Prenatal Lead and Depression Exposures Jointly Influence Birth Outcomes and NR3C1 DNA Methylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measures
2.3. Analytic Plan
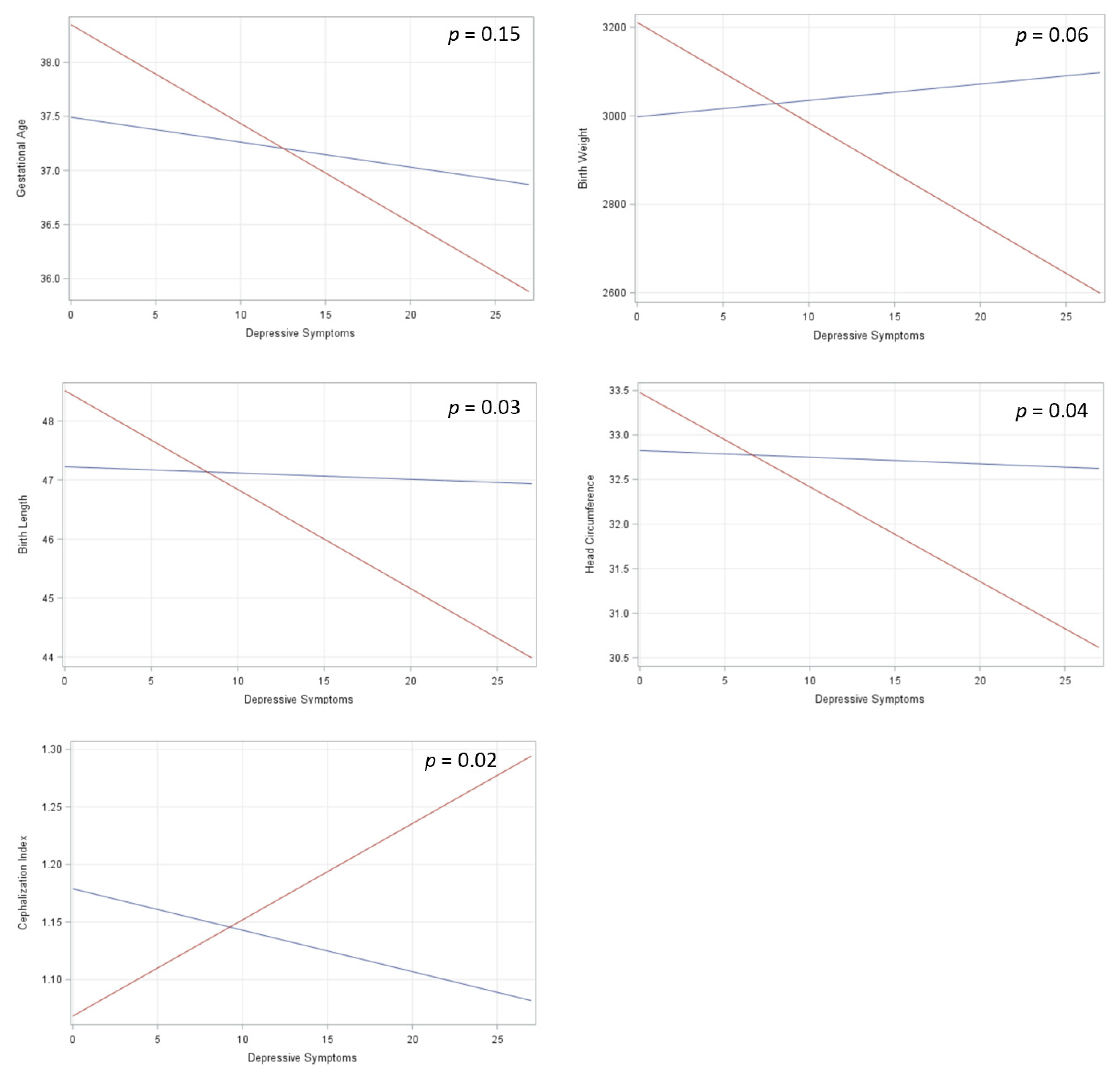
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef]
- Shonkoff, J.P.; Bsoyce, W.T.; McEwen, B.S. Neuroscience, molecular biology, and the childhood roots of health disparities. J. Am. Med. Assoc. 2009, 301, 2252–2259. [Google Scholar] [CrossRef]
- Kundakovic, M.; Jaric, I. The Epigenetic Link between Prenatal Adverse Environments and Neurodevelopmental Disorders. Genes 2017, 8, 104. [Google Scholar] [CrossRef]
- Veenendaal, M.V.E.; Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.M.; van der Post, J.a.M.; Gluckman, P.D.; Hanson, M.A.; Roseboom, T.J. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 548–553. [Google Scholar] [CrossRef]
- Manzari, N.; Matvienko-Sikar, K.; Baldoni, F.; O’Keeffe, G.W.; Khashan, A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr Epidemiol. 2019. [Google Scholar] [CrossRef]
- Marsit, C.J. Placental Epigenetics in Children’s Environmental Health. Semin. Reprod. Med. 2016, 34, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Olvera Alvarez, H.A.; Appleton, A.A.; Fuller, C.H.; Belcourt, A.; Kubzansky, L.D. An Integrated Socio-Environmental Model of Health and Well-Being: A Conceptual Framework Exploring the Joint Contribution of Environmental and Social Exposures to Health and Disease Over the Life Span. Curr. Env. Health Rep. 2018. [Google Scholar] [CrossRef]
- Wright, R.O.; Baccarelli, A. Metals and neurotoxicology. J. Nutr. 2007, 137, 2809–2813. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.M.; Tilling, K.; Golding, J.; Emond, A.M. Low level lead exposure and pregnancy outcomes in an observational birth cohort study: Dose–response relationships. BMC Res. Notes 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. The effects of low-level prenatal lead exposure on birth outcomes. Environ. Pollut. 2013, 175, 30–34. [Google Scholar] [CrossRef]
- Rabito, F.A.; Kocak, M.; Werthmann, D.W.; Tylavsky, F.A.; Palmer, C.D.; Parsons, P.J. Changes in low levels of lead over the course of pregnancy and the association with birth outcomes. Reprod. Toxicol. Elmsford N 2014, 50, 138–144. [Google Scholar] [CrossRef] [PubMed]
- ATSDR Lead (Pb) Toxicity: What Are the U.S. Standards for Lead Levels? | ATSDR–Environmental Medicine & Environmental Health Education—CSEM. Available online: https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=8 (accessed on 4 April 2019).
- Taylor, C.; Golding, J.; Emond, A. Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: A prospective birth cohort study. BJOG 2015, 122, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishioka, E.; Yokoyama, K.; Matsukawa, T.; Vigeh, M.; Hirayama, S.; Ueno, T.; Miida, T.; Makino, S.; Takeda, S. Evidence that birth weight is decreased by maternal lead levels below 5μg/dl in male newborns. Reprod. Toxicol. Elmsford N 2014, 47, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.H.; Samms-Vaughan, M.; Dickerson, A.S.; Hessabi, M.; Bressler, J.; Coore Desai, C.; Shakespeare-Pellington, S.; Reece, J.-A.; Morgan, R.; Loveland, K.A.; et al. Concentration of Lead, Mercury, Cadmium, Aluminum, Arsenic and Manganese in Umbilical Cord Blood of Jamaican Newborns. Int. J. Environ. Res. Public. Health 2015, 12, 4481–4501. [Google Scholar] [CrossRef]
- McDermott, S.; Bao, W.; Aelion, C.M.; Cai, B.; Lawson, A.B. Does the metal content in soil around a pregnant woman’s home increase the risk of low birth weight for her infant? Environ. Geochem. Health 2014, 36, 1191–1197. [Google Scholar] [CrossRef]
- Sun, H.; Chen, W.; Wang, D.; Jin, Y.; Chen, X.; Xu, Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere 2014, 108, 33–39. [Google Scholar] [CrossRef]
- Gharehzadehshirazi, A.; Kadivar, M.; Shariat, M.; Shirazi, M.; Zarkesh, M.R.; Ghanavati Najed, M. Comparative analyses of umbilical cord lead concentration in term and IUGR complicated neonates. J. Matern. -Fetal Neonatal Med. 2019, 34, 867–872. [Google Scholar] [CrossRef]
- Ertel, K.A.; Rich-Edwards, J.W.; Koenen, K.C. Maternal depression in the United States: Nationally representative rates and risks. J. Womens Health 2002 2011, 20, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Accortt, E.E.; Cheadle, A.C.D.; Schetter, C.D. Prenatal Depression and Adverse Birth Outcomes: An Updated Systematic Review. Matern. Child Health J. 2015, 19, 1306–1337. [Google Scholar] [CrossRef]
- Grote, N.K.; Bridge, J.A.; Gavin, A.R.; Melville, J.L.; Iyengar, S.; Katon, W.J. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatry 2010, 67, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Appleton, A.A.; Holdsworth, E.A.; Kubzansky, L.D. A Systematic Review of the Interplay Between Social Determinants and Environmental Exposures for Early-Life Outcomes. Curr. Env. Health Rep. 2016, 3, 287–301. [Google Scholar] [CrossRef]
- Ray, P.D.; Yosim, A.; Fry, R.C. Incorporating epigenetic data into the risk assessment process for the toxic metals arsenic, cadmium, chromium, lead, and mercury: Strategies and challenges. Front. Genet. 2014, 5, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsit, C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015, 218, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccarelli, A.; Bollati, V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009, 21, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nye, M.D.; Fry, R.C.; Hoyo, C.; Murphy, S.K. Investigating Epigenetic Effects of Prenatal Exposure to Toxic Metals in Newborns: Challenges and Benefits. Med. Epigenet. 2014, 2, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.G.; McGowan, P.O. Developmental programming of the HPA axis and related behaviours: Epigenetic mechanisms. J. Endocrinol. 2019, 242, T69–T79. [Google Scholar] [CrossRef]
- Lester, B.M.; Marsit, C.J. Epigenetic mechanisms in the placenta related to infant neurodevelopment. Epigenomics 2018, 10, 321–333. [Google Scholar] [CrossRef]
- Sosnowski, D.W.; Booth, C.; York, T.P.; Amstadter, A.B.; Kliewer, W. Maternal prenatal stress and infant DNA methylation: A systematic review. Dev. Psychobiol. 2018, 60, 127–139. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Conradt, E.; Lester, B.M.; Appleton, A.A.; Armstrong, D.A.; Marsit, C.J. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 2013, 8, 1321–1329. [Google Scholar] [CrossRef] [Green Version]
- Monk, C.; Feng, T.; Lee, S.; Krupska, I.; Champagne, F.A.; Tycko, B. Distress During Pregnancy: Epigenetic Regulation of Placenta Glucocorticoid-Related Genes and Fetal Neurobehavior. Am. J. Psychiatry 2016, 173, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Appleton, A.A.; Lester, B.M.; Armstrong, D.A.; Lesseur, C.; Marsit, C.J. Examining the joint contribution of placental NR3C1 and HSD11B2 methylation for infant neurobehavior. Psychoneuroendocrinology 2015, 52, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Appleton, A.A.; Jackson, B.P.; Karagas, M.; Marsit, C.J. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 2017, 12, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma-Gudiel, H.; Cordova-Palomera, A.; Eixarch, E.; Deuschle, M.; Fananas, L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: A meta-analysis. Epigenetics 2015, 10, 893–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harel, S.; Tomer, A.; Barak, Y.; Binderman, I.; Yavin, E. The cephalization index: A screening device for brain maturity and vulnerability in normal and intrauterine growth retarded newborns. Brain Dev. 1985, 7, 580–584. [Google Scholar] [CrossRef]
- Appleton, A.A.; Kiley, K.; Holdsworth, E.A.; Schell, L.M. Social Support During Pregnancy Modifies the Association Between Maternal Adverse Childhood Experiences and Infant Birth Size. Matern. Child Health J. 2019. [Google Scholar] [CrossRef]
- Ab Razak, N.H.; Praveena, S.M.; Hashim, Z. Toenail as a biomarker of heavy metal exposure via drinking water: A systematic review. Rev. Env. Health 2015, 30, 1–7. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry J. Ment. Sci. 1987, 150, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Leitner, Y.; Fattal-Valevski, A.; Geva, R.; Eshel, R.; Toledano-Alhadef, H.; Rotstein, M.; Bassan, H.; Radianu, B.; Bitchonsky, O.; Jaffa, A.J.; et al. Neurodevelopmental outcome of children with intrauterine growth retardation: A longitudinal, 10-year prospective study. J. Child Neurol. 2007, 22, 580–587. [Google Scholar] [CrossRef]
- Moran, S.; Arribas, C.; Esteller, M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics 2016, 8, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm-Benartzi, C.S.; Houseman, E.A.; Maccani, M.A.; Poage, G.M.; Koestler, D.C.; Langevin, S.M.; Gagne, L.A.; Banister, C.E.; Padbury, J.F.; Marsit, C.J. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Env. Health Perspect. 2012, 120, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Rothman, K.J.; Greenland, S. Modern Epidemiology, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998. [Google Scholar]
- Michaud, D.S.; Skinner, H.G.; Wu, K.; Hu, F.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Fuchs, C.S. Dietary patterns and pancreatic cancer cancer risk in men and women. J. Natl. Cancer Inst. 2005, 97, 518–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Paquette, A.G.; Lester, B.M.; Lesseur, C.; Armstrong, D.A.; Guerin, D.J.; Appleton, A.A.; Marsit, C.J. Placental epigenetic patterning of glucocorticoid response genes is associated with infant neurodevelopment. Epigenomics 2015, 7, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, S.L.; Tamayo, P.; Gaasenbeek, M.; Sturla, L.M.; Angelo, M.; McLaughlin, M.E.; Kim, J.Y.H.; Goumnerova, L.C.; Black, P.M.; Lau, C.; et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 2002, 415, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Anton, L.; Bastek, J.; Brown, A.G. Can microRNA profiling in maternal blood identify women at risk for preterm birth? Am. J. Obstet. Gynecol. 2015, 212, 782.e1–782.e5. [Google Scholar] [CrossRef]
- Horn, J.L. A rationale and test for the number of factors in factor analysis. Psychometrica 1965, 30, 179–185. [Google Scholar] [CrossRef]
- Ledesma, D.R.; Mora, P.V. Determining the number of factors to retain in EFA: An easy-to-use computer program for carrying out parallel analysis. Pract. Assess. Res. Eval. 2007, 12, 1–11. [Google Scholar]
- Zilversmit Pao, L.; Harville, E.W.; Wickliffe, J.K.; Shankar, A.; Buekens, P. The Cumulative Risk of Chemical and Nonchemical Exposures on Birth Outcomes in Healthy Women: The Fetal Growth Study. Int. J. Environ. Res. Public. Health 2019, 16, 3700. [Google Scholar] [CrossRef] [Green Version]


| Characteristic | % or Mean (SD) |
|---|---|
| Depression during pregnancy | |
| Depressive symptoms score | 8.81 (5.50) |
| Depression, yes | 23.08 |
| Depression, no | 76.92 |
| Lead exposure during pregnancy | |
| Lead concentrations, µg/g | 0.80 (1.28) |
| High tertile, µg/g | 1.85 (1.86) |
| Lower/Middle tertiles, µg/g | 0.30 (0.19) |
| Maternal characteristics | |
| Age, years | 28.56 (5.48) |
| Race, Black/Hispanic/Other | 46.54 |
| Race, White/Not Hispanic | 53.46 |
| Education attainment, high school or less | 35.77 |
| Education attainment, more than high school | 64.23 |
| Pre-pregnancy BMI | 28.82 (8.49) |
| Nulliparous, yes | 23.85 |
| Nulliparous, no | 76.15 |
| Mode of delivery, c-section | 33.85 |
| Mode of delivery, vaginal | 66.15 |
| Smoking during pregnancy, yes | 13.85 |
| Smoking during pregnancy, no | 86.15 |
| Maternal Western diet score | 39.81 (15.25) |
| Infant characteristics | |
| Infant sex, male | 50.77 |
| Infant sex, female | 49.23 |
| Gestational age at delivery, weeks | 38.8 (2.14) |
| Birth weight, grams | 3247.83 (639.27) |
| Birth length, centimeters | 48.68 (3.36) |
| Head circumference, centimeters | 33.43 (2.16) |
| Cephalization index | 1.07 (0.23) |
| NR3C1 Factor 1 | NR3C1 Factor 2 | NR3C1 Factor 3 | NR3C1 Factor 4 | NR3C1 Factor 5 | NR3C1 Factor 6 | |
|---|---|---|---|---|---|---|
| Predictor | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) |
| Depression | 0.33 (0.21) [−0.07, 0.74] | 0.11 (0.21) [−0.30, 0.52] | −0.07 (0.20) [−0.46, 0.33] | 0.04 (0.18) * [0.04, 0.76] | 0.06 (0.19) [−0.31, 0.42] | 0.07 (0.19) [−0.30, 0.44] |
| Pb | 0.13 (0.17) [−0.21, 0.47] | 0.14 (0.17) [−0.20, 0.52] | 0.12 (0.17) [−0.22, 0.45] | 0.16 (0.15) [−0.14, 0.46] | 0.18 (0.16) [−0.13, 0.49] | 0.22 (0.16) [−0.10, 0.54] |
| Depression × Pb | −0.58 (0.34) + [−1.25, 0.10] | −0.65 (0.35) + [−1.33, 0.03] | 0.21 (0.34) [−0.45, 0.87] | −0.10 (0.30) [−0.70, 0.50] | 0.17 (0.31) [−0.44, 0.78] | −0.20 (0.31) [−0.82, 0.42] |
| Predictor | Gestational Age | Birth Weight | Birth Length | Head Circ. | Cephalization |
|---|---|---|---|---|---|
| NR3C1 factor 1 | 0.11 (0.12) [−0.14, 0.35] | 18.25 (42.08) [−64.80, 101.23] | 0.01 (0.21) [−0.40, 0.42] | 0.01 (0.13) [−0.26, 0.27] | −0.01 (0.01) [−0.03, 0.02] |
| NR3C1 factor 2 | 0.32 (0.12) ** [0.08, 0.56] | 38.44 (41.60) [−43.49, 120.68] | 0.20 (0.20) [−0.20, 0.60] | 0.08 (0.13) [−0.20, 0.32] | −0.04 (0.01) ** [−0.06, 0.01] |
| NR3C1 factor 3 | −0.09 (0.13) [−0.35, 0.16] | −60.28 (43.12) [−145.34, 24.79] | 0.06 (0.21) [−0.36, 0.48] | −0.14 (0.14) [−0.41, 0.13] | 0.03 (0.01) + [−0.001, 0.05] |
| NR3C1 factor 4 | 0.38 (0.13) ** [0.12, 0.65] | 80.14 (46.51) + [−11.56, 171.91] | 0.26 (0.23) [−0.19, 0.71] | −0.05 (0.15) [−0.34, 0.25] | −0.04 (0.02) * [−0.07, −0.01] |
| NR3C1 factor 5 | −0.34 (0.14) * [−0.60, −0.07] | −73.59 (46.37) [−165.35, 17.66] | −0.09 (0.23) [−0.54, 0.36] | −0.31 (0.15) * [−0.60, −0.02] | 0.03 (0.02) + [−0.004, 0.06] |
| NR3C1 factor 6 | −0.29 (0.13) * [−0.55, −0.03] | −101.13 (45.15) * [−190.04, −12.00] | −0.50 (0.22) * [−0.94, −0.06] | −0.17 (0.15) [−0.46, 0.12] | 0.03 (0.02) + [−0.004, 0.06] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appleton, A.A.; Kiley, K.C.; Schell, L.M.; Holdsworth, E.A.; Akinsanya, A.; Beecher, C. Prenatal Lead and Depression Exposures Jointly Influence Birth Outcomes and NR3C1 DNA Methylation. Int. J. Environ. Res. Public Health 2021, 18, 12169. https://doi.org/10.3390/ijerph182212169
Appleton AA, Kiley KC, Schell LM, Holdsworth EA, Akinsanya A, Beecher C. Prenatal Lead and Depression Exposures Jointly Influence Birth Outcomes and NR3C1 DNA Methylation. International Journal of Environmental Research and Public Health. 2021; 18(22):12169. https://doi.org/10.3390/ijerph182212169
Chicago/Turabian StyleAppleton, Allison A., Kevin C. Kiley, Lawrence M. Schell, Elizabeth A. Holdsworth, Anuoluwapo Akinsanya, and Catherine Beecher. 2021. "Prenatal Lead and Depression Exposures Jointly Influence Birth Outcomes and NR3C1 DNA Methylation" International Journal of Environmental Research and Public Health 18, no. 22: 12169. https://doi.org/10.3390/ijerph182212169
APA StyleAppleton, A. A., Kiley, K. C., Schell, L. M., Holdsworth, E. A., Akinsanya, A., & Beecher, C. (2021). Prenatal Lead and Depression Exposures Jointly Influence Birth Outcomes and NR3C1 DNA Methylation. International Journal of Environmental Research and Public Health, 18(22), 12169. https://doi.org/10.3390/ijerph182212169








