavirus Last Updated. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_19_Rotavirus_R2.pdf?ua=1 (accessed on 21 July 2021).
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossouw, E.; Brauer, M.; Meyer, P.; Plessis, N.M.D.; Avenant, T.; Mans, J. Virus Etiology, Diversity and Clinical Characteristics in South African Children Hospitalised with Gastroenteritis. Viruses 2021, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Lestari, F.B.; Vongpunsawad, S.; Wanlapakorn, N.; Poovorawan, Y. Rotavirus infection in children in Southeast Asia 2008–2018: Disease burden, genotype distribution, seasonality, and vaccination. J. Biomed. Sci. 2020, 27, 66. [Google Scholar] [CrossRef]
- Butz, A.M.; Fosarelli, P.; Dick, J.; Cusack, T.; Yolken, R. Prevalence of rotavirus on high-risk fomites in day-care facilities. J. Pediatr. 1993, 92, 202–205. [Google Scholar]
- Celik, C.; Gozel, M.G.; Turkay, H.; Bakici, M.Z.; Güven, A.S.; Elaldi, N. Rotavirus and adenovirus gastroenteritis: Time series analysis. Pediatr. Int. 2015, 57, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.; Greenberg, H. Rotavirus. In Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1347–1401. [Google Scholar]
- Jagai, J.S.; Sarkar, R.; Castronovo, D.; Kattula, D.; McEntee, J.; Ward, H.; Kang, G.; Naumova, E.N. Seasonality of rotavirus in South Asia: A meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS ONE 2012, 7, e38168. [Google Scholar] [CrossRef]
- Levy, K.; Hubbard, A.E.; Eisenberg, J.N. Seasonality of rotavirus disease in the tropics: A systematic review and meta-analysis. Int. J. Epidemiol. 2009, 38, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Goggins, W.B.; Chan, E.Y. A time-series study of the association of rainfall, relative humidity and ambient temperature with hospitalizations for rotavirus and norovirus infection among children in Hong Kong. Sci. Total Environ. 2018, 643, 414–422. [Google Scholar] [CrossRef]
- Ye, Q.; Fu, J.F.; Mao, J.H.; Shen, H.Q.; Chen, X.J.; Shao, W.X.; Shang, S.Q.; Wu, Y.F. Haze is an important medium for the spread of rotavirus. Environ. Pollut. 2016, 216, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-S.; Huang, Y.-C.; Chen, C.-J.; Shie, S.-S.; Yang, S.-L.; Huang, C.-G.; Tsao, K.-C.; Chiu, C.-H.; Hsieh, Y.-C.; Kuo, C.-Y. Detection of norovirus and rotavirus among inpatients with acute gastroenteritis in a medical center in northern Taiwan, 2013–2018. J. Microbiol. Immunol. Infect. 2020, 53, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Armstrong, B.; Wagatsuma, Y.; Faruque, A.; Hayashi, T.; Sack, D.A. Rotavirus infections and climate variability in Dhaka, Bangladesh: A time-series analysis. Epidemiol. Infect. 2008, 136, 1281–1289. [Google Scholar] [CrossRef] [Green Version]
- Delahoy, M.J.; Cárcamo, C.; Huerta, A.; Lavado, W.; Escajadillo, Y.; Ordoñez, L.; Vasquez, V.; Lopman, B.; Clasen, T.; Gonzales, G.F. Meteorological factors and childhood diarrhea in Peru, 2005–2015: A time series analysis of historic associations, with implications for climate change. Environ. Health. 2021, 20, 22. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM 2.5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar]
- Colston, J.M.; Zaitchik, B.; Kang, G.; Yori, P.P.; Ahmed, T.; Lima, A.; Turab, A.; Mduma, E.; Shrestha, P.S.; Bessong, P. Use of earth observation-derived hydrometeorological variables to model and predict rotavirus infection (MAL-ED): A multisite cohort study. Lancet Planet. Health 2019, 3, e248–e258. [Google Scholar] [CrossRef] [Green Version]
- Hervás, D.; Hervás-Masip, J.; Rosell, A.; Mena, A.; Pérez, J.; Hervás, J. Are hospitalizations for rotavirus gastroenteritis associated with meteorologic factors? Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Carducci, A.; Verani, M.; Lombardi, R.; Casini, B.; Privitera, G. Environmental survey to assess viral contamination of air and surfaces in hospital settings. J. Hosp. Infect. 2011, 77, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Verani, M.; Bigazzi, R.; Carducci, A. Viral contamination of aerosol and surfaces through toilet use in health care and other settings. Am. J. Infect. Control. 2014, 42, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Bi, P.; Dhimal, M.; Sherchand, J.B.; Hanson-Easey, S. Non-linear effect of temperature variation on childhood rotavirus infection: A time series study from Kathmandu, Nepal. Sci. Total Environ. 2020, 748, 141376. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.S.; Astry, C.; Vonderfecht, S.; Jakab, G.; Shen, F.M.; Yolken, R.H. Aerosol transmission of experimental rotavirus infection. Pediatr. Infect. Dis. 1986, 5, 218–222. [Google Scholar] [CrossRef]
- Liou, Y.-A.; Yan, S.-K. Two-year microwave radiometric observations of low-level boundary-layer temperature inversion signatures. In Proceedings of the 2006 IEEE International Symposium on Geoscience and Remote Sensing, Denver, CO, USA, 31 July–4 August 2006; pp. 644–647. [Google Scholar]
- Malek, E.; Davis, T.; Martin, R.S.; Silva, P.J. Meteorological and environmental aspects of one of the worst national air pollution episodes (January 2004) in Logan, Cache Valley, Utah, USA. Atmos. Res. 2006, 79, 108–122. [Google Scholar] [CrossRef]
- Wallace, J.; Kanaroglou, P. The effect of temperature inversions on ground-level nitrogen dioxide (NO2) and fine particulate matter (PM2.5) using temperature profiles from the Atmospheric Infrared Sounder (AIRS). Sci. Total Environ. 2009, 407, 5085–5095. [Google Scholar] [CrossRef]
| Variable | Infection, n/100 Days | Relative Risk 1 (95% Confidence Interval) |
|---|---|---|
| Average daily temperature, °C | ||
| <15 | 52.8 | 1.74 (1.61–1.88) |
| 15–19 | 71.6 | 2.25 (2.11–2.41) |
| 20–24 | 54.9 | 1.72 (1.61–1.84) |
| 25–29 | 33.3 | 1.20 (1.12–1.28) |
| ≥30 | 29.2 | Ref. |
| Average daily PM2.5, µg/m3 | ||
| <17.5 | 30.8 | Ref. |
| 17.5–31.4 | 45.8 | 1.34 (1.29–1.38) |
| 31.5–41.9 | 52.3 | 1.52 (1.46–1.58) |
| ≥42.0 | 63.0 | 1.85 (1.78–1.92) |
| Parental income, NTD | ||
| <250,000 | 27.1 | Ref. |
| 250,000–299,999 | 63.0 | 2.40 (2.33–2.46) |
| ≥300,000 | 108.8 | 4.07 (3.95–4.20) |
| Variable | Adjusted Relative Risk (95% Confidence Interval) 1 | |
|---|---|---|
| ≤5 Years | 6–9 Years | |
| Average temperature, °C | ||
| <15 | 1.74 (1.59–1.89) | 1.75 (1.50–2.03) |
| 15–19 | 2.29 (2.12–2.47) | 2.15 (1.88–2.46) |
| 20–24 | 1.74 (1.62–1.88) | 1.63 (0.42–1.87) |
| 25–29 | 1.22 (1.13–1.32) | 1.13 (0.99–1.30) |
| ≥30 | Ref. | Ref. |
| Average PM2.5, µg/m3 | ||
| <17.5 | Ref. | Ref. |
| 17.5–31.4 | 1.35 (1.30–1.41) | 1.29 (1.20–1.38) |
| 31.5–41.9 | 1.52 (1.45–1.59) | 1.51 (1.39–1.63) |
| ≥42.0 | 1.85 (1.78–1.93) | 1.85 (1.72–1.99) |
| Parental income, NTD | ||
| <250,000 | Ref. | Ref. |
| 250,000–299,999 | 2.21 (2.14–2.27) | 3.21 (3.03–3.40) |
| ≥300,000 | 3.75 (3.62–3.88) | 5.50 (5.15–5.88) |
| Temperature °C | PM2.5, μg/m3 | Rate | Relative Risk Crude | (95% CI) Adjusted 1 |
|---|---|---|---|---|
| <20 | <17.5 | 14.8 | Ref. | Ref. |
| 17.5–31.4 | 14.8 | 1.00 (0.88–1.12) | 1.03 (0.92–1.17) | |
| 31.5–41.9 | 16.4 | 1.11 (0.97–1.26) | 1.26 (1.11–1.43) | |
| ≥42.0 | 15.9 | 1.07 (0.96–1.21) | 1.39 (1.24–1.57) | |
| 20–24 | <17.5 | 7.81 | 0.53 (0.45–0.62) | 0.59 (0.50–0.69) |
| 17.5–31.4 | 10.7 | 0.72 (0.64–0.83) | 0.77 (0.68–0.88) | |
| 31.5–41.9 | 12.0 | 0.81 (0.70–0.93) | 0.91 (0.79–1.05) | |
| ≥42.0 | 16.2 | 1.09 (0.98–1.23) | 1.32 (1.18–1.48) | |
| ≥25 | <17.5 | 4.84 | 0.33 (0.29–0.37) | 0.40 (0.35–0.45) |
| 17.5–31.4 | 8.32 | 0.56 (0.50–0.63) | 0.61 (0.54–0.68) | |
| 31.5–41.9 | 8.95 | 0.60 (0.53–0.69) | 0.70 (0.61–0.80) | |
| ≥42.0 | 9.84 | 0.66 (0.58–0.76) | 0.81 (0.77–0.93) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, H.-C.; Sung, F.-C.; Mou, C.-H.; Chen, C.W.; Tsai, S.P.; Hsieh, D.P.H.; Lu, C.-Y.; Chen, P.-C.; Tzeng, Y.-L. Childhood Rotavirus Infection Associated with Temperature and Particulate Matter 2.5 µm: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 12570. https://doi.org/10.3390/ijerph182312570
Tseng H-C, Sung F-C, Mou C-H, Chen CW, Tsai SP, Hsieh DPH, Lu C-Y, Chen P-C, Tzeng Y-L. Childhood Rotavirus Infection Associated with Temperature and Particulate Matter 2.5 µm: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(23):12570. https://doi.org/10.3390/ijerph182312570
Chicago/Turabian StyleTseng, Hui-Chen, Fung-Chang Sung, Chih-Hsin Mou, Chao W. Chen, Shan P. Tsai, Dennis P. H. Hsieh, Chung-Yen Lu, Pei-Chun Chen, and Ya-Ling Tzeng. 2021. "Childhood Rotavirus Infection Associated with Temperature and Particulate Matter 2.5 µm: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 18, no. 23: 12570. https://doi.org/10.3390/ijerph182312570
APA StyleTseng, H.-C., Sung, F.-C., Mou, C.-H., Chen, C. W., Tsai, S. P., Hsieh, D. P. H., Lu, C.-Y., Chen, P.-C., & Tzeng, Y.-L. (2021). Childhood Rotavirus Infection Associated with Temperature and Particulate Matter 2.5 µm: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 18(23), 12570. https://doi.org/10.3390/ijerph182312570







