High Rate of Depression among Saudi Children with Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patients
2.3. Scoring
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grey, M.; Whittemore, R.; Tamborlane, W. Depression in Type 1 diabetes in children. J. Psychosom. Res. 2002, 53, 907–911. [Google Scholar] [CrossRef]
- Lyoo, I.K.; Yoon, S.J.; Musen, G.; Simonson, D.C.; Weinger, K.; Bolo, N.; Ryan, C.M.; Kim, J.E.; Renshaw, P.F.; Jacobson, A.M. Altered prefrontal glutamate–glutamine–γ-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in Type 1 diabetes mellitus. Arch. Gen. Psychiatry 2009, 66, 878–887. [Google Scholar] [CrossRef]
- Herder, C.; Schmitt, A.; Budden, F.; Reimer, A.; Kulzer, B.; Roden, M.; Haak, T.; Hermanns, N. Association between pro- and anti-inflammatory cytokines and depressive symptoms in patients with diabetes—potential differences by diabetes type and depression scores. Transl. Psychiatry 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.A.; Helgeson, V.S. Children with diabetes compared to peers: Depressed? Distressed? Ann. Behav. Med. 2011, 42, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Gonder-Frederick, L.; Nyer, M.; A Shepard, J.; Vajda, K.; Clarke, W. Assessing fear of hypoglycemia in children with Type 1 diabetes and their parents. Diabetes Manag. 2011, 1, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Zduńczyk, B.; Sendela, J.; Szypowska, A. High prevalence of depressive symptoms in well-controlled adolescents with type 1 diabetes treated with continuous subcutaneous insulin infusion. Diabetes/Metab. Res. Rev. 2014, 30, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Rewers, A. Predictors of acute complications in children with Type 1 diabetes. JAMA 2002, 287, 2511–2518. [Google Scholar] [CrossRef]
- Stewart, S.M.; Rao, U.; Emslie, G.J.; Klein, D.; White, P.C. Depressive symptoms predict hospitalization for adolescents with Type 1 diabetes mellitus. Pediatrics 2005, 115, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Goldston, D.B.; Kovacs, M.; Ho, V.Y.; Parrone, P.L.; Stiffler, L. Suicidal ideation and suicide attempts among youth with insulin-dependent diabetes mellitus. J. Am. Acad. Child Adolesc. Psychiatry 1994, 33, 240–246. [Google Scholar] [CrossRef]
- Hood, K.K.; Huestis, S.; Maher, A.; Butler, D.; Volkening, L.; Laffel, L.M. Depressive symptoms in children and adolescents with Type 1 Diabetes: Association with diabetes-specific characteristics. Diabetes Care 2006, 29, 1389–1391. [Google Scholar] [CrossRef]
- Picozzi, A.; DeLuca, F. Depression and glycemic control in adolescent diabetics: Evaluating possible association between depression and hemoglobin A1c. Public Health 2019, 170, 32–37. [Google Scholar] [CrossRef] [PubMed]
- McGrady, M.E.; Hood, K.K. Depressive symptoms in adolescents with type 1 diabetes: Associations with longitudinal outcomes. Diabetes Res. Clin. Pract. 2010, 88, e35–e37. [Google Scholar] [CrossRef][Green Version]
- Johnson, B.; Eiser, C.; Young, V.; Brierley, S.; Heller, S. Prevalence of depression among young people with Type 1 diabetes: A systematic review. Diabet. Med. 2013, 30, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, G.A.; Beshai, J. Arabic version of the children’s depression inventory: Reliability and validity. J. Clin. Child Psychol. 1989, 18, 323–326. [Google Scholar] [CrossRef]
- Wolfsdorf, J.I.; Glaser, N.; Agus, M.; Fritsch, M.; Hanas, R.; Rewers, A.; Sperling, M.A.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr. Diabetes 2018, 19, 155–177. [Google Scholar] [CrossRef]
- Kovacs, M. Children’s Depression Inventory CDI Manual. New York Multi-Health Systems. 1992, pp. 1–800. Available online: http://www.pearsonclinical.co.uk/Psychology/generic/ChildrensDepressionInventory(CDI)/Resources/Technical.pdf (accessed on 11 September 2021).
- Gale, E.A.M. Type 1 diabetes in the young: The harvest of sorrow goes on. Diabetologia 2005, 48, 1435–1438. [Google Scholar] [CrossRef]
- Green, A.; Hede, S.M.; Patterson, C.C.; Wild, S.H.; Imperatore, G.; Roglic, G.; Beran, D. Type 1 diabetes in 2017: Global estimates of incident and prevalent cases in children and adults. Diabetologia 2021, 64, 2741–2750. [Google Scholar] [CrossRef]
- International Diabetes Federation. 2017. Available online: http://www.diabetesatlas.org (accessed on 11 September 2021).
- AlBuhairan, F.; Nasim, M.; Al Otaibi, A.; Shaheen, N.; Al Jaser, S.; Al Alwan, I. Health related quality of life and family impact of type 1 diabetes among adolescents in Saudi Arabia. Diabetes Res. Clin. Pract. 2016, 114, 173–179. [Google Scholar] [CrossRef]
- Delamater, A.M.; De Wit, M.; McDarby, V.; Malik, J.; Acerini, C. Psychological care of children and adolescents with type 1 diabetes. Pediatr. Diabetes 2014, 15, 232–244. [Google Scholar] [CrossRef]
- La Greca, A.M.; Swales, T.; Klemp, S.; Madigan, S.; Skyler, J. Adolescents with diabetes: Gender differences in psychosocial functioning and glycemic control. Child. Health Care 1995, 24, 61–78. [Google Scholar] [CrossRef]
- AlBuhairan, F.S.; Tamim, H.; Al Dubayee, M.; AlDhukair, S.; Al Shehri, S.; Tamimi, W.; El Bcheraoui, C.; Magzoub, M.E.; de Vries, N.; Al Alwan, I. Time for an adolescent health surveillance system in Saudi Arabia: Findings from “Jeeluna”. J. Adolesc. Health 2015, 57, 263–269. [Google Scholar] [CrossRef]
- Hoey, H.; Aanstoot, H.-J.; Chiarelli, F.; Daneman, D.; Danne, T.; Dorchy, H.; Fitzgerald, M.; Garandeau, P.; Greene, S.; Holl, R.; et al. Good metabolic control is associated with better quality of life in 2101 adolescents with Type 1 diabetes. Diabetes Care 2001, 24, 1923–1928. [Google Scholar] [CrossRef]
- Sendela, J.; Zduńczyk, B.; Trippenbach-Dulska, H.; Szypowska, A. Prevalence of depressive symptoms in school aged children with type 1 diabetes— A questionnaire study. Psychiatr. Polska 2015, 49, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.; Loar, R.; Anderson, B.J.; Heptulla, R.A. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J. Pediatr. 2006, 149, 526–531. [Google Scholar] [CrossRef]
- Khater, D.; Omar, M. Frequency and risk factors of depression in type 1 diabetes in a developing country. J. Pediatr. Endocrinol. Metab. 2017, 30, 917–922. [Google Scholar] [CrossRef]
- McGrady, M.E.; Laffel, L.; Drotar, D.; Repaske, D.; Hood, K.K. Depressive symptoms and glycemic control in adolescents with Type 1 diabetes: Mediational role of blood glucose monitoring. Diabetes Care 2009, 32, 804–806. [Google Scholar] [CrossRef][Green Version]
- Silverstein, J.; Klingensmith, G.; Copeland, K.; Plotnick, L.; Kaufman, F.; Laffel, L.; Deeb, L.; Grey, M.; Anderson, B.; Holzmeister, L.A.; et al. Care of children and adolescents with Type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care 2005, 28, 186–212. [Google Scholar] [CrossRef]
- American Diabetes Association. 13. Children and adolescents: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S180–S199. [Google Scholar] [CrossRef]
- Jaser, S.S. Family interaction in pediatric diabetes. Curr. Diabetes Rep. 2011, 11, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Fazeli, M. Depression in children. Aust. Fam. Physicians 2017, 46, 901–907. [Google Scholar] [PubMed]
| Study Variables | N (%) |
|---|---|
| Age group | |
| 8–12 years | 86 (58.1%) |
| 13–16 years | 62 (41.9%) |
| Gender | |
| Male | 69 (46.6%) |
| Female | 79 (53.4%) |
| Nationality | |
| Saudi | 140 (94.6%) |
| Non-Saudi | 08 (05.4%) |
| HbA1c | |
| ≤7.5% | 32 (21.6%) |
| >7.5% | 116 (78.4%) |
| BMI level | |
| Underweight (<5th) | 12 (08.1%) |
| Normal (5th–<85th) | 94 (63.5%) |
| Overweight (85th–<95th) | 26 (17.6%) |
| Obese (>95th) | 16 (10.8%) |
| DM Duration | |
| 6 months–1 year | 39 (26.4%) |
| 1–2 years | 24 (16.2%) |
| 2–3 years | 19 (12.8%) |
| 3–4 years | 15 (10.1%) |
| >4 years | 51 (34.5%) |
| Method of insulin administration | |
| Multiple daily injections without carbohydrate calculation | 102 (68.9%) |
| Multiple daily injections with carbohydrate calculation | 19 (12.8%) |
| Insulin pump | 02 (01.4%) |
| Premixed insulin | 25 (16.9%) |
| Episodes of DKA in the past year? | |
| Never | 79 (53.4%) |
| One time | 40 (27.0%) |
| Twice times | 14 (09.5%) |
| ≥Three times | 15 (10.1%) |
| Depression | N (%) |
|---|---|
| Level of depression | |
| Depressed | 40 (27.0%) |
| Not depressed | 108 (73.0%) |
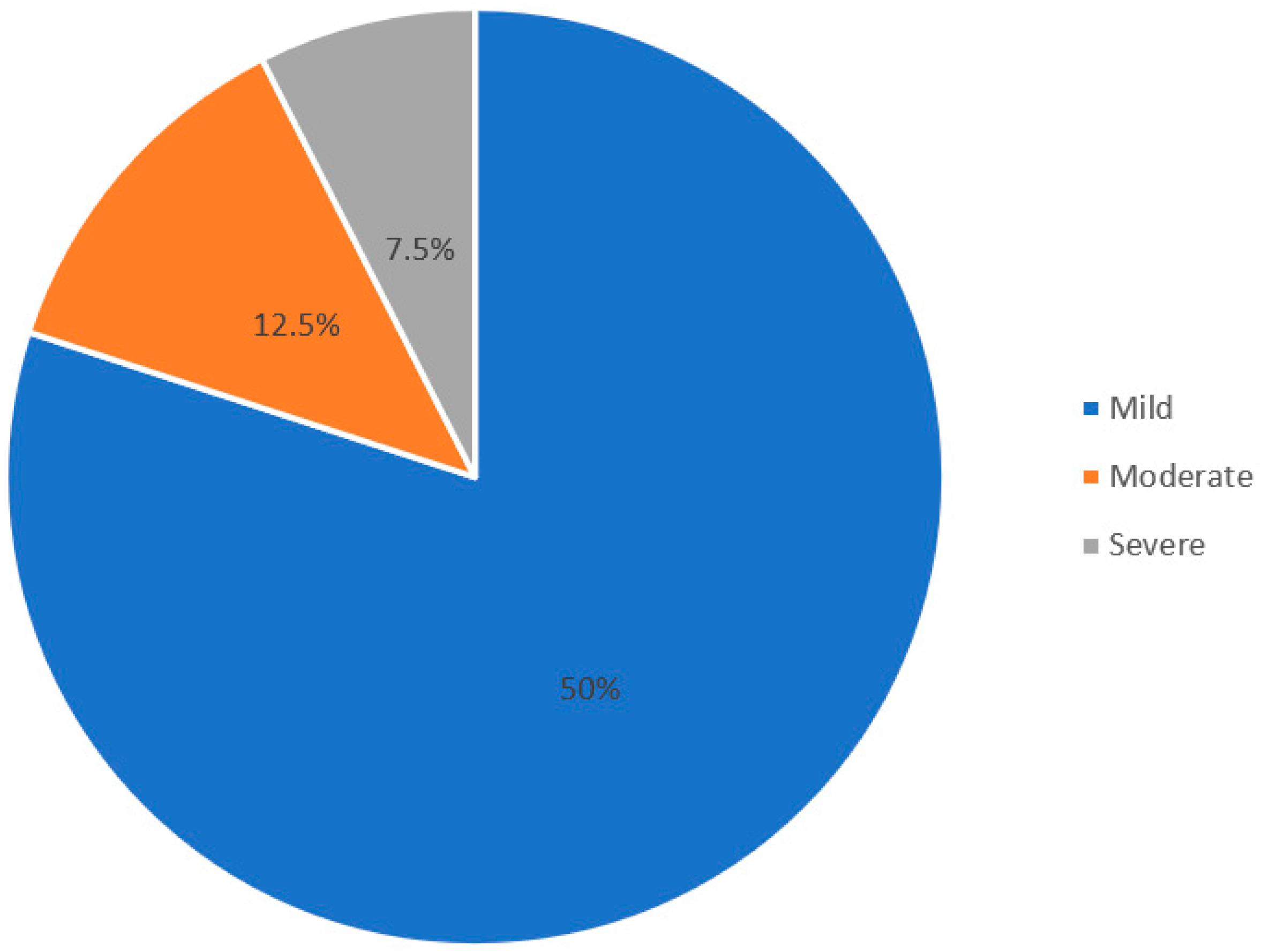
| Severity of depression (n = 40) | |
| Mild | 32 (80.0%) |
| Moderate | 05 (12.5%) |
| Severe | 03 (07.5%) |
| Factor | Depressed N (%) (n = 40) | Not Depressed N (%) (n = 108) | p-Value § |
|---|---|---|---|
| Age group | |||
| 8–12 years | 20 (50.0%) | 66 (61.1%) | 0.224 |
| 13–16 years | 20 (50.0%) | 42 (38.9%) | |
| Gender | |||
| Male | 12 (30.0%) | 57 (52.8%) | 0.014 ** |
| Female | 28 (70.0%) | 51 (47.2%) | |
| Nationality | |||
| Saudi | 38 (95.0%) | 102 (94.4%) | 0.894 |
| Non-Saudi | 02 (05.0%) | 06 (05.6%) | |
| HbA1c | |||
| ≤7.5% | 04 (10.0%) | 28 (25.9%) | 0.037 ** |
| >7.5% | 36 (90.0%) | 80 (74.1%) | |
| BMI level | |||
| Underweight | 04 (10.0%) | 08 (07.4%) | 0.593 |
| Normal | 27 (67.5%) | 67 (62.0%) | |
| Overweight/Obese | 09 (22.5%) | 33 (30.6%) | |
| DM Duration | |||
| 6 months–1 year | 04 (10.0%) | 35 (32.4%) | 0.013 ** |
| 1–2 years | 06 (15.0%) | 18 (16.7%) | |
| 2–3 years | 05 (12.5%) | 14 (13.0%) | |
| 3–4 years | 03 (07.5%) | 12 (11.1%) | |
| >4 years | 22 (55.0%) | 29 (26.9%) | |
| Method of insulin administration | |||
| Multiple daily injections without carbohydrate calculation | 32 (80.0%) | 70 (64.8%) | 0.234 |
| Multiple daily injections with carbohydrate calculation | 03 (07.5%) | 16 (14.8%) | |
| Insulin pump | 01 (02.5%) | 01 (0.90%) | |
| Premixed insulin | 04 (10.0%) | 21 (19.4%) | |
| History of DKA event | |||
| Yes | 19 (47.5%) | 50 (46.3%) | 0.896 |
| No | 21 (52.5%) | 58 (53.7%) |
| Factor | AOR | 95% CI | p-Value |
|---|---|---|---|
| Gender | |||
| Male | Ref | ||
| Female | 4.552 | 1.805–11.476 | 0.001 ** |
| HbA1c | |||
| ≤7.5% | Ref | ||
| >7.5% | 7.122 | 1.927–26.320 | 0.003 ** |
| DM Duration | |||
| 6 months–1 year | Ref | ||
| 1–2 years | 7.620 | 2.145–27.066 | 0.002 ** |
| 2–3 years | 1.895 | 0.557–6.448 | 0.306 |
| 3–4 years | 1.469 | 0.412–5.238 | 0.553 |
| >4 years | 4.820 | 1.073–21.653 | 0.040 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaqeel, A.; Almijmaj, M.; Almushaigeh, A.; Aldakheel, Y.; Almesned, R.; Al Ahmadi, H. High Rate of Depression among Saudi Children with Type 1 Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11714. https://doi.org/10.3390/ijerph182111714
Alaqeel A, Almijmaj M, Almushaigeh A, Aldakheel Y, Almesned R, Al Ahmadi H. High Rate of Depression among Saudi Children with Type 1 Diabetes. International Journal of Environmental Research and Public Health. 2021; 18(21):11714. https://doi.org/10.3390/ijerph182111714
Chicago/Turabian StyleAlaqeel, Aqeel, Muna Almijmaj, Abdulaziz Almushaigeh, Yasser Aldakheel, Raghad Almesned, and Husam Al Ahmadi. 2021. "High Rate of Depression among Saudi Children with Type 1 Diabetes" International Journal of Environmental Research and Public Health 18, no. 21: 11714. https://doi.org/10.3390/ijerph182111714
APA StyleAlaqeel, A., Almijmaj, M., Almushaigeh, A., Aldakheel, Y., Almesned, R., & Al Ahmadi, H. (2021). High Rate of Depression among Saudi Children with Type 1 Diabetes. International Journal of Environmental Research and Public Health, 18(21), 11714. https://doi.org/10.3390/ijerph182111714






