Control and Prevention of SARS-CoV-2 Outbreaks among Healthcare Workers from 129 Healthcare Facilities in Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population Cohort
2.2. Inclusion and Exclusion Criteria
2.3. Molecular and Immunological SARS-CoV-2 Test
2.4. COVID-19 Control Program
- Triage in facilities
- 2.
- Screening in facilities
- 3.
- SARS-CoV-2 test scheduling
- Identification and isolation of positive cases
- 2.
- Epidemiologic study
- 3.
- Disinfection of surfaces and facilities
- Mild case: people who were asymptomatic or presented symptoms that were tolerable, temporary, and did not put their lives at risk, such as headache, fatigue, myalgia, anosmia, ageusia, runny nose, or sore throat.
- Moderate case: people who received ambulatory assisted oxygenation having difficulty breathing, standing difficulty, and excessive cough that did not allow exertion.
- Hospitalized case: people who presented symptoms as a moderate case and needed specialized assistance in public or private health institutions.
- Respiratory support case: people who needed high flow oxygen due to respiratory insufficiency.
- ICU case: people who needed mechanical ventilation and intensive care due to severe respiratory insufficiency.
- Death case: people died due to COVID-19.
2.5. Criteria for Discharge HCW from Follow-Up and Back to Work
2.6. Statistical Analysis
3. Results
3.1. Enrollment of Healthcare Workers in the Study
3.2. COVID-19 Incidence and Contagions Origin
3.3. Baseline Characteristics of the Followed-Up Cohort
3.4. Training Plan and Use of Personal Protective Equipment (PPE)
3.5. Follow-Up and Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Agren, D. Understanding Mexican health worker COVID-19 deaths. Lancet 2020, 396, 807. [Google Scholar] [CrossRef]
- de Salud, S. Informes Sobre el Personal de Salud COVID19 en México 2021. Available online: http://www.gob.mx/salud/documentos/informes-sobre-el-personal-de-salud-covid19-en-mexico-2021 (accessed on 30 August 2021).
- Bandyopadhyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; Kamath, A.; Parepalli, S.A.; Brown, G.; Iharchane, S.; et al. Infection and mortality of healthcare workers worldwide from COVID-19: A systematic review. BMJ Glob. Health 2020, 5, e003097. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Villa, N.E.; Bello-Chavolla, O.Y.; Vargas-Vazquez, A.; Fermin-Martinez, C.A.; Marquez-Salinas, A.; Pisanty-Alatorre, J.; Bahena-Lopez, J.P. Assessing the Burden of Coronavirus Disease 2019 (COVID-19) Among Healthcare Workers in Mexico City: A Data-Driven Call to Action. Clin. Infect. Dis. 2021, 73, e191–e198. [Google Scholar] [CrossRef]
- Atici, S.; Ek, Ö.F.; Yildiz, M.S.; Şikgenç, M.M.; Güzel, E.; Soysal, A. Symptomatic recurrence of SARS-CoV-2 infection in healthcare workers recovered from COVID-19. J. Infect. Dev. Ctries. 2021, 15, 69–72. [Google Scholar] [CrossRef]
- Al Maskari, Z.; Al Blushi, A.; Khamis, F.; Al Tai, A.; Al Salmi, I.; Al Harthi, H.; Al Saadi, M.; Al Mughairy, A.; Gutierrez, R.; Al Blushi, Z. Characteristics of healthcare workers infected with COVID-19: A cross-sectional observational study. Int. J. Infect. Dis. 2021, 102, 32–36. [Google Scholar] [CrossRef]
- Phan, L.T.; Sweeney, D.M.; Maita, D.; Moritz, D.C.; Bleasdale, S.C.; Jones, R.M.; CDC Prevention Epicenters Program. Respiratory viruses in the patient environment. Infect. Control Hosp. Epidemiol. 2020, 41, 259–266. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Pelissier, C.; Gagnaire, J.; Pillet, S.; Pozzetto, B.; Botelho-Nevers, E.; Berthelot, P. SARS-CoV-2 infection: Advocacy for training and social distancing in healthcare settings. J. Hosp. Infect. 2020, 106, 610–612. [Google Scholar] [CrossRef]
- Arenas, M.D.; Villar, J.; González, C.; Cao, H.; Collado, S.; Crespo, M.; Horcajada, J.P.; Pascual, J. Management of the SARS-CoV-2 (COVID-19) coronavirus epidemic in hemodialysis units. Nefrología 2020, 40, 258–264. [Google Scholar] [CrossRef]
- Salazar, M.Á.; Chavez-Galan, L.; Castorena-Maldonado, A.; Mateo-Alonso, M.; Diaz-Vazquez, N.O.; Vega-Martínez, A.M.; Martínez-Orozco, J.A.; Becerril-Vargas, E.; Sosa-Gómez, F.M.; Patiño-Gallegos, H.; et al. Low Incidence and Mortality by SARS-CoV-2 Infection Among Healthcare Workers in a Health National Center in Mexico: Successful Establishment of an Occupational Medicine Program. Front. Public Health 2021, 9, 651144. [Google Scholar] [CrossRef]
- WHO. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays; WHO: Geneva, Switzerland, 2020; p. 9. [Google Scholar]
- Angulo-Zamudio, U.A.; Martinez-Villa, F.M.; Leon-Sicairos, N.; Flores-Villasenor, H.; Velazquez-Roman, J.; Campos-Romero, A.; Alcantar-Fernandez, J.; Urrea, F.; Muro-Amador, S.; Medina-Serrano, J.; et al. Analysis of Epidemiological and Clinical Characteristics of COVID-19 in Northwest Mexico and the Relationship Between the Influenza Vaccine and the Survival of Infected Patients. Front. Public Health 2021, 9, 570098. [Google Scholar] [CrossRef]
- Treskova-Schwarzbach, M.; Haas, L.; Reda, S.; Pilic, A.; Borodova, A.; Karimi, K.; Koch, J.; Nygren, T.; Scholz, S.; Schonfeld, V.; et al. Pre-existing health conditions and severe COVID-19 outcomes: An umbrella review approach and meta-analysis of global evidence. BMC Med. 2021, 19, 212. [Google Scholar] [CrossRef]
- Galang, R.R.; Newton, S.M.; Woodworth, K.R.; Griffin, I.; Oduyebo, T.; Sancken, C.L.; Olsen, E.O.; Aveni, K.; Wingate, H.; Shephard, H.; et al. Risk Factors for Illness Severity Among Pregnant Women with Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection-Surveillance for Emerging Threats to Mothers and Babies Network, 22 State, Local, and Territorial Health Departments, 29 March 2020–5 March 2021. Clin. Infect. Dis. 2021, 73, S17–S23. [Google Scholar] [CrossRef]
- Suleyman, G.; Fadel, R.A.; Malette, K.M.; Hammond, C.; Abdulla, H.; Entz, A.; Demertzis, Z.; Hanna, Z.; Failla, A.; Dagher, C.; et al. Clinical Characteristics and Morbidity Associated with Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw. Open 2020, 3, e2012270. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, M.A.; Esparza, M.A.L.-R.; Campos-Romero, A.; Calva-Espinosa, D.Y.; Moreno-Camacho, J.L.; Langle-Martínez, A.P.; García-Gil, A.; Solís-González, C.J.; Canizalez-Román, A.; León-Sicairos, N.; et al. Epidemiology of COVID-19 in Mexico: Symptomatic profiles and presymptomatic people. Int. J. Infect. Dis. 2021, 104, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Muhlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Moncke-Buchner, E.; Muller, M.A.; et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef]
- Secretaria de Salud (Mexico). Listado de Pruebas de Antígeno, Útiles Para SARS CoV 2 en Puntos de Atención. Available online: https://www.gob.mx/salud/documentos/listado-de-pruebas-de-antigeno-para-sars-cov-2 (accessed on 15 August 2020).
- Forster, P.; Forster, L.; Renfrew, C.; Forster, M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. USA 2020, 117, 9241. [Google Scholar] [CrossRef] [Green Version]
- de Salud, S. Estudio Epidemiológico de Caso Sospechoso de Enfermedad Respiratoria Viral. Mexico. 2020. Available online: https://www.gob.mx/salud/documentos/lineamiento-estandarizado-para-la-vigilancia-epidemiologica-y-por-laboratorio-de-la-enfermedad-respiratoria-viral (accessed on 10 April 2020).
- Alajmi, J.; Jeremijenko, A.M.; Abraham, J.C.; Alishaq, M.; Concepcion, E.G.; Butt, A.A.; Abou-Samra, A.-B. COVID-19 infection among healthcare workers in a national healthcare system: The Qatar experience. Int. J. Infect. Dis. 2020, 100, 386–389. [Google Scholar] [CrossRef]
- Gholami, M.; Fawad, I.; Shadan, S.; Rowaiee, R.; Ghanem, H.; Hassan Khamis, A.; Ho, S.B. COVID-19 and healthcare workers: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Díez-Manglano, J.; Solís-Marquínez, M.N.; Álvarez García, A.; Alcalá-Rivera, N.; Maderuelo Riesco, I.; Gericó Aseguinolaza, M.; Beato Pérez, J.L.; Méndez Bailón, M.; Labirua-Iturburu Ruiz, A.-E.; García Gómez, M.; et al. Healthcare workers hospitalized due to COVID-19 have no higher risk of death than general population. Data from the Spanish SEMI-COVID-19 Registry. PLoS ONE 2021, 16, e0247422. [Google Scholar] [CrossRef]
- Guerrero-Torres, L.; Caro-Vega, Y.; Crabtree-Ramírez, B.; Sierra-Madero, J.G. Clinical Characteristics and Mortality of Healthcare Workers with SARS-CoV-2 infection in Mexico City. Clin. Infect. Dis. 2020, 73, e199–e205. [Google Scholar] [CrossRef] [PubMed]
- CONACyT. COVID-19 México. Available online: https://datos.covid-19.conacyt.mx/ (accessed on 25 August 2021).
- Ioannidis, J.P.A.; Axfors, C.; Contopoulos-Ioannidis, D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Robles-Perez, E.; Gonzalez-Diaz, B.; Miranda-Garcia, M.; Borja-Aburto, V.H. Infection and death by COVID-19 in a cohort of healthcare workers in Mexico. Scand. J. Work Environ. Health 2021, 47, 349–355. [Google Scholar] [CrossRef]
- Cook, T.M. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic—A narrative review. Anaesthesia 2020, 75, 920–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Cheng, S.-Z.; Xu, K.-W.; Yang, Y.; Zhu, Q.-T.; Zhang, H.; Yang, D.-Y.; Cheng, S.-Y.; Xiao, H.; Wang, J.-W.; et al. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: Cross sectional study. BMJ 2020, 369, m2195. [Google Scholar] [CrossRef] [PubMed]
- Calò, F.; Russo, A.; Camaioni, C.; De Pascalis, S.; Coppola, N. Burden, risk assessment, surveillance and management of SARS-CoV-2 infection in health workers: A scoping review. Infect. Dis. Poverty 2020, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, D.; Vos, M.; Stolmeijer, R.; Lameijer, H.; Schönberger, T.; Gaakeer, M.I.; de Groot, B.; Eikendal, T.; Wansink, L.; Ter Avest, E. Association between personal protective equipment and SARS-CoV-2 infection risk in emergency department healthcare workers. Eur. J. Emerg. Med. 2021, 28, 202–209. [Google Scholar] [CrossRef]
- Rajme-López, S.; González-Lara, M.F.; Ortiz-Brizuela, E.; Román-Montes, C.M.; Santiago-Cruz, J.; Mendoza-Rojas, M.Á.; Méndez-Ramos, S.; Tamez-Torres, K.M.; Pérez-García, E.; Martínez-Guerra, B.A.; et al. Large-scale screening for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) among healthcare workers: Prevalence and risk factors for asymptomatic and pauci-symptomatic carriers, with emphasis on the use of personal protective equipment (PPE). Infect. Control Hosp. Epidemiol. 2021, 1–5. [Google Scholar] [CrossRef]
- World Health Organization (WHO). When and How to Use Masks. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks (accessed on 25 May 2020).
- Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019. Available online: https://www.cdc.gov/media/releases/2020/p0714-americans-to-wear-masks.html (accessed on 25 May 2020).
- Tomas, J.; Rego, A.; Viciano-Tudela, S.; Lloret, J. Incorrect Facemask-Wearing Detection Using Convolutional Neural Networks with Transfer Learning. Healthcare 2021, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Teboulbi, S.; Messaoud, S.; Hajjaji, M.A.; Mtibaa, A. Real-Time Implementation of AI-Based Face Mask Detection and Social Distancing Measuring System for COVID-19 Prevention. Sci. Program. 2021, 2021, 8340779. [Google Scholar] [CrossRef]
- Hussain, S.; Yu, Y.; Ayoub, M.; Khan, A.; Rehman, R.; Wahid, J.A.; Hou, W. IoT and Deep Learning Based Approach for Rapid Screening and Face Mask Detection for Infection Spread Control of COVID-19. Appl. Sci. 2021, 11, 3495. [Google Scholar] [CrossRef]
- Mbunge, E.; Simelane, S.; Fashoto, S.G.; Akinnuwesi, B.; Metfula, A.S. Application of deep learning and machine learning models to detect COVID-19 face masks—A review. Sustain. Oper. Comput. 2021, 2, 235–245. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Eikenberry, S.E.; Mancuso, M.; Iboi, E.; Phan, T.; Eikenberry, K.; Kuang, Y.; Kostelich, E.; Gumel, A.B. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect. Dis. Model. 2020, 5, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Thakre, S.S.; Thakre, S.B.; Jadhao, A.; Dass, R.; Dhoble, M.A.; Tiwari, P.N. Evaluation of effectiveness of COVID-19 training of tertiary health care workers. Int. J. Community Med. Public Health 2020, 7, 2635–2639. [Google Scholar] [CrossRef]
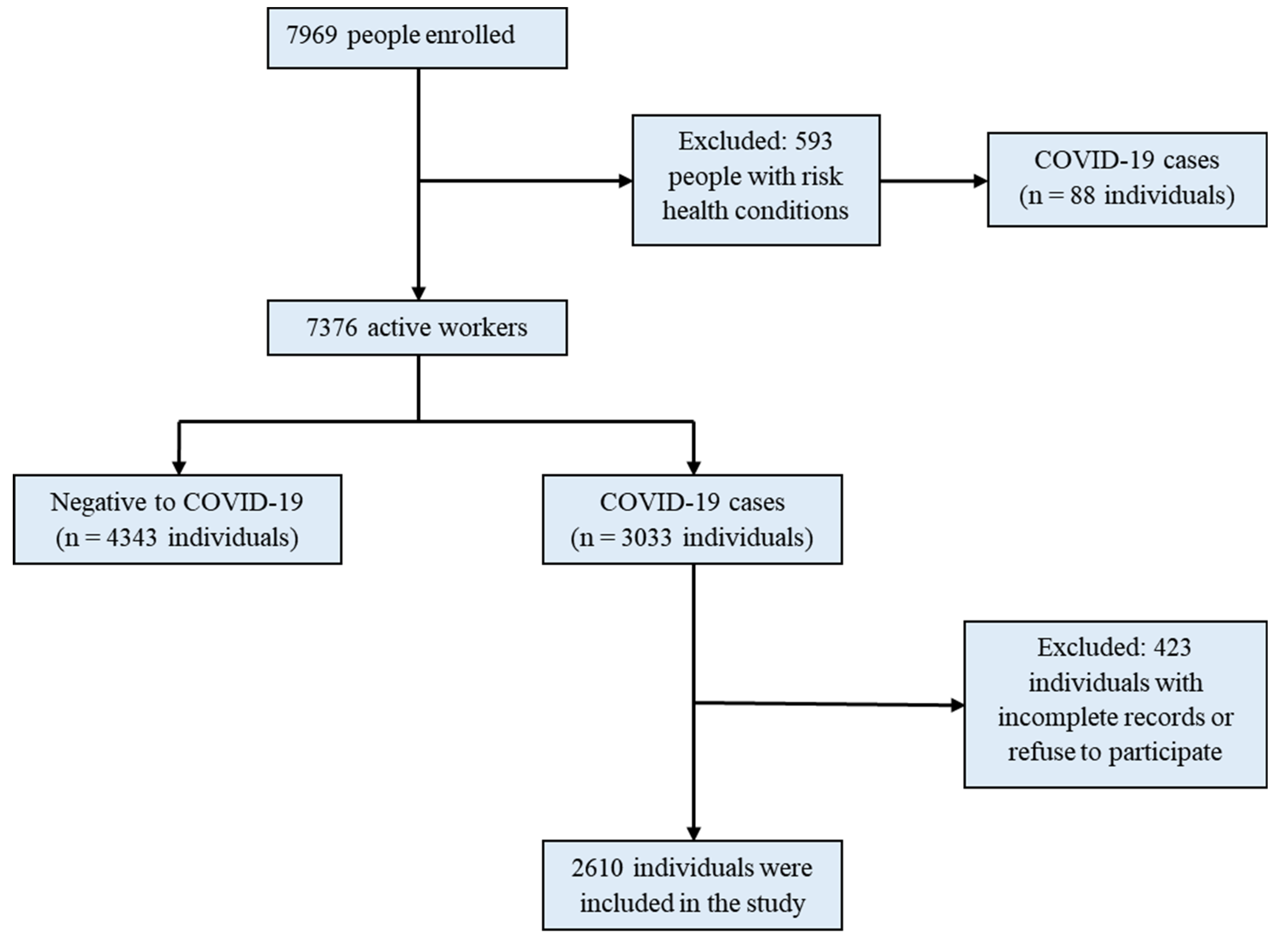
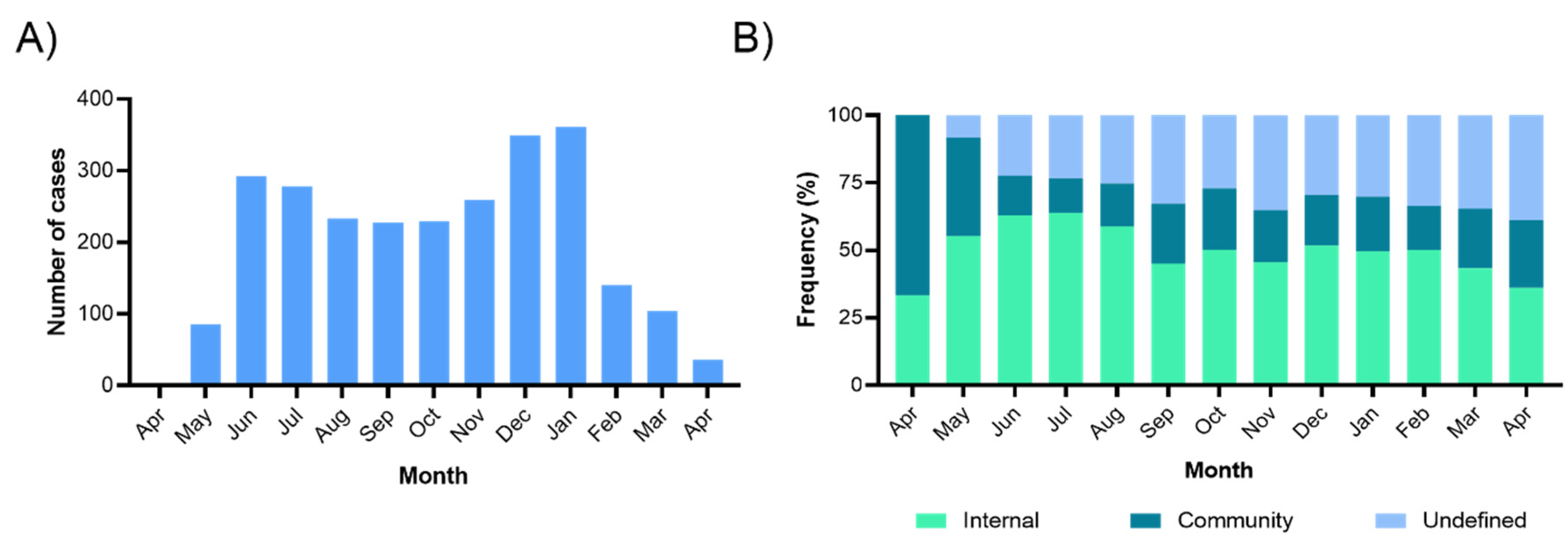
| Characteristic | Number of People | Number of COVID-19 Cases | Infection per 100 HCWs | 95% CI | p-Value |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 5414 | 1946 | 35.9 | 34.7–37.2 | 0.0983 |
| Male | 1962 | 664 | 33.8 | 31.8–36.0 | |
| Overall | 7376 | 2610 | 35.4 | 34.3–36.5 | - |
| Age (years) | |||||
| <30 | 3556 | 1570 | 44.2 | 42.5–45.8 | <0.0001 * |
| 30–39 | 2781 | 828 | 29.8 | 28.1–31.5 | |
| 40–49 | 760 | 172 | 22.6 | 19.8–25.7 | |
| 50–59 | 279 | 40 | 14.3 | 10.7–19.0 | |
| Type of worker | |||||
| Front-line HCWs | 2251 | 1481 | 65.8 | 63.8–67.7 | <0.0001 |
| Non-front-line HCWs | 5125 | 1129 | 22.0 | 20.9–23.2 |
| Characteristic (n = 2610) | Number of People | % (95% CI) |
|---|---|---|
| Age | ||
| Median (IQR) | 28 (25–33) | --- |
| Sex | ||
| Female | 1946 | 74.6 (72.9–76.2) |
| Male | 664 | 25.4 (23.8–27.2) |
| Symptoms | ||
| No | 263 | 10.1 (9.0–11.3) |
| Yes | 2347 | 89.9 (88.7–91.0) |
| Comorbidities | ||
| No | 2196 | 84.1 (82.7–85.5) |
| Yes | 414 | 15.9 (14.5–17.3) |
| Smokinga | ||
| Non-Smokers | 2208 | 84.6 (83.2–85.9) |
| Passive smokers | 100 | 3.8 (3.2–4.6) |
| Smokers | 300 | 11.5 (10.3–12.8) |
| Type of Transporta | ||
| Public | 1708 | 65.4 (63.6–67.2) |
| Private | 779 | 29.9 (28.1–31.6) |
| Not use | 121 | 4.6 (3.9–5.5) |
| Characteristic a (n = 2610) | Number of People | % (95% CI) |
|---|---|---|
| Use of PPE | ||
| No | 15 | 0.6 (0.4–0.9) |
| Yes | 2595 | 99.4 (99.1–99.7) |
| PPE typeb | ||
| Polycarbonate face shield | 2520 | 96.6 (95.8–97.2) |
| Surgical mask | 2582 | 98.9 (98.5–99.3) |
| N95 mask | 2132 | 81.7 (80.2–83.1) |
| Hand gel | 1243 | 47.6 (45.7–49.5) |
| Gloves | 1087 | 41.7 (39.8–43.6) |
| Disposable gown | 611 | 23.4 (21.8–25.1) |
| Shoe cover | 562 | 21.5 (20.0–23-.2) |
| Training | ||
| No | 2 | 0.08 (0.02–0.28) |
| Yes | 2608 | 99.9 (99.7–100) |
| Training typec | ||
| Hand washing | 2450 | 93.9 (92.9–94.7) |
| Work area disinfection | 2465 | 94.4 (93.5–95.3) |
| Use of mask | 2238 | 85.8 (84.4–87.0) |
| Use of antibacterial gel | 1404 | 53.8 (51.9–55.7) |
| Use of face mask | 1213 | 46.5 (44.6–48.4) |
| COVID-19 symptoms recognition | 857 | 32.8 (31.1–34.7) |
| Training mediad | ||
| Institutional website | 282 | 10.8 (9.7–12.1) |
| Platform Universidad Salud Digna | 1440 | 55.2 (53.3–57.1) |
| Video recording | 1350 | 51.7 (49.8–53.6) |
| Video call | 1093 | 41.9 (40.0–43.8) |
| Slide presentation | 660 | 25.3 (23.7–27.0) |
| Institutional bulletin | 405 | 15.5 (14.2–17.0) |
| 364 | 13.9 (12.7–15.3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Santoyo, C.; Campos-Romero, A.; Luna-Ruiz Esparza, M.A.; López-Luna, L.E.; Sánchez-Zarate, M.E.; Zepeda-González, A.; Fernández-Rojas, M.A.; Alcántar-Fernández, J. Control and Prevention of SARS-CoV-2 Outbreaks among Healthcare Workers from 129 Healthcare Facilities in Mexico. Int. J. Environ. Res. Public Health 2021, 18, 11772. https://doi.org/10.3390/ijerph182211772
Pineda-Santoyo C, Campos-Romero A, Luna-Ruiz Esparza MA, López-Luna LE, Sánchez-Zarate ME, Zepeda-González A, Fernández-Rojas MA, Alcántar-Fernández J. Control and Prevention of SARS-CoV-2 Outbreaks among Healthcare Workers from 129 Healthcare Facilities in Mexico. International Journal of Environmental Research and Public Health. 2021; 18(22):11772. https://doi.org/10.3390/ijerph182211772
Chicago/Turabian StylePineda-Santoyo, César, Abraham Campos-Romero, Marco A. Luna-Ruiz Esparza, Liliana E. López-Luna, Martha E. Sánchez-Zarate, Abraham Zepeda-González, Miguel A. Fernández-Rojas, and Jonathan Alcántar-Fernández. 2021. "Control and Prevention of SARS-CoV-2 Outbreaks among Healthcare Workers from 129 Healthcare Facilities in Mexico" International Journal of Environmental Research and Public Health 18, no. 22: 11772. https://doi.org/10.3390/ijerph182211772
APA StylePineda-Santoyo, C., Campos-Romero, A., Luna-Ruiz Esparza, M. A., López-Luna, L. E., Sánchez-Zarate, M. E., Zepeda-González, A., Fernández-Rojas, M. A., & Alcántar-Fernández, J. (2021). Control and Prevention of SARS-CoV-2 Outbreaks among Healthcare Workers from 129 Healthcare Facilities in Mexico. International Journal of Environmental Research and Public Health, 18(22), 11772. https://doi.org/10.3390/ijerph182211772







