A Long-Term Follow-Up of Dental and Craniofacial Disturbances after Cancer Therapy in a Pediatric Rhabdomyosarcoma Patient: Case Report
Abstract
1. Introduction
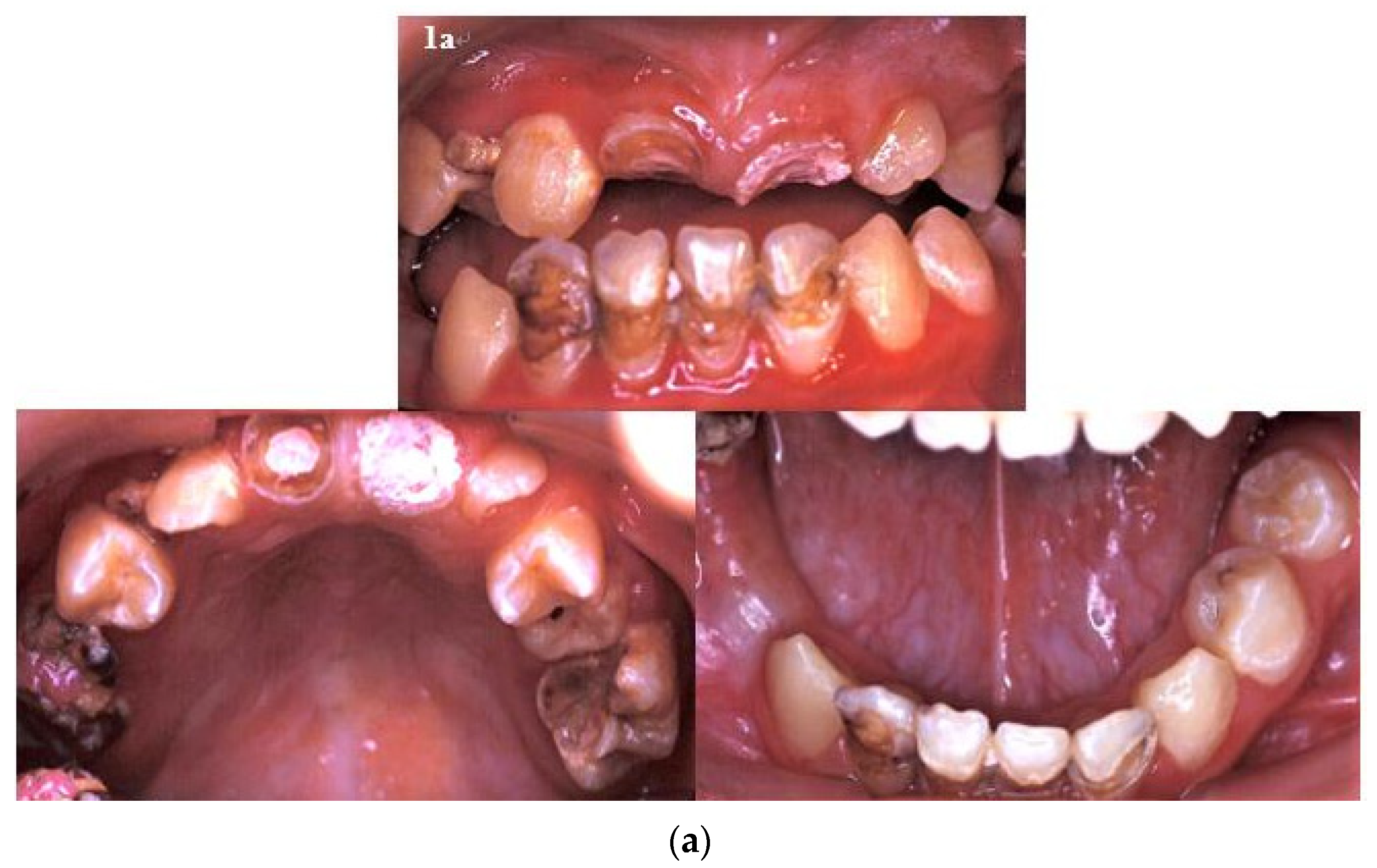
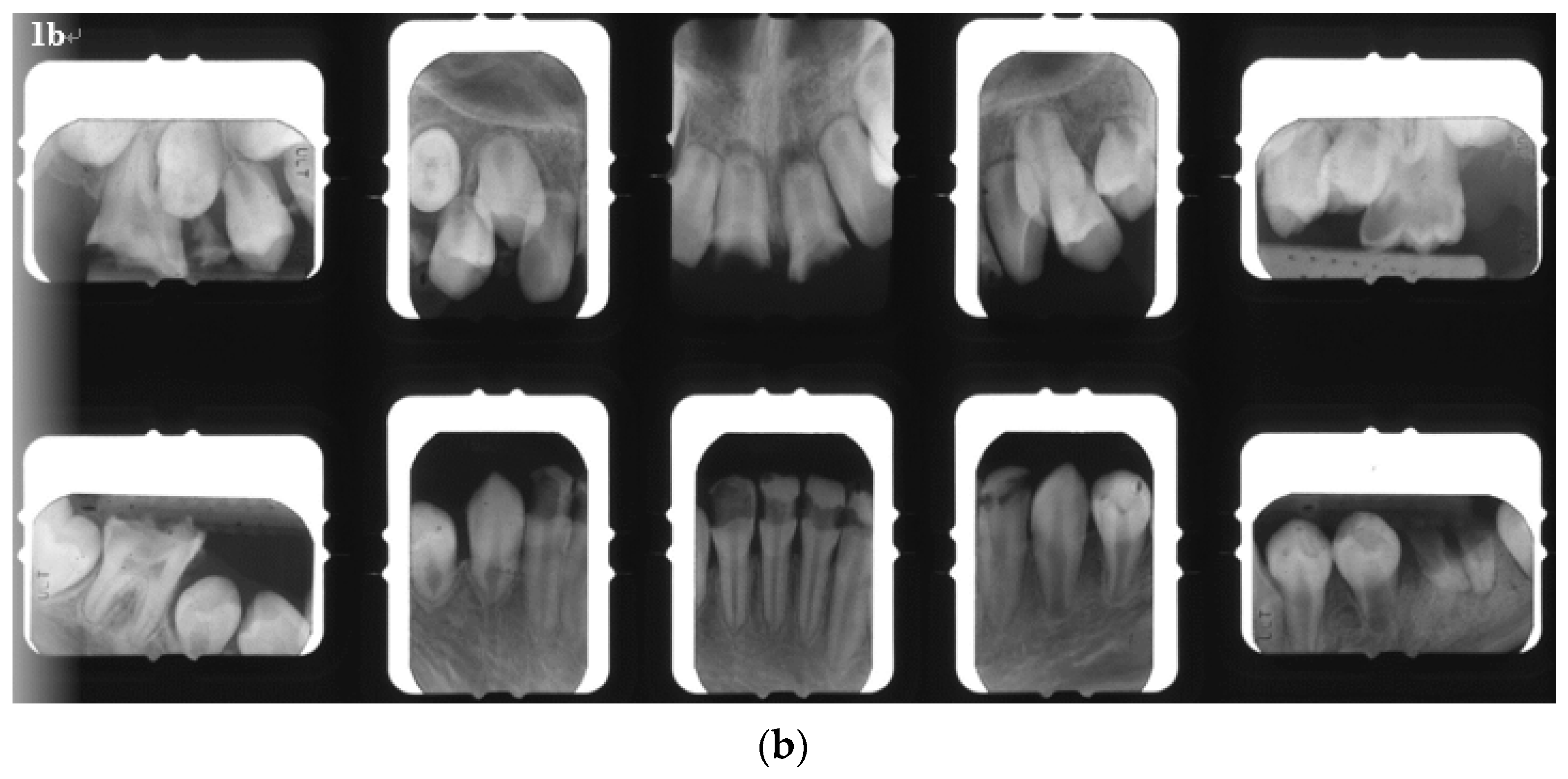
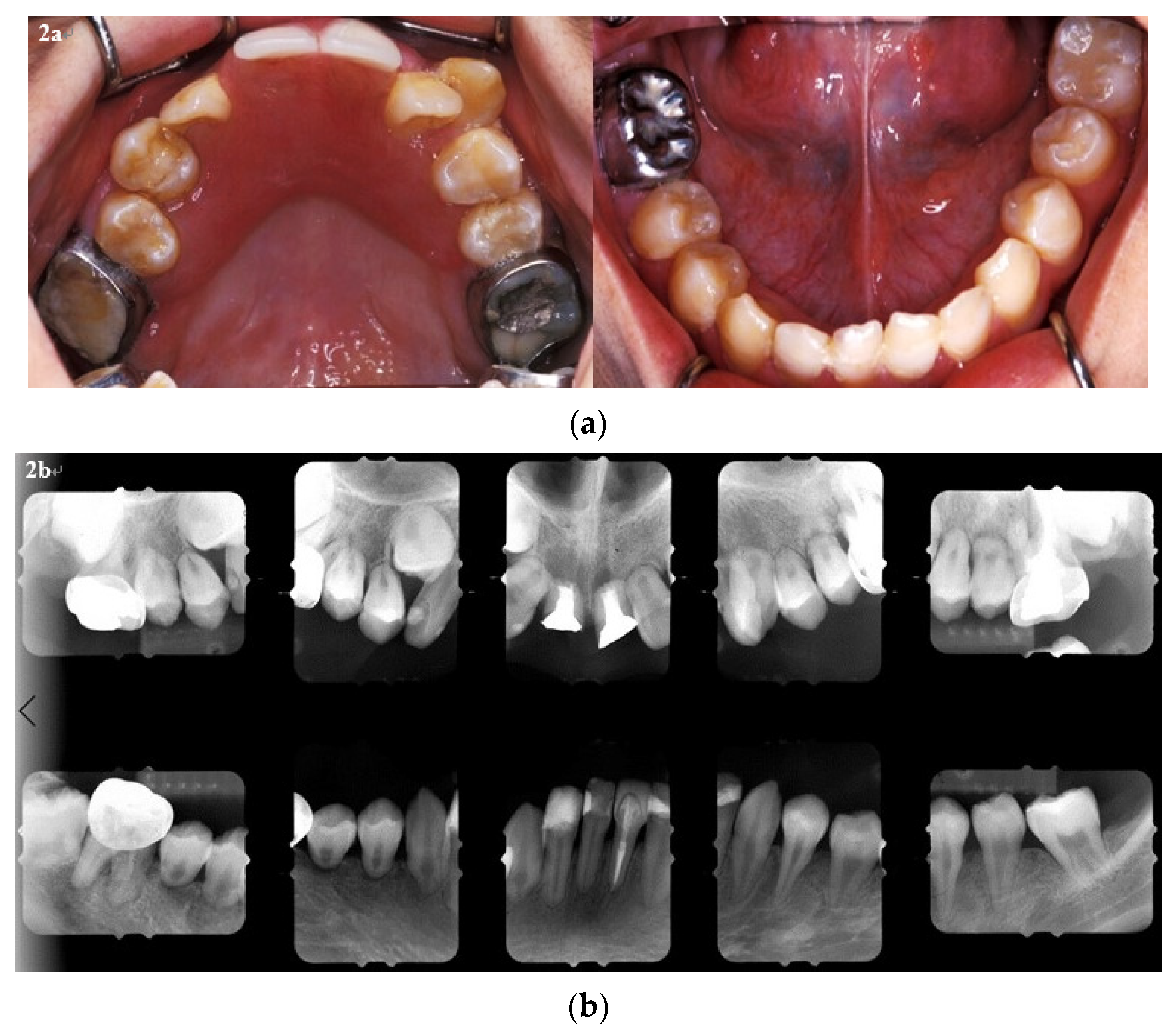
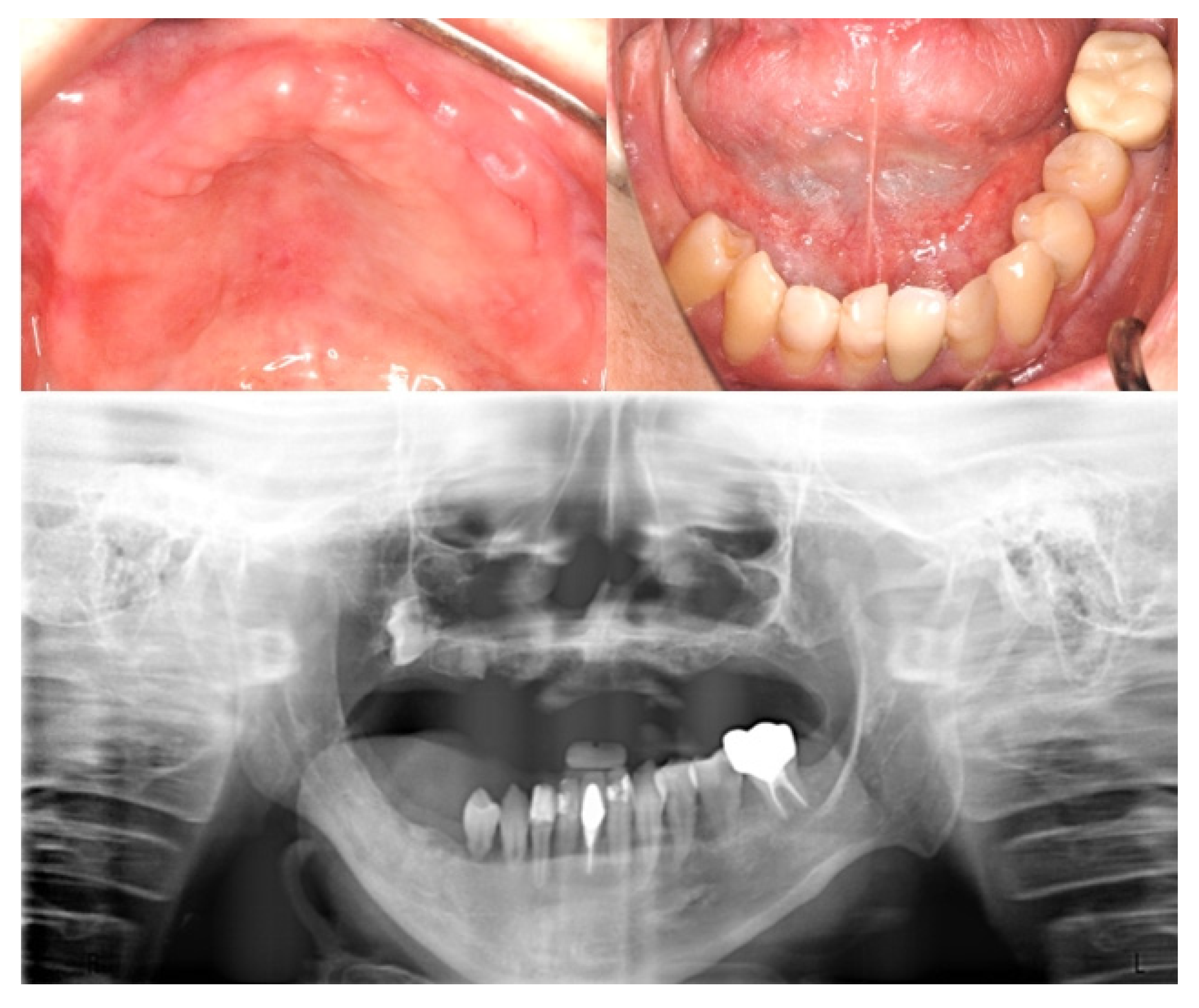
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Göbel, U.; Calaminus, G.; Engert, J.; Kaatsch, P.; Gadner, H.; Bökkerink, J.P.; Hass, R.J.; Waag, K.; Blohm, M.E.; Dippert, S.; et al. Teratomas in infancy and childhood. Med. Pediatric Oncol. 1998, 31, 8–15. [Google Scholar] [CrossRef]
- Moretti, G.; Guimarães, R.; de Oliveira, K.M.; Sanjar, F.; Voegels, R.L. Rhabdomyosarcoma of the head and neck: 24 cases and literature review. Braz. J. Otorhinolaryngol. 2010, 76, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Ritz, B.; Ognjanovic, S.; Lombardi, C.A.; Wilhelm, M.; Heck, J.E. Early life factors and risk of childhood rhabdomyosarcoma. Front. Public Health 2013, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Womer, R.B.; Pressey, J.G. Rhabdomyosarcoma and soft tissue sarcoma in childhood. Curr. Opin. Oncol. 2000, 12, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.; Flaitz, C. Rhabdomyosarcoma of the head and neck in children. Oral Oncol. 2002, 38, 450–459. [Google Scholar] [CrossRef]
- Smith, M.A.; Altekruse, S.F.; Adamson, P.C.; Reaman, G.H.; Seibel, N.L. Declining childhood and adolescent cancer mortality. Cancer 2014, 120, 2497–2506. [Google Scholar] [CrossRef]
- Malempati, S.; Hawkins, D.S. Rhabdomyosarcoma: Review of the Children’s Oncology Group (COG) soft-tissue Sarcoma committee experience and rationale for current COG studies. Pediatric Blood Cancer 2012, 59, 5–10. [Google Scholar] [CrossRef]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.; Burlage, F.R.; Coppes, R.P. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef]
- Oberlin, O.; Rey, A.; Sanchez de Toledo, J.; Martelli, H.; Jenney, M.E.; Scopinaro, M.; Bergeron, C.; Merks, J.H.; Bouvet, N.; Ellershaw, C.; et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: Long-term results from the International Society of Pediatric Oncology MMT95 study. J. Clin. Oncol. 2012, 30, 2457–2465. [Google Scholar]
- van der Pas-van Voskuilen, I.G.; Veerkamp, J.S.; Raber-Durlacher, J.E.; Bresters, D.; van Wijk, A.J.; Barasch, A.; McNeal, S.; Gortzak, R.A. Long-term adverse effects of hematopoietic stem cell transplantation on dental development in children. Supportive Care Cancer 2009, 17, 1169–1175. [Google Scholar] [CrossRef]
- Carl, W. Local radiation and systemic chemotherapy: Preventing and managing the oral complications. J. Am. Dent. Assoc. 1993, 124, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Hölttä, P.; Alaluusua, S.; Saarinen-Pihkala, U.M.; Peltola, J.; Hovi, L. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer 2005, 103, 181–190. [Google Scholar] [CrossRef]
- Dens, F.; Boogaerts, M.; Boute, P.; Declerck, D.; Demuynck, H.; Vinckier, F.; Belgium, B. Caries-related salivary microorganisms and salivary flow rate in bone marrow recipients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996, 81, 38–43. [Google Scholar]
- Hutton, A.; Bradwell, M.; English, M.; Chapple, I. The oral health needs of children after treatment for a solid tumour or lymphoma. Int. J. Paediatr. Dent. 2010, 20, 15–23. [Google Scholar] [CrossRef]
- Satoh, H.; Uesugi, Y.; Kawabata, T.; Mori, K.; Fujii, F.; Kashimoto, Y.; Kajimura, T.; Furuhama, K. Morphological classification of dental lesions induced by various antitumor drugs in mice. Toxicol. Pathol. 2001, 29, 292–299. [Google Scholar] [CrossRef]
- Gawade, P.L.; Hudson, M.M.; Kaste, S.C.; Neglia, J.P.; Constine, L.S.; Robison, L.L.; Ness, K.K. A systematic review of dental late effects in survivors of childhood cancer. Pediatric Blood Cancer 2014, 61, 407–416. [Google Scholar] [CrossRef]
- Näsman, M.; Björk, O.; Söderhäll, S.; Ringdén, O.; Dahllöf, G. Disturbances in the oral cavity in pediatric long-term survivors after different forms of antineoplastic therapy. Pediatric Dent. 1994, 16, 217–223. [Google Scholar]
- Allen, K.L. Wheeler’s Dental Anatomy, Physiology, and Occlusion. N. Y. State Dent. J. 2003, 69, 58. [Google Scholar]
- Collett, W.K.; Thonard, J.C. The Effect of Fractional Radiation on Dentinogenesis in the Rat. J. Dent. Res. 1965, 44, 84–90. [Google Scholar] [CrossRef]
- Arsenault, A.L.; Robinson, B.W. The dentino-enamel junction: A structural and microanalytical study of early mineralization. Calcif. Tissue Int. 1989, 45, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, L.; Libersa, P.; Lambilliotte, A.; Pichon, F.; Turck, D.; Lafforgue, P.; Libersa, J.C. Dental anomalies following anticancer chemotherapy. Arch Pediatr. 2001, 8, 754–756. [Google Scholar] [PubMed]
- Näsman, M.; Forsberg, C.M.; Dahllöf, G. Long-term dental development in children after treatment for malignant disease. Eur. J. Orthod. 1997, 19, 151–159. [Google Scholar] [CrossRef]
- Morrish, R.B., Jr.; Chan, E.; Silverman, S., Jr.; Meyer, J.; Fu, K.K.; Greenspan, D. Osteonecrosis in patients irradiated for head and neck carcinoma. Cancer 1981, 47, 1980–1983. [Google Scholar] [CrossRef]
- Schubert, M.M.; Epstein, J.B.; Peterson, D.E. Oral complications of cancer therapy. In Pharmacology and Therapeutics for Dentistry, 4th ed.; Yagiela, J.A., Neidle, E.A., Dowd, F.J., Eds.; Mosby-Year Book Inc.: St. Louis, MO, USA, 1998; pp. 644–655. [Google Scholar]
- Epstein, J.B.; Schubert, M.M. Oral mucositis in myelosuppressive cancer therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 273–276. [Google Scholar] [PubMed]
- Kennedy, H.F.; Morrison, D.; Tomlinson, D.; Gibson, B.E.; Bagg, J.; Gemmell, C.G. Gingivitis and toothbrushes: Potential roles in viridans streptococcal bacteraemia. J. Infect. 2003, 46, 67–70. [Google Scholar] [CrossRef]
- Da Fonseca, M.A. Dental care of the pediatric cancer patient. Pediatric Dent. 2004, 26, 53–57. [Google Scholar]
- American Academy of Pediatric Dentistry. Guideline on dental management of pediatric patients receiving chemotherapy, hematopoietic cell transplantation, and/or radiation. Pediatric Dent. 2018, 40, 392–400.
- Addems, A.; Epstein, J.B.; Damji, S.; Spinelli, J. The lack of efficacy of a foam brush in maintaining gingival health: A controlled study. Spec. Care Dentist. 1992, 12, 103–106. [Google Scholar] [CrossRef]
- Ransier, A.; Epstein, J.B.; Lunn, R.; Spinelli, J. A combined analysis of a toothbrush, foam brush, and a chlorhexidine-soaked foam brush in maintaining oral hygiene. Cancer Nurs. 1995, 18, 393–396. [Google Scholar] [CrossRef]
- Daeffler, R. Oral hygiene measures for patients with cancer. I. Cancer Nurs. 1980, 3, 347–356. [Google Scholar] [CrossRef]
- Spak, C.J.; Johnson, G.; Ekstrand, J. Caries incidence, salivary flow rate and efficacy of fluoride gel treatment in irradiated patients. Caries Res. 1994, 28, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.J. Current practices in the oral management of the patient undergoing chemotherapy or bone marrow transplantation. Support. Care Cancer 1999, 7, 17–20. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Guideline on Fluoride Therapy. Pediatric Dent. 2016, 38, 181–184. [Google Scholar]
- American Academy of Pediatric Dentistry. Guideline on Caries-risk Assessment and Management for Infants, Children, and Adolescents. Pediatric Dent. 2017, 15, 197–204. [Google Scholar]
- Hong, C.H.; da Fonseca, M. Considerations in the Pediatric Population with Cancer. Dent. Clin. N. Am. 2008, 52, 155–181. [Google Scholar] [CrossRef]
- Llena, C.; Forner, L.; Baca, P. Anticariogenicity of casein phosphopeptide-amorphous calcium phosphate: A review of the literature. J. Contemp. Dent. Pract. 2009, 10, 1–9. [Google Scholar] [CrossRef]
- Konings, A.W.; Coppes, R.P.; Vissink, A. On the mechanism of salivary gland radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1187–1194. [Google Scholar] [CrossRef]
- Gornitsky, M.; Shenouda, G.; Sultanem, K.; Katz, H.; Hier, M.; Black, M.; Velly, A.M. Double-blind randomized, placebo-controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 45–52. [Google Scholar] [CrossRef]
- Rapidis, A.D.; Dijkstra, P.U.; Roodenburg, J.L.; Rodrigo, J.P.; Rinaldo, A.; Strojan, P.; Takes, R.P.; Ferlito, A. Trismus in patients with head and neck cancer: Etiopathogenesis, diagnosis and management. Clin. Otolaryngol. 2015, 40, 516–526. [Google Scholar] [CrossRef]
- Wang, C.J.; Huang, E.Y.; Hsu, H.C.; Chen, H.C.; Fang, F.M.; Hsiung, C.Y. The degree and time-course assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope 2005, 115, 1458–1460. [Google Scholar] [CrossRef]
- Dental Management of Pediatric Patients Receiving Immunosuppressive Therapy and/or Radiation Therapy. Pediatric Dent. 2018, 40, 392–400.
- Karsila-Tenovuo, S.; Jahnukainen, K.; Peltomäki, T.; Minn, H.; Kulmala, J.; Salmi, T.T.; Rönning, O. Disturbances in craniofacial morphology in children treated for solid tumors. Oral Oncol. 2001, 37, 586–592. [Google Scholar] [CrossRef]
- Dahllöf, G.; Forsberg, C.M.; Ringdén, O.; Bolme, P.; Borgström, B.; Näsman, M.; Heimdahl, A.; Modéer, T. Facial growth and morphology in long-term survivors after bone marrow transplantation. Eur. J. Orthod. 1989, 11, 332–340. [Google Scholar] [CrossRef]
- Sonis, A.L.; Tarbell, N.; Valachovic, R.W.; Gelber, R.; Schwenn, M.; Sallan, S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia. A Comp. Three Treat. Modalities Cancer 1990, 66, 2645–2652. [Google Scholar]
- Guyuron, B.; Dagys, A.P.; Munro, I.R.; Ross, R.B. Effect of irradiation on facial growth: A 7- to 25-year follow-up. Ann. Plast. Surg. 1983, 11, 423–427. [Google Scholar] [CrossRef]
- Pruzinsky, T. Social and psychological effects of major craniofacial deformity. Cleft Palate Craniofacial J. 1992, 29, 578–584; discussion 570. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.; Littman, P.; Raney, R.B.; Nelson, L.; Handler, S.; Diamond, G.; Stanley, C. Late effects after treatment of twenty children with soft tissue sarcomas of the head and neck. Cancer 1986, 57, 2070–2076. [Google Scholar] [CrossRef]
- De Smet, A.A.; Kuhns, L.R.; Fayos, J.V.; Holt, J.F. Effects of radiation therapy on growing long bones. Am. J. Roentgenol. 1976, 127, 935–939. [Google Scholar] [CrossRef][Green Version]
- Samuelsson, B.O.; Márky, I.; Rosberg, S.; Albertsson-Wikland, K. Growth and growth hormone secretion after treatment for childhood non-Hodgkin’s lymphoma. Med. Pediatric Oncol. 1997, 28, 27–34. [Google Scholar] [CrossRef]
- Denys, D.; Kaste, S.C.; Kun, L.E.; Chaudhary, M.A.; Bowman, L.C.; Robbins, K.T. The effects of radiation on craniofacial skeletal growth: A quantitative study. Int. J. Pediatric Otorhinolaryngol. 1998, 45, 7–13. [Google Scholar] [CrossRef]
- Mishra, S. Orthodontic Therapy for Paediatric Cancer Survivors: A Review. J. Clin. Diagn. Res. 2017, 11, Ze01–Ze04. [Google Scholar] [CrossRef] [PubMed]
- Sheller, B.; Williams, B. Orthodontic management of patients with hematologic malignancies. Am. J. Orthod. Dentofac. Orthop. 1996, 109, 575–580. [Google Scholar] [CrossRef]
- Costa, M.T.; Lenza, M.A.; Gosch, C.S.; Costa, I.; Ribeiro-Dias, F. In vitro evaluation of corrosion and cytotoxicity of orthodontic brackets. J. Dent. Res. 2007, 86, 441–445. [Google Scholar] [CrossRef]
- Hamula, D.W.; Hamula, W.; Sernetz, F. Pure titanium orthodontic brackets. J. Clin. Orthod. 1996, 30, 140–144. [Google Scholar] [PubMed]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef] [PubMed]
- Levander, E.; Malmgren, O.; Eliasson, S. Evaluation of root resorption in relation to two orthodontic treatment regimes. A clinical experimental study. Eur. J. Orthod. 1994, 16, 223–228. [Google Scholar] [CrossRef]
- Dahllöf, G.; Jönsson, A.; Ulmner, M.; Huggare, J. Orthodontic treatment in long-term survivors after pediatric bone marrow transplantation. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 459–465. [Google Scholar] [CrossRef]
- Rosenberg, S.W. Oral complications of cancer therapies. Chronic dental complications. NCI Monogr. 1990, 9, 173–178. [Google Scholar]
- Kaste, S.C.; Hopkins, K.P. Micrognathia after radiation therapy for childhood facial tumors: Report of two cases with long-term follow-up. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 95–99. [Google Scholar] [CrossRef]
- Lockney, N.A.; Friedman, D.N.; Wexler, L.H.; Sklar, C.A.; Casey, D.L.; Wolden, S.L. Late Toxicities of Intensity-Modulated Radiation Therapy for Head and Neck Rhabdomyosarcoma. Pediatric Blood Cancer 2016, 63, 1608–1614. [Google Scholar] [CrossRef]
- Raney, R.B.; Anderson, J.R.; Kollath, J.; Vassilopoulou-Sellin, R.; Klein, M.J.; Heyn, R.; Glicksman, A.S.; Wharam, M.; Crist, W.M.; Maurer, H.M. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: Report from the Intergroup Rhabdomyosarcoma Study (IRS)-III, 1984–1991. Med. Pediatric Oncol. 2000, 34, 413–420. [Google Scholar] [CrossRef]
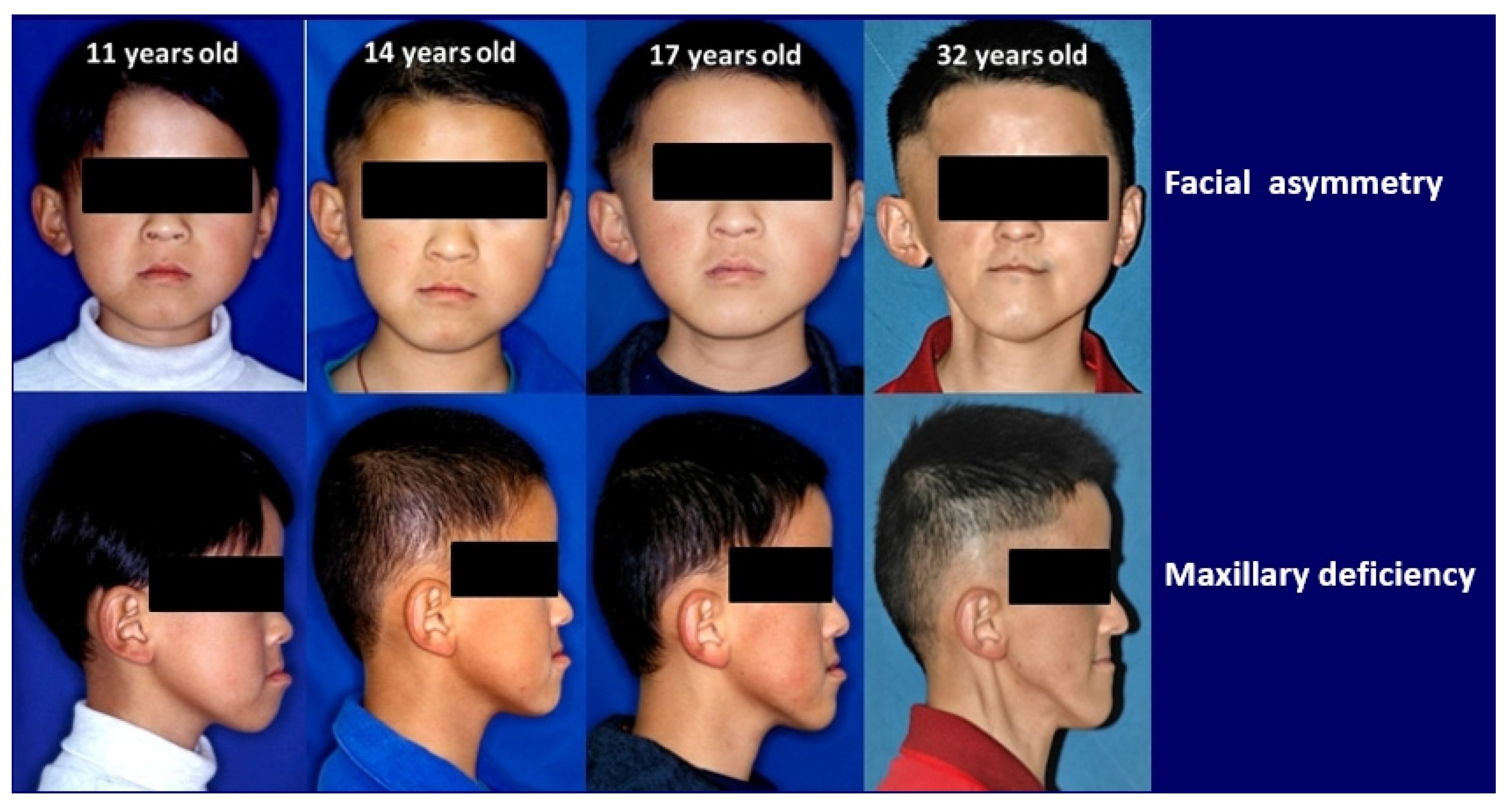
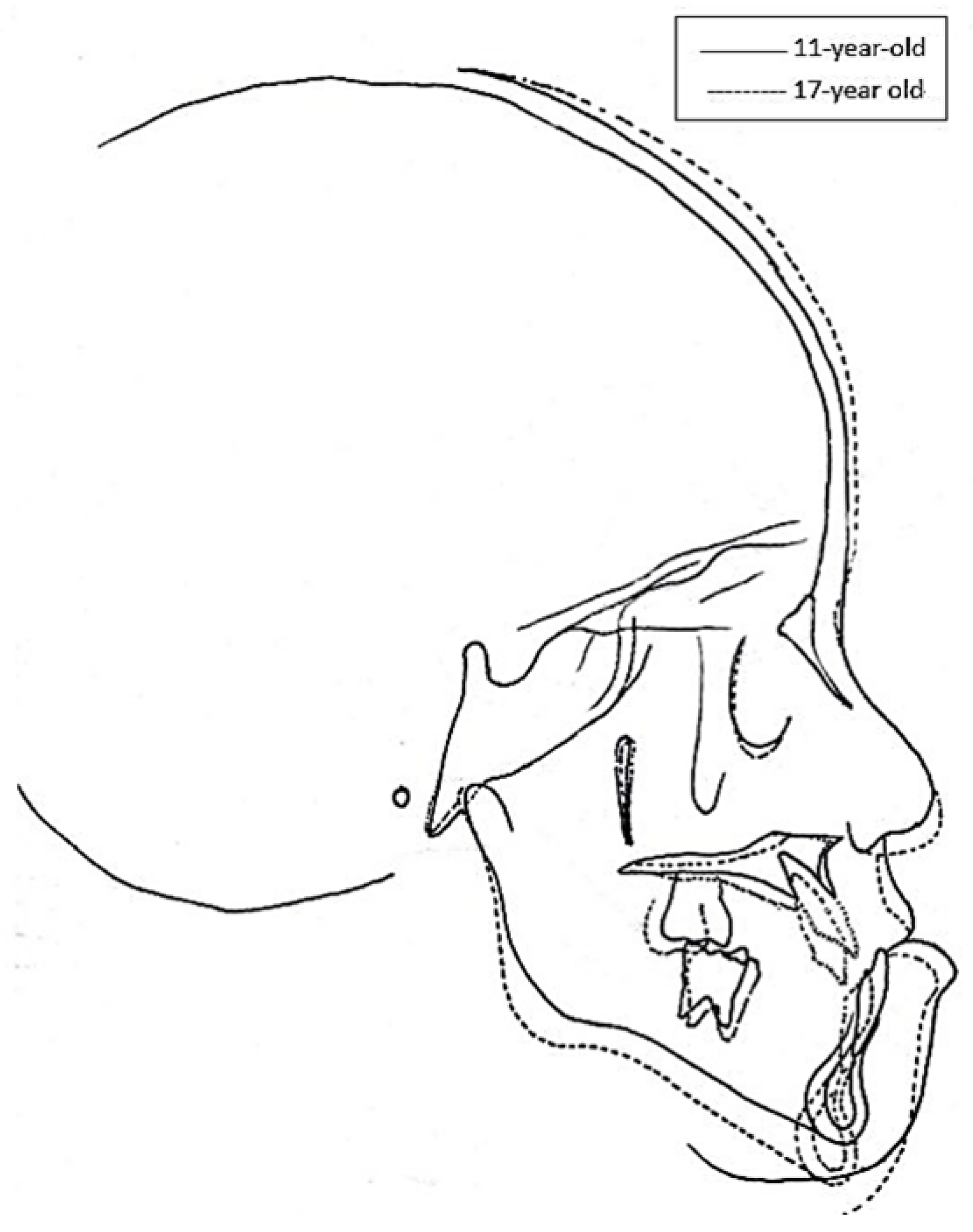
| 11 y/o | 17 y/o | |||
|---|---|---|---|---|
| P’t | Mean ± SD | P’t | Mean ± SD | |
| SN | 58 | 67.67 ± 2.82 | 58 | 73.37 ± 3.64 |
| SN- FH | 5.5 | 7.36 ± 2.67 | 6.5 | 6.04 ± 3.32 |
| SN- MP | 41 | 33.10 ± 4.69 | 39 | 26.38 ± 6.34 |
| SNA | 80 | 82.00 ± 3.24 | 80 | 83.99 ± 4.20 |
| SNB | 82 | 77.60 ± 3.21 | 81 | 81.76 ± 4.03 |
| ANB | −2 | 4.40 ± 1.56 | −1 | 2.24 ± 2.13 |
| A-Nv | −5 | −0.81 ± 2.36 | −3.5 | 0.02 ± 3.59 |
| B-Nv | −4 | −8.91 ± 4.07 | −4 | −4.24 ± 5.12 |
| S-Go (PFH) | 64 | 75.18 ± 3.99 | 72 | 94.53 ± 6.89 |
| N- Me (AFH) | 108 | 117.41 ± 4.84 | 115 | 134.09 ± 5.16 |
| N-A (UFH) | 51 | 59.27 ± 3.12 | 52 | 67.66 ± 2.79 |
| A-Me (LFH) | 57 | 59.17 ± 3.52 | 63 | 66.99 ± 4.13 |
| PFH/AFH % | 59.26% | 64.11 ± 3.78 | 62.61% | 70.61 ± 5.92 |
| UFH/LFH% | 89.47% | 100.50 ± 7.64 | 82.54% | 101.28 ± 6.65 |
| Go-Gn | 67 | 71.62 ± 3.09 | 67 | 85.36 ± 4.65 |
| Ar-A (mm) | 69 | 83.35 ± 3.41 | 69 | 94.29 ± 4.82 |
| Ar- Gn (mm) | 99 | 101.73 ± 3.69 | 104 | 122.18 ± 6.61 |
| Ar-Go (mm) | 39 | 42.97 ± 3.00 | 46 | 58.15 ± 6.15 |
| Ar-Go-Gn | 137 | 123.35 ± 3.87 | 133 | 115.96 ± 8.31 |
| U1-SN | 111 | 105.13 ± 5.92 | 101 | 110.46 ± 6.35 |
| L1-MP | 77 | 98.31 ± 5.67 | 75 | 101.56 ± 8.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-C.; Lin, S.-Y. A Long-Term Follow-Up of Dental and Craniofacial Disturbances after Cancer Therapy in a Pediatric Rhabdomyosarcoma Patient: Case Report. Int. J. Environ. Res. Public Health 2021, 18, 12158. https://doi.org/10.3390/ijerph182212158
Chang P-C, Lin S-Y. A Long-Term Follow-Up of Dental and Craniofacial Disturbances after Cancer Therapy in a Pediatric Rhabdomyosarcoma Patient: Case Report. International Journal of Environmental Research and Public Health. 2021; 18(22):12158. https://doi.org/10.3390/ijerph182212158
Chicago/Turabian StyleChang, Pei-Ching, and Shiao-Yu Lin. 2021. "A Long-Term Follow-Up of Dental and Craniofacial Disturbances after Cancer Therapy in a Pediatric Rhabdomyosarcoma Patient: Case Report" International Journal of Environmental Research and Public Health 18, no. 22: 12158. https://doi.org/10.3390/ijerph182212158
APA StyleChang, P.-C., & Lin, S.-Y. (2021). A Long-Term Follow-Up of Dental and Craniofacial Disturbances after Cancer Therapy in a Pediatric Rhabdomyosarcoma Patient: Case Report. International Journal of Environmental Research and Public Health, 18(22), 12158. https://doi.org/10.3390/ijerph182212158






