Gamified Dual-Task Training for Individuals with Parkinson Disease: An Exploratory Study on Feasibility, Safety, and Efficacy
Abstract
1. Background and Purpose
2. Method
2.1. Participants
2.2. Procedures
2.3. Statistical Analysis
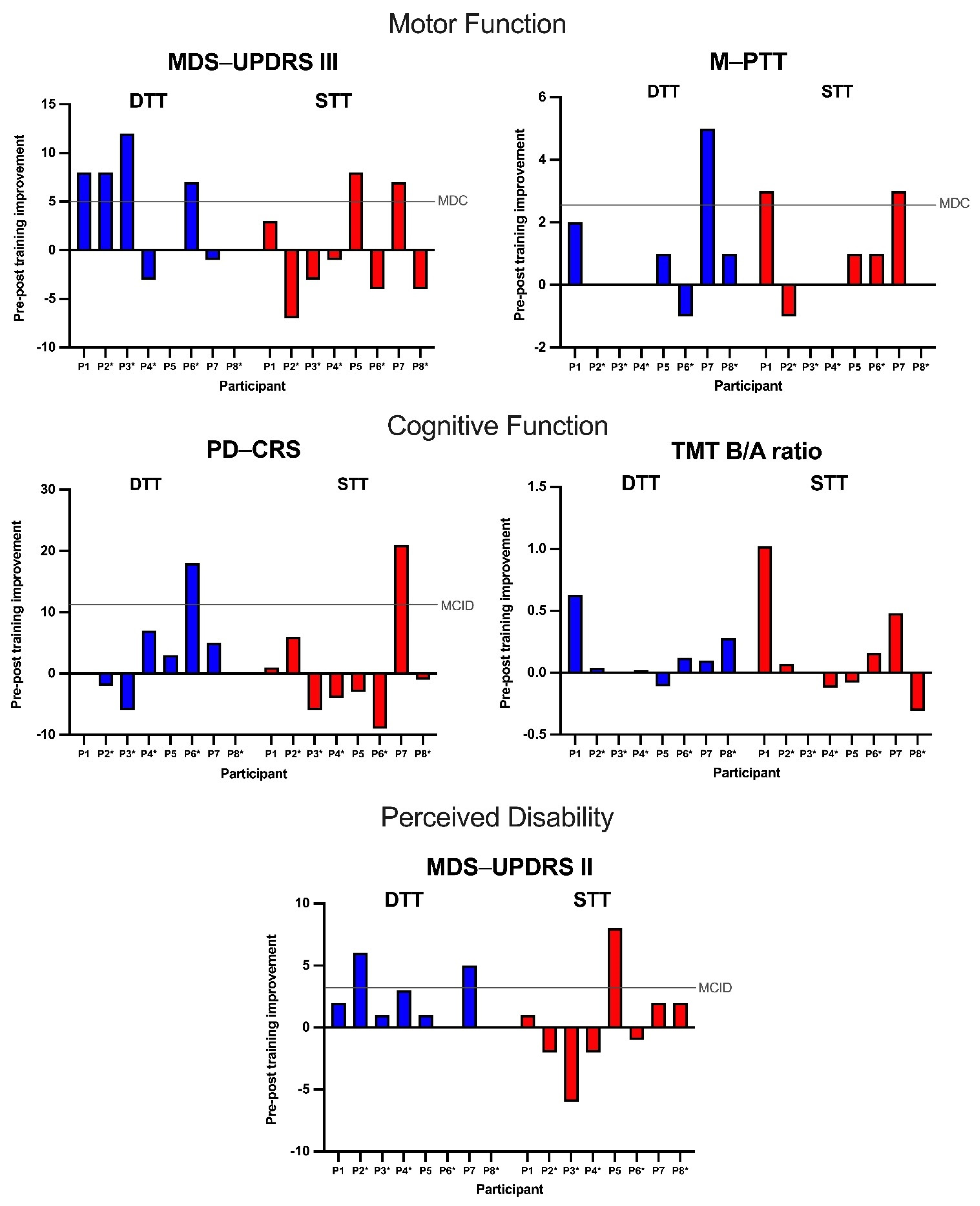
3. Results
3.1. Feasibility and Safety
3.2. Motor Measures
3.2.1. MDS-UPDRS III Score
3.2.2. M-PPT Score
3.3. Cognitive Measures
3.3.1. PD-CRS Score
3.3.2. TMT B/A Ratio
3.4. Perceived Disability Measure
MDS-UPDRS II Score
4. Discussion
4.1. Potential Predictive Characteristics for Differential Response to Gamified DTT versus STT
4.2. Limitations
4.3. Potential Advantages and Implications in Traditional Clinical Setting and Telerehabilitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Appendix A
| Task A | Task B | |
|---|---|---|
| Pair 1 | Sit-to-stand | Multi-plane locomotion (obstacle course) |
| Pair 2 | Gait | Reach and grasp |
| Pair 3 | Floor-to-stand; stand-to-floor | Single limb standing |
| Name of Game | Targeted Cognitive Processes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selective Attention | Sustained Attention | Inhibitory Control | Cognitive Flexibility | Decision-Making | Visual Search | Visual-Perceptual Processing | Visuospatial Working Memory | Language Processing | Information Processing Speed | |
| Category: Chase | ||||||||||
| Chase the Color | 1 | 2 | 1 | 1 | ||||||
| Chase the Target | 1 | 1 | 2 | 2 | 1 | 1 | ||||
| Category: Equations | ||||||||||
| Addition, Subtraction | 2 | 1 | 2 | 2 | ||||||
| Squares, Division, Multiplication | 2 | 2 | 2 | 1 | 1 | 2 | ||||
| Category: Memory | ||||||||||
| Color, Numbers, Shapes | 2 | 2 | 1 | 2 | ||||||
| Dice, Symbols | 2 | 2 | 1 | |||||||
| Category: Seek | ||||||||||
| Seek the Color | 1 | 2 | 2 | 2 | ||||||
| Seek the Letter | 1 | 2 | 2 | 2 | ||||||
| Seek the Smiley | 1 | 2 | 2 | |||||||
| Category: Track | ||||||||||
| Track Left, Right, Both | 2 | 1 | 2 | 2 | 1 | 2 | 2 | |||
| Track the Letter | 1 | 2 | 1 | 2 | ||||||
| Track the Color | 1 | 2 | 2 | |||||||
| Non-Categorized Games | ||||||||||
| Lights Out | 1 | 2 | 1 | |||||||
| Pattern Recognition | 2 | 1 | 1 | |||||||
| Rally | 2 | 2 | 1 | |||||||
| Spelling | 1 | |||||||||
| Pair 1 Task A: Sit-to-Stand | ||||||
|---|---|---|---|---|---|---|
| Level | Amplitude/Speed | Endurance | Balance | Vision | Accuracy | |
| 1 | Perform task well enough so that the task is able to be completed in one attempt—18-inch height chair | Complete 10 repetitions | Both feet on the ground and allowed to use upper extremity support | No manipulation | Not using back of legs on the chair and standing up to full upright posture; no “toes up” | |
| 2 | Perform task well enough so that the task is able to be completed in one attempt—16-inch height chair | Complete 20 repetitions | Both feet on the ground with goal of no upper extremity support | Look left/right | ||
| 3 | Perform task well enough so that the task is able to be completed in one attempt—14-inch height chair | Complete 40+ repetitions | No upper extremity support with pelvis or feet on an unstable surface (i.e., DynaDisk cushion; Airex pad) | Eyes closed | Maintain end upright posture—no wobbling, no “toes up” end position | |
| Pair 1 Task B: Multi-plane locomotion (turns, stepping over, around, and between obstacles, steps, or stairs) | ||||||
| Level | Amplitude/speed | Endurance | Balance | Vision | Accuracy | |
| 1 | Largest step at comfortable speed | 15 min with any number of rest breaks | PT UE support | Stable surface but obstructed path with obstacles to walk around and over (short objects) | No manipulation | Not knocking over any obstacle |
| 2 | Maintain largest step length and increase speed | 15 min with 1–2 rest breaks | Cane | Stable with obstacles to walk around and over (tall objects), and unstable surfaces (thick floor mat) | Look left/right | Predictable start and stops and 90° turns |
| 3 | Maintain largest step length and as fast as possible safely | 15 min with no rest break | No Support | Stable with obstacles to walk around and over (tall objects), and unstable surfaces (step on Airex pads or river stones) | Scanning environment with head turns | Unpredictable start and stops and turns of 90° (2-steps), 180° (max of 4 steps), or 360° (max of 8 steps) |
| Pair 2 Task A: Gait | ||||||
| Level | Amplitude/speed | Endurance | Balance | Vision | Accuracy | |
| 1 | Largest step at comfortable speed | 15 min with any number of rest breaks | Forward, side-ways, and backward walking with PT in front giving UE support | No manipulation | No accuracy manipulation | |
| 2 | Maintain largest step length and increase speed | 15 min with 1–2 rest breaks | Forward, side-ways, and backward walking with a cane | Look left/right | PT directed stop and start | |
| 3 | Maintain largest step length and as fast as possible safely | 15 min with no rest break | Forward, side-ways, and backward walking with no UE support | Scanning environment with head turns | Stop and start and regular turns (left, right, 180° or reversal from forward to back or side-stepping direction | |
| Pair 2 Task B: Reach and grasp | ||||||
| Level | Amplitude/speed | Endurance | Balance | Vision | Accuracy | |
| 1 | Sitting—maximum excursion (DISTANCE) of the reach with variations in both HEIGHT: Shoulder height, up and down AND DIRECTION: forward, lateral, and across | 10 reaches each arm | Blocked direction and blocked height | No manipulation | Grasp large object (e.g., water bottle) with palmer grasp | |
| 2 | Standing—maximum excursion (DISTANCE) of the reach with variations in both HEIGHT: Shoulder height, up and down AND DIRECTION: forward, lateral, and across | 20 reaches each arm | Blocked height variable direction | Look left/right | Grasp medium-sized object (e.g., highlighter) with tripod grasp | |
| 3 | Standing: Step and reach Maximum excursion (DISTANCE) of the reach with variations in both HEIGHT: Shoulder height, up and down AND DIRECTION: forward, lateral, and across | 40 reaches each arm | Variable height and direction | Look at object and then close eyes for reach and grasp | Grasp small object (e.g., paper clip) with pincer grasp | |
| Pair 3 Task A: Floor-to-stand; stand-to-floor | ||||||
| Level | Amplitude/speed | Endurance | Balance | Vision | Accuracy | |
| 1 | With chair in front—down to knees and back up | 5 repetitions | Using chair for upper extremity support | No manipulation | Best posture possible but OK if trunk is flexed; minimal sway or instability through the transition and in end positions | |
| 2 | Down onto hands and knees and back up | 10 repetitions | Not using chair | Look left/right | Maintenance of extension in trunk; minimal sway or instability through the transition and in end positions | |
| 3 | All the way down into prone and back up | 20 repetitions | Using least number of limbs possible (arms and legs) | Eyes closed | Maintenance of extension in trunk; minimal sway or instability through the transition and in end positions | |
| Pair 3 Task B: Single limb standing | ||||||
| Level | Amplitude/speed | Endurance | Balance | Vision | Accuracy | |
| 1 | Lift one foot up slightly to hip/knee flexion 15°, and to hip abduction 10° | 5 repetitions | Hand contact on an object as support | No manipulation | Maintain for 5 s per repetition | |
| 2 | Lift one foot up to hip/knee flexion 30°, and to hip abduction 20° | 10 repetitions | Hands on hip | Look left/right | Maintain for 10 s per repetition | |
| 3 | Lift one foot up to hip/knee flexion 45°, and to hip abduction 30° | 20 repetitions | With bilateral shoulder abduction/flexion 90° | Eyes closed | Maintain for 20 s per repetition | |
Appendix B
| Category of Treatment Response | Participant | Order of Training Conditions | Dx (y) a | HoF b | ABC c | LAPAQ d | |
|---|---|---|---|---|---|---|---|
| Responders | DS | P1 | STT-DTT | 5.7 | 0 (NF) | 97.2 (H) | 286 (A) |
| P7 | STT-DTT | 1.9 | 0 (NF) | 76.3 (L) | 300 (A) | ||
| D | P2 | DTT-STT | 0.3 | 3 (F) | 94.1 (H) | 220 (A) | |
| P3 | DTT-STT | 1.6 | 2 (F) | 85.8 (H) | 107 (I) | ||
| P6 | DTT-STT | 1.8 | 1 (F) | 98.4 (H) | 264 (A) | ||
| S | P5 | STT-DTT | 0.6 | 0 (NF) | 95.0 (H) | 86 (I) | |
| Non- Responders | P4 | DTT-STT | 6.9 | 0 (NF) | 95.6 (H) | 103 (I) | |
| P8 | DTT-STT | 6.5 | 0 (NF) | 87.3 (H) | 220 (A) |
References
- Parkinson’s Foundation. Statistics. Available online: http://www.parkinson.org/Understanding-Parkinsons/Statistics (accessed on 11 August 2021).
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Postuma, R.B.; Aarsland, D.; Barone, P.; Burn, D.J.; Hawkes, C.H.; Oertel, W.; Ziemssen, T. Identifying prodromal Parkinson’s disease: Pre-motor disorders in Parkinson’s disease. Mov. Disord. 2012, 27, 617–626. [Google Scholar] [CrossRef]
- Bernal-Pacheco, O.; Limotai, N.; Go, C.L.; Fernandez, H.H. Nonmotor manifestations in parkinson disease. Neurologist 2012, 18, 1–16. [Google Scholar] [CrossRef]
- Aarsland, D.; Larsen, J.P.; Tandberg, E.; Laake, K. Predictors of nursing home placement in Parkinson’s disease: A population-based, prospective study. J. Am. Geriatr. Soc. 2000, 48, 938–942. [Google Scholar] [CrossRef]
- Levy, G.; Tang, M.X.; Louis, E.D.; Côté, L.J.; Alfaro, B.; Mejia, H.; Stern, Y.; Marder, K. The association of incident dementia with mortality in PD. Neurology 2002, 59, 1708–1713. [Google Scholar] [CrossRef]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Lolk, A.; Kragh-Sørensen, P. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Arch. Neurol. 2003, 60, 387–392. [Google Scholar] [CrossRef]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef]
- Litvan, I.; Aarsland, D.; Adler, C.H.; Goldman, J.G.; Kulisevsky, J.; Mollenhauer, B.; Rodriguez-Oroz, M.C.; Tröster, A.I.; Weintraub, D. MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov. Disord. 2011, 26, 1814–1824. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Foltynie, T.; Brayne, C.E.G.; Robbins, T.W.; Barker, R.A. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 2007, 130, 1787–1798. [Google Scholar] [CrossRef]
- Wu, T.; Mark, H. Dual task interference in Parkinson’s disease. US Neurol. 2009, 5, 30–33. [Google Scholar] [CrossRef][Green Version]
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. CMAJ 2016, 188, 1157–1165. [Google Scholar] [CrossRef]
- Emre, M.; Ford, P.J.; Bilgic, B.; Uc, E.Y. Cognitive impairment and dementia in Parkinson’s disease: Practical issues and management. Mov. Disord. 2014, 29, 663–672. [Google Scholar] [CrossRef]
- Sanchez-Luengos, I.; Balboa-Bandeira, Y.; Lucas-Jiménez, O.; Ojeda, N.; Peña, J.; Ibarretxe-Bilbao, N. Effectiveness of cognitive rehabilitation in Parkinson’s disease: A systematic review and meta-analysis. J. Pers. Med. 2021, 11, 429. [Google Scholar] [CrossRef]
- Fritz, N.E.; Cheek, F.M.; Nichols-Larsen, D.S. Motor-cognitive dual-task training in persons with neurologic disorders: A systematic review. J. Neurol. Phys. Ther. 2015, 39, 142–153. [Google Scholar] [CrossRef]
- Strouwen, C.; Molenaar, E.A.L.M.; Münks, L.; Keus, S.H.J.; Bloem, B.R.; Rochester, L.; Nieuwboer, A. Dual tasking in Parkinson’s disease: Should we train hazardous behavior? Expert. Rev. Neurother. 2015, 15, 1031–1039. [Google Scholar] [CrossRef]
- De Freitas, T.B.; Leite, P.H.W.; Doná, F.; Pompeu, J.E.; Swarowsky, A.; Torriani-Pasin, C. The effects of dual task gait and balance training in Parkinson’s disease: A systematic review. Physiother. Theory. Pract. 2020, 36, 1088–1096. [Google Scholar] [CrossRef]
- Janssen, J.; Verschuren, O.; Renger, W.J.; Ermers, J.; Ketelaar, M.; van Ee, R. Gamification in physical therapy: More than using games. Pediatr. Phys. Ther. 2017, 29, 95–99. [Google Scholar] [CrossRef]
- Adcock, M.; Sonder, F.; Schättin, A.; Gennaro, F.; de Bruin, E.D. A usability study of multicomponent video game-based training for older adults. Eur. Rev. Aging Phys. Act. 2020, 17, 3. [Google Scholar] [CrossRef]
- Garcia-Agundez, A.; Folkerts, A.-K.; Konrad, R.; Caserman, P.; Tregel, T.; Goosses, M.; Göbel, S.; Kalbe, E. Recent advances for Parkinson’s disease with exergames: A systematic review. J. Neuroeng. Rehabil. 2019, 16, 17. [Google Scholar] [CrossRef]
- Li, Z.; Wang, T.; Liu, H.; Jiang, Y.; Wang, Z.; Zhuang, J. Dual-task training on gait, motor symptoms, and balance in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 1355–1367. [Google Scholar] [CrossRef]
- Rodriguez-Blazquez, C.; Rojo-Abuin, J.M.; Alvarez-Sanchez, M.; Arakaki, T.; Bergareche-Yarza, A.; Chade, A.; Garretto, N.; Gershanik, O.; Kurtis, M.M.; Martinez-Castrillo, J.C.; et al. The MDS-UPDRS Part II (motor experiences of daily living) resulted useful for assessment of disability in Parkinson’s disease. Parkinsonism. Relat. Disord. 2013, 10, 889–893. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; MacAskill, M.R.; Nakas, C.T.; Livingston, L.; Graham, C.; Crucian, G.P.; Melzer, T.R.; Kirwan, J.; Keenan, R.; Wells, S.; et al. The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010, 75, 1717–1725. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiat. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The activities-specific balance confidence (ABC) scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50A, M28–M34. [Google Scholar] [CrossRef]
- van Nimwegen, M.; Speelman, A.D.; Hofman-van Rossum, E.J.M.; Overeem, S.; Deeg, D.J.H.; Borm, G.F.; van der Horst, M.H.L.; Bloem, B.R.; Munneke, M. Physical inactivity in Parkinson’s disease. J. Neurol. 2011, 258, 2214–2221. [Google Scholar] [CrossRef]
- King, L.A.; Horak, F.B. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys. Ther. 2009, 89, 384–393. [Google Scholar] [CrossRef]
- Soke, F.; Guclu-Gunduz, A.; Kocer, B.; Fidan, I.; Keskinoglu, P. Task-oriented circuit training combined with aerobic training improves motor performance and balance in people with Parkinson’s disease. Acta Neurol. Belg. 2021, 121, 535–543. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; King, L.A. Effects of exercise on mobility in people with Parkinson’s disease. Mov. Disord. 2013, 28, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Target Heart Rates Chart. Available online: http://www.heart.org/en/healthy-living/fitness-basics/target-heart-rates (accessed on 11 August 2021).
- Wulf, G.; Lewthwaite, R. Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychon. Bull. Rev. 2016, 23, 1382–1414. [Google Scholar] [CrossRef]
- Loftus, G.R.; Masson, M.E. Using confidence intervals in within-subject designs. Psychon. Bull. Rev. 1994, 1, 476–490. [Google Scholar] [CrossRef]
- Julious, S.A. Using confidence intervals around individual means to assess statistical significance between two means. Pharmaceut. Statist. 2004, 3, 217–222. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Schrag, A.; Sampaio, C.; Counsell, N.; Poewe, W. Minimally clinically important change on the Unified Parkinson’s Disease Rating Scale. Mov. Disord. 2006, 21, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M.; Gruber-Baldini, A.L.; Anderson, K.E.; Fishman, P.S.; Reich, S.G.; Weiner, W.J. The clinically important difference on the unified Parkinson’s disease rating scale. Arch. Neurol. 2010, 67, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Sinacore, D.R.; Binder, E.F.; Kohrt, W.M. Physical and performance measures for the identification of mild to moderate frailty. J. Geron Med. Sci. A 2000, 55, 350–355. [Google Scholar] [CrossRef]
- King, L.A.; Wilhelm, J.; Chen, Y.; Blehm, R.; Nutt, J.; Chen, Z.; Serder, A.; Horak, F.B. Effects of group, individual, and home exercise in persons with Parkinson disease: A randomized clinical trial. J. Neurol. Phys. Ther. 2015, 39, 204–212. [Google Scholar] [CrossRef]
- Paschal, K.A.; Oswald, A.R.; Siegmund, R.W.; Siegmund, S.E.; Threlkeld, A.J. Test-retest reliability of the physical performance test for persons with Parkinson disease. J. Geriatr. Phys. Ther. 2006, 29, 82–86. [Google Scholar] [CrossRef]
- Pagonabarraga, J.; Kulisevsky, J.; Llebaria, G.; García_Sánchez, C.; Pascual-Sedano, B.; Gironell, A. Parkinson’s disease-cognitive rating scale: A new cognitive scale for Parkinson’s disease. Mov. Disord. 2008, 23, 998–1005. [Google Scholar] [CrossRef]
- de Bobadilla, R.F.; Pagonbarraga, J.; Martínez-Horta, S.; Pascual-Sedano, B.; Campolongo, A.; Kulisevsky, J. Parkinson’s disease-cognitive rating scale: Psychometrics for mild cognitive impairment. Mov. Disord. 2013, 28, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.T.; Piper, B.J. The Psychology Experiment Building Language (PEBL) and PEBL Test Battery. J. Neurosci. Methods 2014, 222, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Horváth, K.; Aschermann, Z.; Kovács, M.; Makkos, A.; Harmat, M.; Janszky, J.; Komoly, S.; Karádi, K.; Kovács, N. Minimal clinically important differences for the experiences of daily living parts of movement disorder society-sponsored unified Parkinson’s disease rating scale. Mov. Disord. 2017, 32, 789–793. [Google Scholar] [CrossRef]
- Brod, M.; Mendelsohn, G.A.; Roberts, B. Patients’ experiences of Parkinson’s disease. J. Gerontol. B Psychol. Sci. Soc. Sci. 1998, 53, P213–P222. [Google Scholar] [CrossRef] [PubMed]
- Hariz, G.M.; Forsgren, L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol. Scand. 2011, 123, 20–27. [Google Scholar] [CrossRef]
- Nonnekes, J.; Růžička, E.; Nieuwboer, A.; Hallett, M.; Fasano, A.; Bloem, B.R. Compensation strategies for gait impairments in Parkinson disease: A review. JAMA Neurol. 2019, 76, 718–725. [Google Scholar] [CrossRef]
- Jung, S.H.; Hasegawa, N.; Mancini, M.; King, L.A.; Carlson-Kuhta, P.; Smulders, K.; Peterson, D.S.; Barlow, N.; Harker, G.; Morris, R.; et al. Effects of the agility boot camp with cognitive challenge (ABC-C) exercise program for Parkinson’s disease. NPJ Parkinson’s Dis. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.R.; Cheng, S.J.; Lee, Y.J.; Liu, Y.C.; Wang, R.Y. Cognitive and motor dual task gait training exerted specific training effects on dual task gait performance in individuals with Parkinson’s disease: A randomized controlled pilot study. PLoS ONE 2019, 14, e0218180. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.S.; King, L.A.; Cohen, R.G.; Horak, F.B. Cognitive contributions to freezing of gait in Parkinson disease: Implications for physical rehabilitation. Phys. Ther. 2016, 96, 659–670. [Google Scholar] [CrossRef]
- Takeuchi, H.; Magistro, D.; Kotozaki, Y.; Motoki, K.; Nejad, K.K.; Nouchi, R.; Jeong, H.; Sato, C.; Sessa, S.; Nagatomi, R.; et al. Effects of simultaneously performed dual-task training with aerobic exercise and working memory training on cognitive functions and neural systems in the elderly. Neural. Plast. 2020, 2020, 3859824. [Google Scholar] [CrossRef]
- Heinzel, S.; Maechtel, M.; Hasmann, S.E.; Hobert, M.A.; Heger, T.; Berg, D.; Maetzler, W. Motor dual-tasking deficits predict falls in Parkinson’s disease: A prospective study. Parkinsonism. Relat. Disord. 2016, 26, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Strouwen, C.; Molenaar, E.; Münks, L.; Broeder, S.; Ginis, P.; Bloem, B.R.; Nieuwboer, A.; Heremans, E. Determinants of dual-task training effect seize in Parkinson disease: Who will benefit most? J. Neurol. Phys. Ther. 2019, 43, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Marco, S.D.; Serrano, M.; Villena, D.; Granda, D. Validation of the Parkinson’s Disease-Cognitive Rating Scale applying the Movement Disorder Society Task Force criteria for dementia associated with Parkinson’s disease. Mov. Disord. Clin. Pract. 2017, 4, 51–57. [Google Scholar]
- Domingos, J.M.; Godinho, C.; Dean, J.; Coelho, M.; Pinto, A.; Bloem, B.R.; Ferreira, J.J. Cognitive impairment in fall-related studies in Parkinson’s disease. J. Parkinson’s Dis. 2015, 5, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.P.; Williams, S.M.; Strine, T.W.; Anda, R.F.; Moore, M.J. Dementia and its implications for public health. Prev. Chronic. Dis. 2006, 3, A34. [Google Scholar] [PubMed]
- Barry, G.; Galna, B.; Rochester, L. The role of exergaming in Parkinson’s disease. J. Neuroeng. Rehabil. 2014, 11, 1–10. [Google Scholar] [CrossRef]
- Dockx, K.; Bekkers, E.M.J.; Van den Bergh, V.; Ginis, P.; Rochester, L.; Hausdorff, J.M.; Mirelman, A.; Nieuwboer, A. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst. Rev. 2016, 12, CD010760. [Google Scholar] [CrossRef]
- van Beek, J.J.W.; van Wegen, E.E.H.; Bohlhalter, S.; Vanbellingen, T. Exergaming-based dexterity training in persons with Parkinson disease: A pilot feasibility study. J. Neurol. Phys. Ther. 2019, 43, 168–174. [Google Scholar] [CrossRef]
- Lumsden, J.; Edwards, E.A.; Lawrence, N.S.; Coyle, D.; Munafò, M.R. Gamification of cognitive assessment and cognitive training: A systematic review of applications and efficacy. JMIR Serious Games 2016, 4, e11. [Google Scholar] [CrossRef]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fund, V.S.C. Postural instability in patients with Parkinson’s disease. Epidemiology, pathophysiology and management. CNS Drugs 2013, 27, 97–112. [Google Scholar] [CrossRef] [PubMed]

| Participant | Age (y) | Sex | Dx (y) | H&Y Stage | MoCA | HoF | ABC | GDS | LAPAQ | Perceived Disability | Motor Function | Cognitive Function | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDS-UPDRS II d | MDS- UPDRS III d | M-PPT e | PD-CRS e | TMT B/A d | ||||||||||
| P1 b | 80 | F | 5.7 | 3 | 30 | 0 | 97.2 | 1 | 286 | 3 | 38 | 29 | 106 | 2.33 |
| P2 c | 53 | F | 0.3 | 2 | 30 | 3 | 94.1 | 2 | 220 | 7 | 24 | 34 | 113 | 1.31 |
| P3 c | 72 | F | 1.6 | 3 | 28 | 2 | 85.8 | 0 | 107 | 12 | 26 | 33 | 114 | 1.39 |
| P4 c | 60 | F | 6.9 | 2 | 26 | 0 | 95.6 | 5 | 103 | 4 | 17 | 33 | 113 | 1.15 |
| P5 b | 68 | F | 0.6 | 2 | 26 | 0 | 95.0 | 4 | 86 | 1 | 25 | 32 | 95 | 1.72 |
| P6 c | 75 | F | 1.8 | 2 | 28 | 1 | 98.4 | 1 | 264 | 0 | 33 | 33 | 100 | 1.32 |
| P7 b | 58 | F | 1.9 | 2 | 19 b | 0 | 76.3 | 19 c | 300 | 7 | 47 | 22 | 53 | 2.05 |
| P8 c | 69 | M | 6.5 | 2 | 27 | 0 | 87.3 | 5 | 220 | 13 | 45 | 28 | 102 | 1.95 |
| P9 d | 73 | M | 0.1 | 2 | 28 | 0 | 98.8 | 2 | 294 | 1 | 13 | 33 | 106 | 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, L.-K.; Chung, Y.-C.; Bellard, D.; Swan, L.; Gobreial, N.; Romano, A.; Glatt, R.; Bonaguidi, M.A.; Lee, D.J.; Jin, Y.; et al. Gamified Dual-Task Training for Individuals with Parkinson Disease: An Exploratory Study on Feasibility, Safety, and Efficacy. Int. J. Environ. Res. Public Health 2021, 18, 12384. https://doi.org/10.3390/ijerph182312384
Chua L-K, Chung Y-C, Bellard D, Swan L, Gobreial N, Romano A, Glatt R, Bonaguidi MA, Lee DJ, Jin Y, et al. Gamified Dual-Task Training for Individuals with Parkinson Disease: An Exploratory Study on Feasibility, Safety, and Efficacy. International Journal of Environmental Research and Public Health. 2021; 18(23):12384. https://doi.org/10.3390/ijerph182312384
Chicago/Turabian StyleChua, Lee-Kuen, Yu-Chen Chung, David Bellard, Laura Swan, Nicole Gobreial, Amanda Romano, Ryan Glatt, Michael A. Bonaguidi, Darrin J. Lee, Yi Jin, and et al. 2021. "Gamified Dual-Task Training for Individuals with Parkinson Disease: An Exploratory Study on Feasibility, Safety, and Efficacy" International Journal of Environmental Research and Public Health 18, no. 23: 12384. https://doi.org/10.3390/ijerph182312384
APA StyleChua, L.-K., Chung, Y.-C., Bellard, D., Swan, L., Gobreial, N., Romano, A., Glatt, R., Bonaguidi, M. A., Lee, D. J., Jin, Y., Liu, C. Y., & Fisher, B. E. (2021). Gamified Dual-Task Training for Individuals with Parkinson Disease: An Exploratory Study on Feasibility, Safety, and Efficacy. International Journal of Environmental Research and Public Health, 18(23), 12384. https://doi.org/10.3390/ijerph182312384







