Construction and Evaluation of a High-Frequency Hearing Loss Screening Tool for Community Residents
Abstract
:1. Introduction
2. Materials and Methods
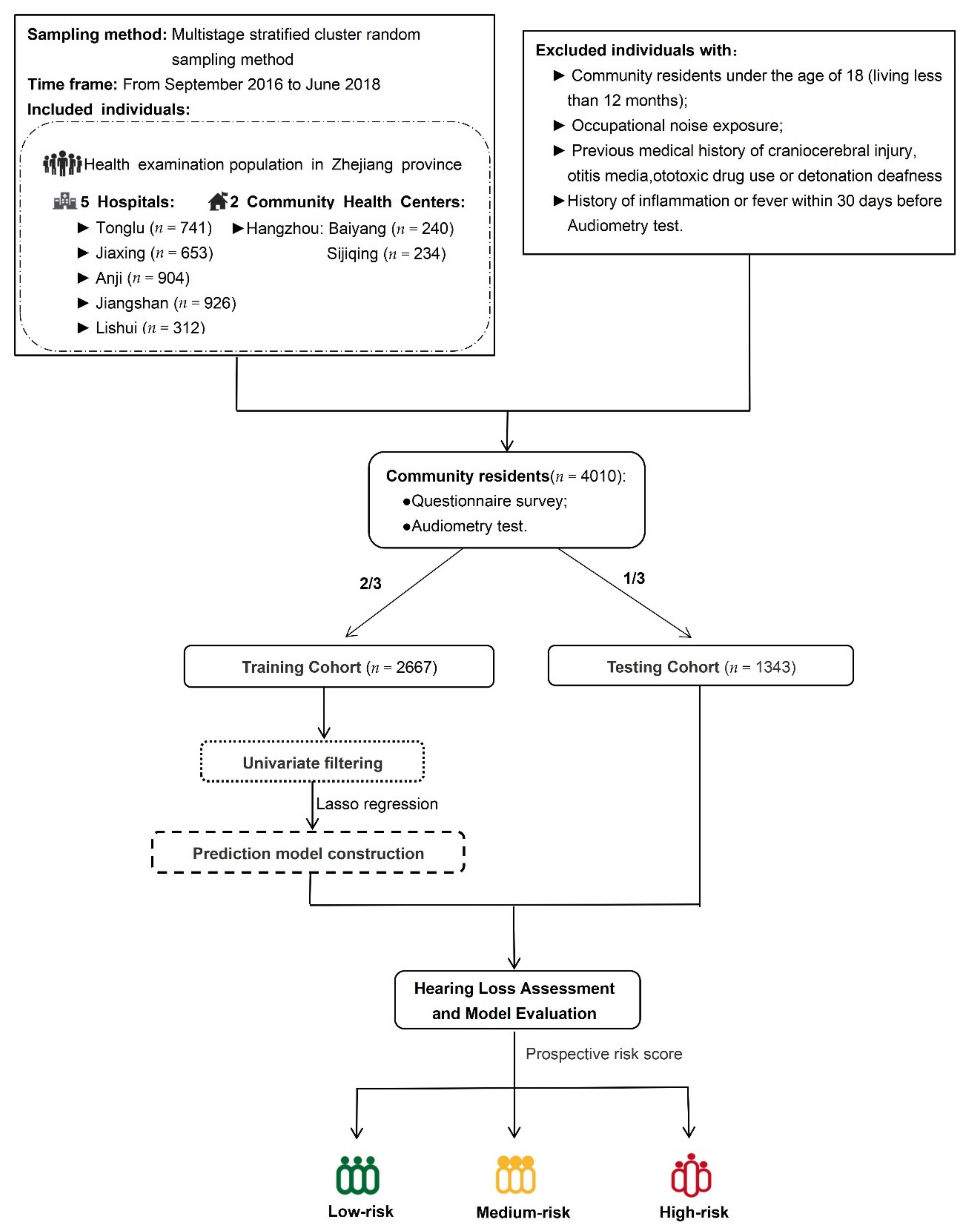
2.1. Participants
2.2. Audiometry Test
2.3. Questionnaire Survey
2.4. Statistical Analysis
2.4.1. Features Selection
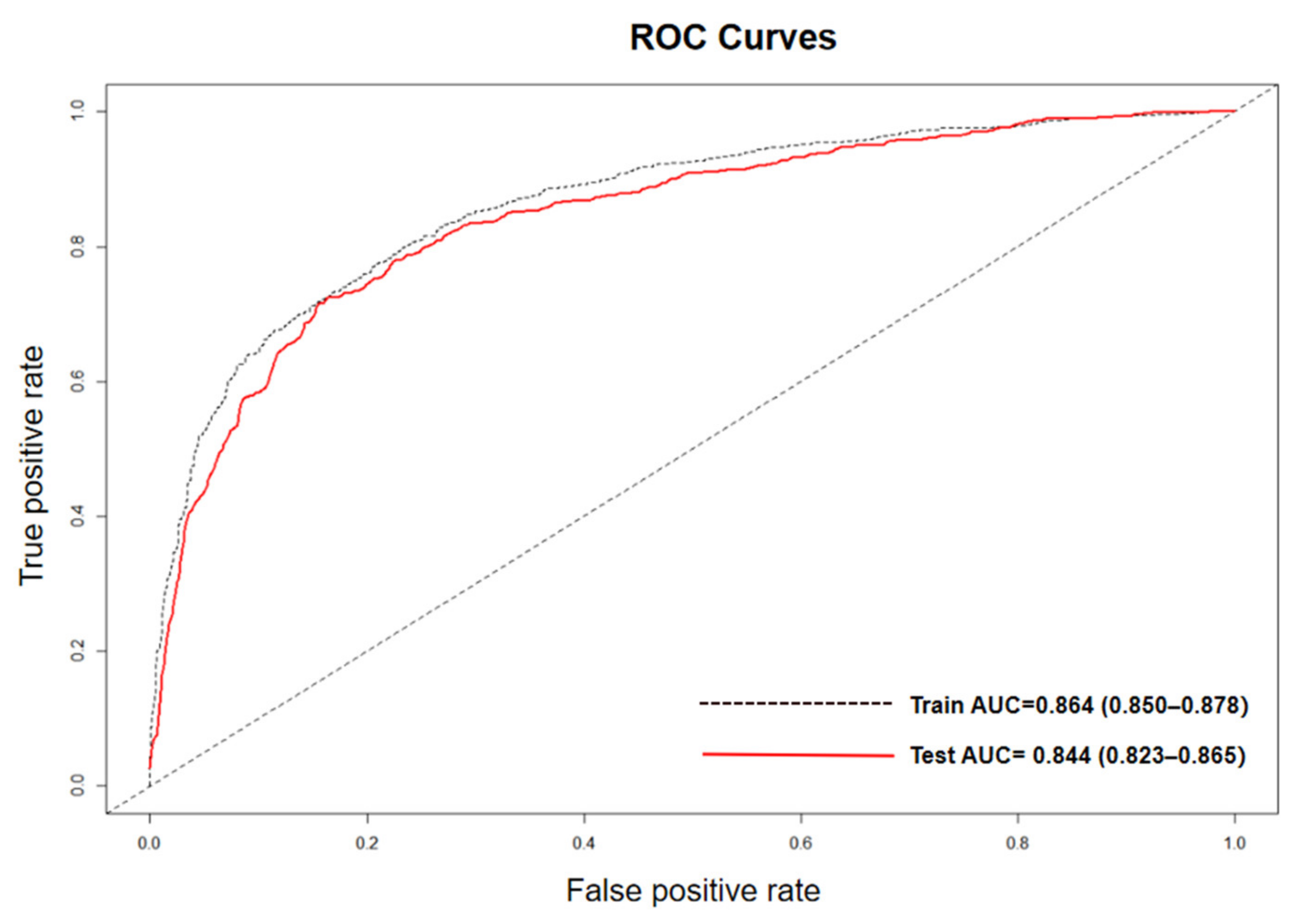
2.4.2. Model Construction and Evaluation
3. Results
3.1. Demographic Characteristics and Variable Selection
3.2. Model Performance
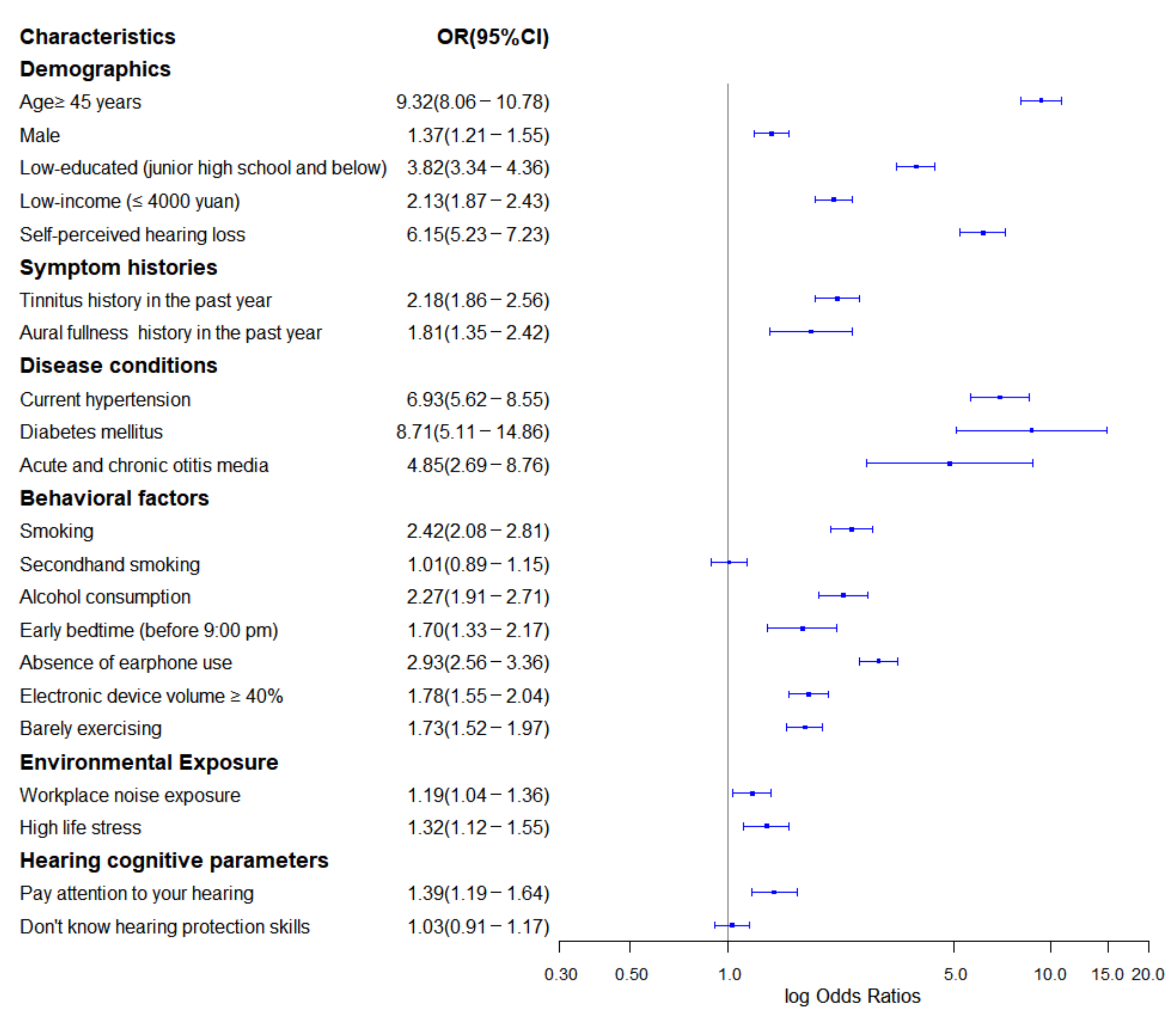
3.3. Significant Features
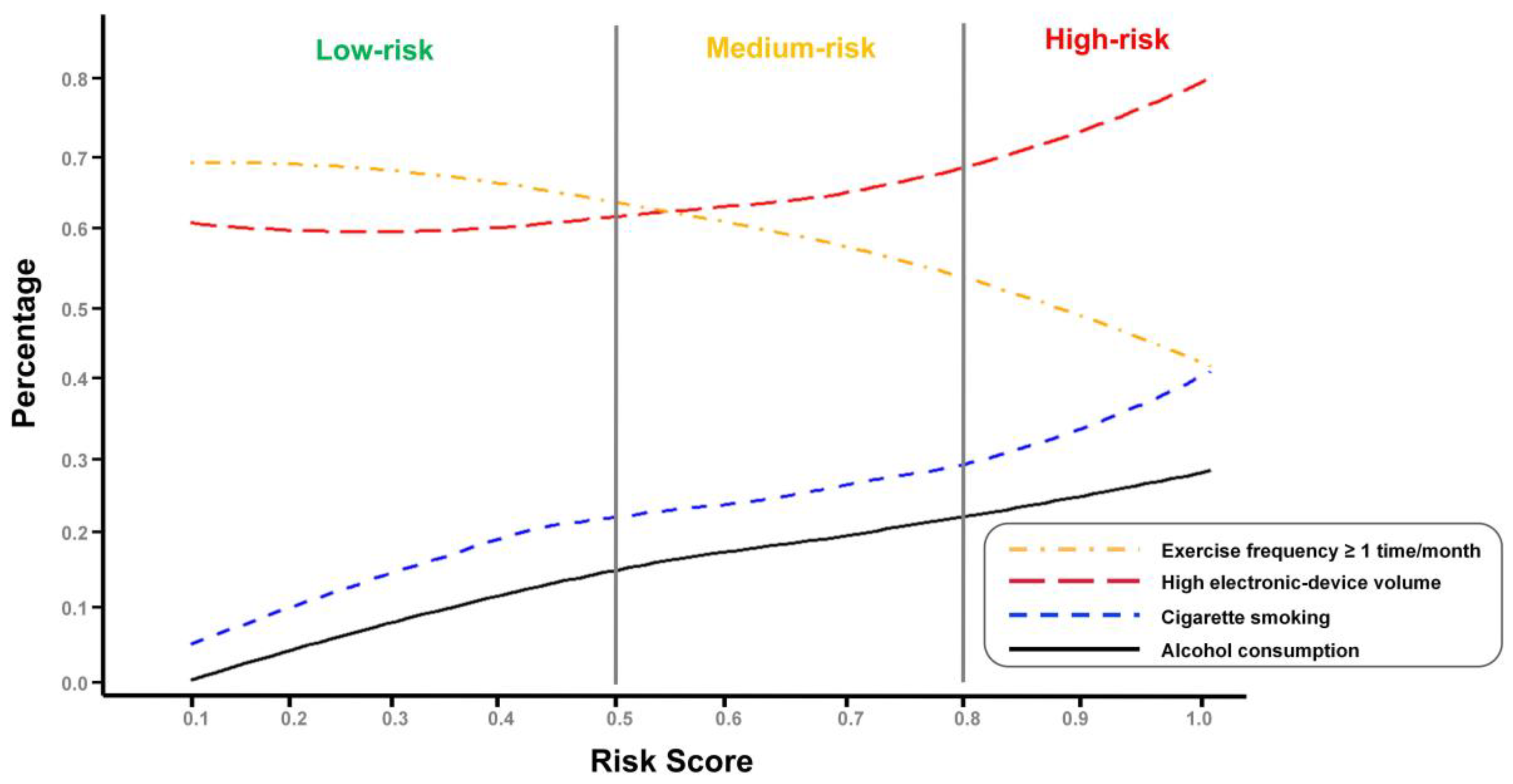
3.4. Distribution Patterns Stratified According to Risk Categories
3.4.1. Lifestyle-Related Feature Difference
3.4.2. Age-Related Feature Difference
3.4.3. Disease-Related Feature Difference
4. Discussion
4.1. Summary of Main Findings
4.2. Interpretation of Meaningful Risk Predictors and Its Implications for Prevention and Early Intervention
4.2.1. Social Determinants and Lifestyles
4.2.2. Age and Gender
4.2.3. Multiple Diseases and Symptom Histories
4.3. Comparison with Other Studies
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, L.L.; Tucci, D.L. Hearing Loss in Adults. N. Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef]
- Tsimpida, D.; Kontopantelis, E.; Ashcroft, D.; Panagioti, M. Socioeconomic and lifestyle factors associated with hearing loss in older adults: A cross-sectional study of the English Longitudinal Study of Ageing (ELSA). BMJ Open 2019, 9, e030–e031. [Google Scholar] [CrossRef] [Green Version]
- Vlaming, M.S.; MacKinnon, R.C.; Jansen, M.; Moore, D.R. Automated screening for high-frequency hearing loss. Ear Hear. 2014, 35, 667–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, G.H.; Frederick, M.T.; Silverman, S.C.; Penman, T.; Gardner, A.; Chisolm, T.H.; Escabi, C.D.; Oree, P.H.; Westermann, L.C.; Sanchez, V.A.; et al. Hearing Screening in the Community. J. Am. Acad. Audiol. 2019, 30, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lycke, M.; Debruyne, P.R.; Lefebvre, T.; Martens, E.; Ketelaars, L.; Pottel, H.; VanEygen, K.; Derijcke, S.; Werbrouck, P.; Vergauwe, P.; et al. The use of uHear™ to screen for hearing loss in older patients with cancer as part of a comprehensive geriatric assessment. Acta Clin. Belg. 2018, 73, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Masalski, M.; Grysiński, T.; Kręcicki, T. Hearing Tests Based on Biologically Calibrated Mobile Devices: Comparison with Pure-Tone Audiometry. JMIR Mhealth Uhealth 2018, 6, e10. [Google Scholar] [CrossRef] [PubMed]
- Cassarly, C.; Matthews, L.J.; Simpson, A.N.; Dubno, J.R. The Revised Hearing Handicap Inventory and Screening Tool Based on Psychometric Reevaluation of the Hearing Handicap Inventories for the Elderly and Adults. Ear Hear. 2018, 41, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Na, W.; Kim, G.; Han, W.; Kim, J. The development and standardization of self-assessment for hearing screening of the elderly. Clin. Interv. Aging 2016, 11, 787–795. [Google Scholar]
- You, S.; Han, W.; Kim, S.; Maeng, S.; Seo, Y.J. Reliability and Validity of Self-Screening Tool for Hearing Loss in Older Adults. Clin. Interv. Aging 2020, 15, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Pelegrin, A.C.; Canuet, L.; Rodríguez, Á.A.; Morales, M.P. Predictive factors of occupational noise-induced hearing loss in Spanish workers: A prospective study. Noise Health 2015, 17, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bing, D.; Ying, J.; Miao, J.; Lan, L.; Wang, D.; Zhao, L.; Yin, Z.; Yu, L.; Guan, J.; Wang, Q. Predicting the hearing outcome in sudden sensorineural hearing loss via machine learning models. Clin. Otolaryngol. 2018, 43, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, C.; Yin, S.; Wang, Y.; Zhou, Y.; Wang, S.; Xu, X.; Liu, W.; Xu, L. Environmental exposure to organochlorine pesticides and its association with the risk of hearing loss in the Chinese adult population: A case-control study. Sci. Total Environ. 2021, 767, 145–153. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, S.K. Environmental Exposures to Lead, Mercury, and Cadmium and Hearing Loss in Adults and Adolescents: KNHANES 2010–2012. Environ. Health Perspect. 2017, 125, 067003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Report of the Informal Working Group on Prevention of Deafness and Hearing Impairment Programme Planning. Available online: https://apps.who.int/iris/bitstream/handle/10665/58839/WHO_PDH_91.1.pdf (accessed on 9 November 2021).
- He, A.; He, S.; Peng, D.; Zhan, Y.; Li, Y.; Chen, Z.; Gong, Y.; Li, X.; Zhou, L. Prognostic value of long non-coding RNA signatures in bladder cancer. Aging 2019, 11, 6237–6251. [Google Scholar] [CrossRef]
- Byhoff, E.; Tripodis, Y.; Freund, K.M.; Garg, A. Gender Differences in Social and Behavioral Determinants of Health in Aging Adults. J. Gen. Intern. Med. 2019, 34, 2310–2312. [Google Scholar] [CrossRef]
- Thornton, R.L.; Glover, C.M.; Cené, C.W.; Glik, D.C.; Henderson, J.A.; Williams, D.R. Evaluating Strategies for Reducing Health Disparities by Addressing the Social Determinants of Health. Health Aff. 2016, 35, 1416–1423. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.F.; Beckles, G.L.; Zhang, X.; Saaddine, J.B. Association of Socioeconomic Position With Sensory Impairment Among US Working-Aged Adults. Am. J. Public Health 2015, 105, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Cruickshanks, K.J.; Dhar, S.; Dinces, E.; Fifer, R.C.; Gonzalez, F., 2nd; Heiss, G.; Hoffman, H.J.; Lee, D.J.; Newhoff, M.; Tocci, L.; et al. Hearing Impairment Prevalence and Associated Risk Factors in the Hispanic Community Health Study/Study of Latinos. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Emmett, S.D.; Francis, H.W. The socioeconomic impact of hearing loss in U.S. adults. Otol. Neurotol. 2015, 36, 545–550. [Google Scholar] [CrossRef]
- He, P.; Luo, Y.; Hu, X.; Gong, R.; Wen, X.; Zheng, X. Association of socioeconomic status with hearing loss in Chinese working-aged adults: A population-based study. PLoS ONE. 2018, 13, e195–e227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruickshanks, K.J.; Nondahl, D.M.; Tweed, T.S.; Wiley, T.L.; Klein, B.E.; Klein, R.; Chappell, R.; Dalton, D.S.; Nash, S.D. Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear. Res. 2010, 264, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artiga, S.; Hinton, E. Beyond Health Care: The Role of Social Determinants in Promoting Health and Health Equity; Kaiser Family Foundation: Oakland, CA, USA, 2018. [Google Scholar]
- Browning, G.G.; Gatehouse, S.; Lowe, G.D. Blood viscosity as a factor in sensorineural hearing impairment. Lancet 1986, 1, 121–123. [Google Scholar] [CrossRef]
- Lin, B.M.; Wang, M.; Stankovic, K.M.; Eavey, R.; McKenna, M.J.; Curhan, G.C.; Curhan, S.G. Cigarette Smoking, Smoking Cessation, and Risk of Hearing Loss in Women. Am. J. Med. 2020, 133, 1180–1186. [Google Scholar] [CrossRef]
- Sulaiman, A.H.; Husain, R.; Seluakumaran, K. Evaluation of early hearing damage in personal listening device users using extended high-frequency audiometry and otoacoustic emissions. Eur. Arch. Otorhinolaryngol. 2014, 271, 1463–1470. [Google Scholar] [CrossRef]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef]
- Breinbauer, H.A.; Anabalón, J.L.; Gutierrez, D.; Cárcamo, R.; Olivares, C.; Caro, J. Output capabilities of personal music players and assessment of preferred listening levels of test subjects: Outlining recommendations for preventing music-induced hearing loss. Laryngoscope 2012, 122, 2549–2556. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, H.G.; Jang, J.H.; Sim, S.; Hong, S.K.; Lee, H.J.; Park, B.; Kim, H.J. Analysis of Predisposing Factors for Hearing Loss in Adults. J. Korean Med. Sci. 2015, 30, 1175–1182. [Google Scholar] [CrossRef]
- Dawes, P.; Cruickshanks, K.J.; Moore, D.R.; Edmondson-Jones, M.; McCormack, A.; Fortnum, H.; Munro, K.J. Cigarette smoking, passive smoking, alcohol consumption, and hearing loss. J. Assoc. Res. Otolaryngol. 2014, 15, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Ding, D.; Lopez, M.C.; Manohar, S.; Zhang, Y.; Kim, M.J.; Park, H.J.; White, K.; Kim, Y.H.; Linser, P.; et al. Effects of Long-Term Exercise on Age-Related Hearing Loss in Mice. J. Neurosci. 2016, 36, 11308–11319. [Google Scholar] [CrossRef] [Green Version]
- Balducci, S.; Sacchetti, M.; Haxhi, J.; Orlando, G.; D’Errico, V.; Fallucca, S.; Menini, S.; Pugliese, G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2014, 30, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Park, S.H.; Jeong, J.H.; Choung, T.; Kim, K.Y. Association between transportation noise and blood pressure in adults living in multi-storey residential buildings. Environ. Int. 2019, 132, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hynie, M. The Social Determinants of Refugee Mental Health in the Post-Migration Context: A Critical Review. Can. J. Psychiatry 2018, 63, 297–303. [Google Scholar] [CrossRef]
- Ren, H.M.; Re, N.J.; Liu, W. Recognition and control of the progression of age-related hearing loss. Rejuvenation Res. 2013, 16, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvestad, E.; Czajkowski, N.; Krog, N.H.; Engdahl, B.; Tambs, K. Heritability of hearing loss. Epidemiology 2012, 23, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Fu, X.; Bi, J.; Li, Y.; Liu, B.; Zhang, L. Alteration of auditory function in type 2 diabetic and pre-diabetic patients. Acta Otolaryngol. 2018, 138, 542–547. [Google Scholar] [CrossRef]
- Gupta, S.; Eavey, R.D.; Wang, M.; Curhan, S.G.; Curhan, G.C. Type 2 diabetes and the risk of incident hearing loss. Diabetologia 2019, 62, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef]
- Curhan, S.G.; Halpin, C.; Wang, M.; Eavey, R.D.; Curhan, G.C. Tinnitus and 3-Year Change in Audiometric Hearing Thresholds. Ear Hear. 2021, 42, 886–895. [Google Scholar] [CrossRef]
- Diao, M.; Sun, J.; Jiang, T.; Tian, F.; Jia, Z.; Liu, Y.; Chen, D. Comparison between self-reported hearing and measured hearing thresholds of the elderly in China. Ear Hear. 2014, 35, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Ikeda, H.; Hanaie, K.; Morikawa, M.; Iwamoto, J.; Okamoto, N.; Saeki, K.; Kurumatani, N. The Hearing Handicap Inventory for Elderly-Screening (HHIE-S) versus a single question: Reliability, validity, and relations with quality of life measures in the elderly community, Japan. Qual. Life Res. 2013, 22, 1151–1159. [Google Scholar] [CrossRef] [PubMed]

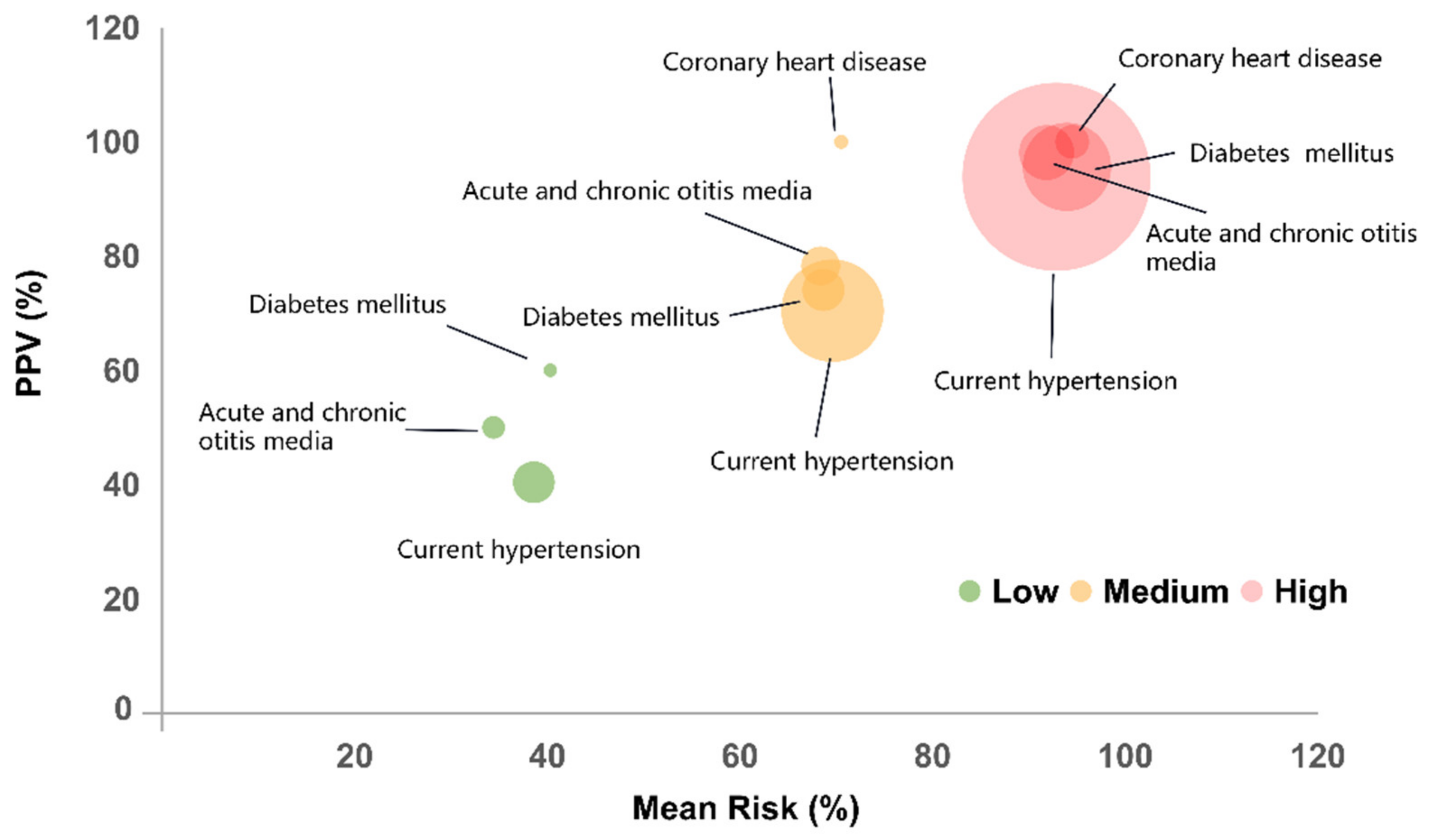
| Risk Category | Low | Medium | High | Total |
|---|---|---|---|---|
| Intervals | [0,0.50] | [0.50,0.80] | [0.80,1.00] | |
| Total, n | 1815 | 1016 | 1179 | 4010 |
| Case, n | 457 | 681 | 1094 | 2232 |
| PPV, % | 25.18 | 67.03 | 92.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ye, C.; Wang, D.; Li, C.; Wang, S.; Li, J.; Wu, J.; Wang, X.; Xu, L. Construction and Evaluation of a High-Frequency Hearing Loss Screening Tool for Community Residents. Int. J. Environ. Res. Public Health 2021, 18, 12311. https://doi.org/10.3390/ijerph182312311
Wang Y, Ye C, Wang D, Li C, Wang S, Li J, Wu J, Wang X, Xu L. Construction and Evaluation of a High-Frequency Hearing Loss Screening Tool for Community Residents. International Journal of Environmental Research and Public Health. 2021; 18(23):12311. https://doi.org/10.3390/ijerph182312311
Chicago/Turabian StyleWang, Yi, Chengyin Ye, Dahui Wang, Chenhui Li, Shichang Wang, Jinmei Li, Jinghua Wu, Xiaozhen Wang, and Liangwen Xu. 2021. "Construction and Evaluation of a High-Frequency Hearing Loss Screening Tool for Community Residents" International Journal of Environmental Research and Public Health 18, no. 23: 12311. https://doi.org/10.3390/ijerph182312311
APA StyleWang, Y., Ye, C., Wang, D., Li, C., Wang, S., Li, J., Wu, J., Wang, X., & Xu, L. (2021). Construction and Evaluation of a High-Frequency Hearing Loss Screening Tool for Community Residents. International Journal of Environmental Research and Public Health, 18(23), 12311. https://doi.org/10.3390/ijerph182312311








