Individual and Contextual Factors Associated with Malaria among Children 6–59 Months in Nigeria: A Multilevel Mixed Effect Logistic Model Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Source of Data
2.3. Sampling Techniques
2.4. Outcome Variable
- (i)
- Rapid diagnostic tests (RDTs) were conducted on blood samples taken from pricking the finger or heal of the child using SD Bioline Ag Pf (HRP-II). The RDTs detect the Histidine-rich protein-II (HRT-II) human whole blood (antigen). The results were either classified as positive or negatives for Pasmodium falciparum (Pf).
- (ii)
- Laboratory microscopy investigation on thick blood smears was performed for a three quarter of the households where RDTs was done. Malaria results were also classified as either positive or negative.
2.5. Independent Variables
2.5.1. Child-Related Variables
2.5.2. Parental-Related Variables
2.5.3. Household-Related Variables
2.5.4. Cluster-Related Variables
2.5.5. Area-Related Va31riables
2.6. Statistical Analysis
2.6.1. Multilevel Model Description for the Three-Level Survey on Malaria Status
2.6.2. Model Building
2.6.3. Measure of Association
2.6.4. Measures of Variations
2.6.5. Intraclass Correlation Coefficient (ICC)
3. Results
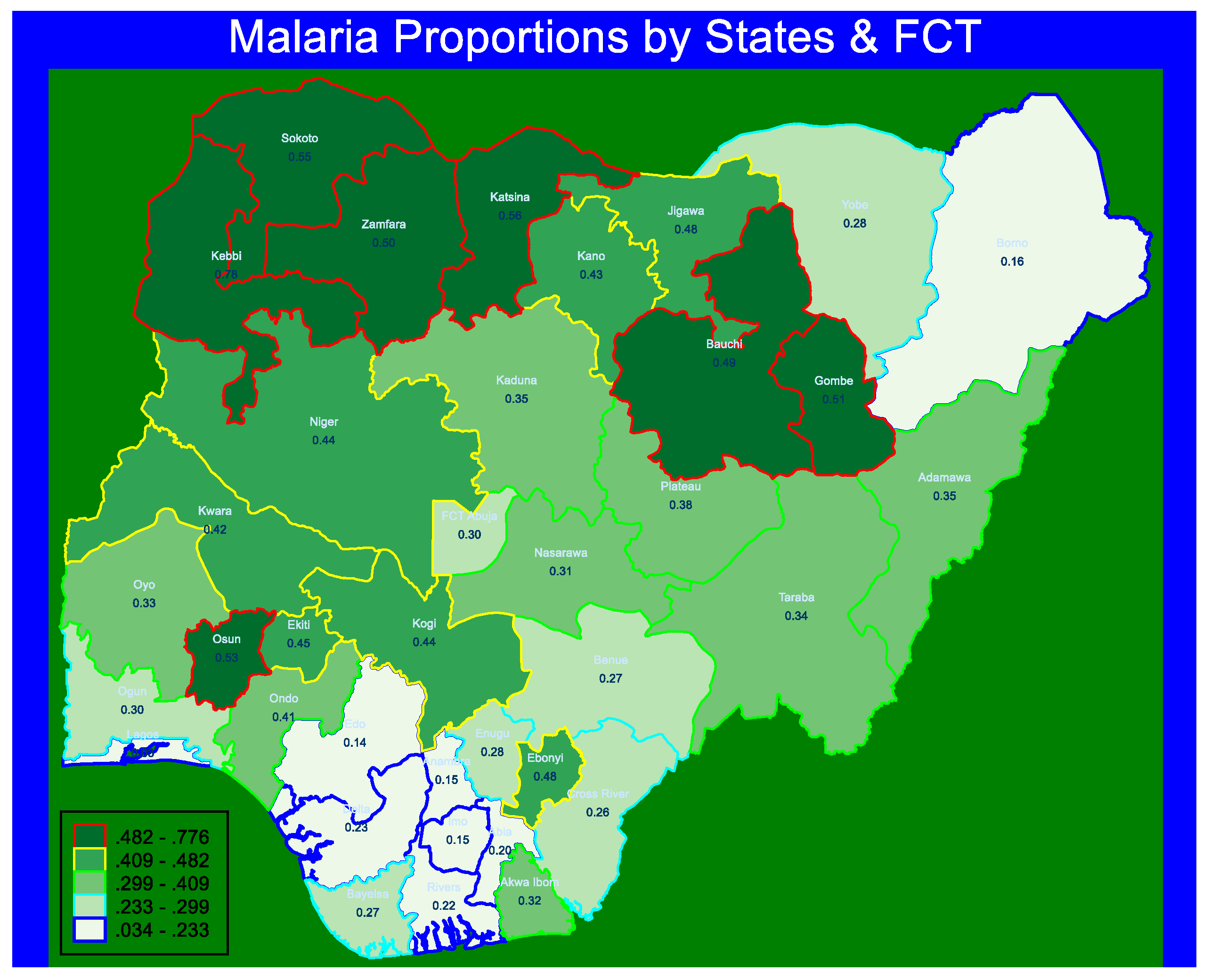
3.1. Prevalence of Malaria Fever
3.2. Bivariate Analysis of Proportion and Associations between Predictors and Malaria Status
3.3. Multilevel Multivariable Models of Predictors of Malaria Fever Status
3.3.1. Multilevel Model Results
A Measure of Variation (Random Effects)
Measures of Association (Fixed Effects)
4. Discussion
4.1. Strengths and Limitations of the Study
4.2. Policy Implications
- (i)
- The findings show that older children are often at risk compared to younger siblings. These children are allowed by their parents to walk about freely unattended to, perhaps to obtain more feeding opportunities elsewhere. These children have been weaned from breast feeding, nutritious foods are often not provided at home because some households cannot afford it. Most of these children scavenge about to find food. It is recommended that the school feeding program of the Government should also be extended to what may be called “community feeding program” where children of pre-school age can access food and boost their immunity.
- (ii)
- Parenting is a very serious matter in our society. The age at which most women first become a mother is often very low, thereby making them “baby-mothers”. Some of these mothers are not even mature enough to take care of themselves, particularly becoming mothers at that tender age, and not even able to care of their babies. Policies and the political will should be in place to discourage early girl-child marriage that often result into early childbirth. Girl-child education often delay the age at which most females become married and give birth. Therefore, female education should be made free and compulsory for up to secondary education. Any parent who withdraws their girl child from school for early marriage or whose daughter gets pregnant while still in school should be liable for prosecution.
- (iii)
- Most families sleep in over-crowded apartments. The governmental housing policies in some states see some buildings constructed but are never allotted to anyone until they are eventually vandalized. The Government’s policies should encourage early allocation of these buildings to those that need them to ease off over-crowding households and communities. This will reduce cross-infections in communicable diseases such as malaria
- (iv)
- Due to the problem of accessibility to most rural areas (especially during the raining season when malaria infection rates are usually very high), these communities are often neglected in the distribution of scare palliatives from the Government and agencies (such as medicals and bed nets), to addressing the issue of malaria. The Government can also invest in the use technologies available for logistics such as drone to transport these items to the hard-to-reach communities.
- (v)
- When community distance to health facility is not a “big problem” to the people, they become prompt in getting the much-needed timely medical attention for their children. Therefore, having identified areas of high malaria prevalence, the governments for these areas should among other suggestions ensure that increased proportion of the people do not travel long distances before they can access prompt medical attention. Therefore, more functional health centres are available in such localities.
- (vi)
- Lastly, the results also indicates that children from a high category among low cluster wealth areas had significantly higher proportion of malaria positive status compared to the proportion among the low category ((d) in Table 1). Therefore, the Government can investigate and implement ways to bring many homes out of poverty lines. Many of the governmental programs in the past toward poverty alleviation have ended up without achieving their aims. These measures do not find their way into the hands of those that need them. Sometimes they are distributed as political campaign “juices” for political party supporters. There should be sincerity in the part of both the program implementers and the beneficiaries in driving the program to success.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. A Scoping Review of Selected Studies on Predictor Variables Associated with the Malaria Status among Children under Five Years in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2021, 18, 2119. [Google Scholar] [CrossRef] [PubMed]
- Oguoma, V.M.; Anyasodor, A.E.; Adeleye, A.O.; Eneanya, O.A.; Mbanefo, E.C. Multilevel Modelling of the Risk of Malaria among Children Aged under Five Years in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Aychiluhm, S.B.; Gelaye, K.A.; Angaw, D.A.; Dagne, G.A.; Tadesse, A.W.; Abera, A.; Dillu, D. Determinants of Malaria among Under-Five Children in Ethiopia: Bayesian Multilevel Analysis. BMC Public Health 2020, 20, 1468. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Bisanzio, D.; Yukich, J.O.; Mappin, B.; Fergus, C.A.; Lynch, M.; Cibulskis, R.E.; Bhatt, S.; Weiss, D.J.; Cameron, E.; et al. Population Coverage of Artemisinin-Based Combination Treatment in Children Younger than 5 Years with Fever and Plasmodium Falciparum Infection in Africa, 2003–2015: A Modelling Study Using Data from National Surveys. Lancet Glob. Health 2017, 5, e418–e427. [Google Scholar] [CrossRef]
- Ugwu, C.L.J.; Zewotir, T. Evaluating the Effects of Climate and Environmental Factors on Under-5 Children Malaria Spatial Distribution Using Generalized Additive Models (GAMs). J. Epidemiol. Glob. Health 2020, 10, 304. [Google Scholar] [CrossRef]
- World Health Organisation. World Malaria Report. 2014. Available online: https://www.who.int/malaria/publications/world_malaria_report_2014/en/ (accessed on 21 February 2019).
- Gup, I. Malaria Morbidity among Under-Five Nigerian Children: A Study of Its Prevalence and Health Practices of Primary Care Givers (Mothers) in a Resource-Poor Setting of a Rural Hospital in Eastern Nigeria. Eur. J. Prev. Med. 2013, 1, 50. [Google Scholar] [CrossRef][Green Version]
- Ready to Beat Malaria (RBM). Commonwealth Leaders Respond to a Global Call to Action and Commit to Halve Malaria across the Commonwealth by 2023. Available online: https://endmalaria.org/news/commonwealth-leaders-respond-global-call-action-and-commit-halve-malaria-across-commonwealth (accessed on 8 June 2021).
- National Malaria Elimination Program (NMEP); National Population Commission (NPopC); National Bureau; ICF International. Nigeria Malaria Indicator Survey 2015; NMEP, NPopC, and ICF International: Abuja, Nigeria; Rockville, MD, USA, 2016. [Google Scholar]
- National Population Commission (NPC) [Nigeria]; National Malaria Control Programme (NMCP) [Nigeria]; ICF International. Nigeria Malaria Indicator Survey 2010; NMEP, NPopC, and ICF International: Abuja, Nigeria; Rockville, MD, USA, 2012. [Google Scholar]
- National Population Commission; ICF International. Nigeria Demographic and Health Survey 2018; NPC: Abuja, Nigeria; ICF: Rockville, MD, USA, 2019. [Google Scholar]
- Anumudu, C.I.; Okafor, C.M.F.; Ngwumohaike, V.; Afolabi, K.A.; Nwuba, R.I.; Nwagwu, M. Epidemiological Factors That Promote the Development of Severe Malaria Anaemia in Children in Ibadan. Afr. Health Sci. 2007, 7, 80–85. [Google Scholar] [CrossRef]
- Berendsen, M.L.; van Gijzel, S.W.; Smits, J.; de Mast, Q.; Aaby, P.; Benn, C.S.; Netea, M.G.; van der Ven, A.J. BCG Vaccination Is Associated with Reduced Malaria Prevalence in Children under the Age of 5 Years in Sub-Saharan Africa. BMJ Glob. Health 2019, 4, e001862. [Google Scholar] [CrossRef] [PubMed]
- Chitunhu, S.; Musenge, E. Direct and Indirect Determinants of Childhood Malaria Morbidity in Malawi: A Survey Cross-Sectional Analysis Based on Malaria Indicator Survey Data for 2012. Malar. J. 2015, 14, 265. [Google Scholar] [CrossRef]
- Morakinyo, O.M.; Balogun, F.M.; Fagbamigbe, A.F. Housing Type and Risk of Malaria among Under-Five Children in Nigeria: Evidence from the Malaria Indicator Survey. Malar. J. 2018, 17, 311. [Google Scholar] [CrossRef]
- Siri, J.G. Independent Associations of Maternal Education and Household Wealth with Malaria Risk in Children. Ecol. Soc. 2014, 19, 33. [Google Scholar] [CrossRef]
- Semakula, H.M.; Song, G.B.; Zhang, S.S.; Achuu, S.P. Potential of Household Environmental Resources and Practices in Eliminating Residual Malaria Transmission: A Case Study of Tanzania, Burundi, Malawi and Liberia. Afr. Health Sci. 2015, 15, 819–827. [Google Scholar] [CrossRef]
- Njau, J.D.; Stephenson, R.; Menon, M.P.; Kachur, S.P.; McFarland, D.A. Investigating the Important Correlates of Maternal Education and Childhood Malaria Infections. Am. J. Trop. Med. Hyg. 2014, 91, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Njau, J.D.; Stephenson, R.; Menon, M.; Kachur, S.P.; McFarland, D.A. Exploring the Impact of Targeted Distribution of Free Bed Nets on Households Bed Net Ownership, Socio-Economic Disparities and Childhood Malaria Infection Rates: Analysis of National Malaria Survey Data from Three Sub-Saharan Africa Countries. Malar. J. 2013, 12, 245. [Google Scholar] [CrossRef]
- Asia Pacific Leaders Malaria Alliance Bangladesh: New Plan for Malaria Elimination (2017–2021). Available online: https://www.aplma.org/blog/42/bangladesh-new-plan-for-malaria-elimination-2017-2021.html (accessed on 3 January 2021).
- Wanzira, H.; Katamba, H.; Okullo, A.E.; Agaba, B.; Kasule, M.; Rubahika, D. Factors Associated with Malaria Parasitaemia among Children under 5 Years in Uganda: A Secondary Data Analysis of the 2014 Malaria Indicator Survey Dataset. Malar. J. 2017, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Adigun, A.B.; Gajere, E.N.; Oresanya, O.; Vounatsou, P. Malaria Risk in Nigeria: Bayesian Geostatistical Modelling of 2010 Malaria Indicator Survey Data. Malar. J. 2015, 14, 156. [Google Scholar] [CrossRef]
- Kayode, G.A.; Adekanmbi, V.T.; Uthman, O.A. Risk Factors and a Predictive Model for Under-Five Mortality in Nigeria: Evidence from Nigeria Demographic and Health Survey. BMC Pregnancy Childbirth 2012, 12, 10. [Google Scholar] [CrossRef]
- Macrotrends. Nigeria Population Growth Rate 1950–2020. Available online: https://www.macrotrends.net/countries/NGA/nigeria/population-growth-rate (accessed on 27 July 2020).
- Tradingeconomics. Nigeria—Population Density (People Per Sq. Km)—1961–2018 Data 2020 Forecast. Available online: https://tradingeconomics.com/nigeria/population-density-people-per-sq-km-wb-data.html (accessed on 27 July 2020).
- Mustapha, A.R. Ethnic Structure, Inequality and Governance of the Public Sector in Nigeria; Centre for Research on Inequality, Human Security and Ethnicity (CRISE); University of Oxford: Oxford, UK, 2005; p. 18. [Google Scholar]
- OpenStreetMap Wiki Contributors. WikiProject Nigeria [Internet]. Open Street/Map. Available online: https://wiki.openstreetmap.org/wiki/WikiProject_Nigeria (accessed on 23 January 2020).
- United Nations Development Programme (UNDP). National Human Development Report 2018: Nigeria Human Development Reports; United Nations Development Programme (UNDP): Abuja, Nigeria, 2018. [Google Scholar]
- Demographic and Health Surveys. Understanding and Using the Demographic and Health Surveys DHS Curriculum Facilitator’s Guide; ICF International: Rockville, MD, USA, 2014. [Google Scholar]
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. A Scoping Review of the Risk Factors Associated with Anaemia among Children Under Five Years in Sub-Saharan African Countries. Int. J. Environ. Res. Public Health 2020, 17, 8829. [Google Scholar] [CrossRef] [PubMed]
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. Risk Factors Associated with Malnutrition among Children Under-Five Years in Sub-Saharan African Countries: A Scoping Review. Int. J. Environ. Res. Public Health 2020, 17, 8782. [Google Scholar] [CrossRef]
- Demographic and Health Survey. The DHS Program—Analysis FAQs. Available online: https://dhsprogram.com/data/analysis-faqs.cfm (accessed on 14 October 2021).
- What are Health Determinants?—Individuals & Society Study.Com. Available online: https://study.com/academy/lesson/what-are-health-determinants-individuals-society.html (accessed on 3 July 2021).
- Black, S.E. New Evidence on the Impacts of Birth Order. Available online: https://www.nber.org/reporter/2017number4/new-evidence-impacts-birth-order (accessed on 2 October 2021).
- Northern Ireland Breastfeeding | Department of Health. Available online: https://www.health-ni.gov.uk/articles/breastfeeding (accessed on 2 October 2021).
- Bamiwuye, S.O.; Wet, N.D.; Adedini, S.A. Linkages between Autonomy, Poverty and Contraceptive Use in Two Sub-Saharan African Countries. Afr. Popul. Stud. 2013, 27, 164–173. [Google Scholar] [CrossRef]
- Nandy, S.; Daoud, A.; Gordon, D. Examining the Changing Profile of Undernutrition in the Context of Food Price Rises and Greater Inequality. Soc. Sci. Med. 2016, 149, 153–163. [Google Scholar] [CrossRef]
- Nandy, S.; Jaime Miranda, J. Overlooking Undernutrition? Using a Composite Index of Anthropometric Failure to Assess How Underweight Misses and Misleads the Assessment of Undernutrition in Young Children. Soc. Sci. Med. 2008, 66, 1963–1966. [Google Scholar] [CrossRef]
- Myrskylä, M.; Fenelon, A. Maternal Age and Offspring Adult Health: Evidence From the Health and Retirement Study. Demography 2012, 49, 1231–1257. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Lee, K.T.H.; Rosales-Rueda, M.; Kalil, A. Maternal Age and Child Development. Demography 2018, 55, 2229–2255. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, S.T.; Uthman, O.A. Individual and Contextual Correlates of Mosquito Net Use among Women in Nigeria. Malar. J. 2020, 19, 138. [Google Scholar] [CrossRef]
- Jennings-Edquist, G. Does the Age You Become a Parent Really Matter? We Asked Three Mums—ABC Everyday. Available online: https://www.abc.net.au/everyday/does-the-age-you-become-a-parent-actually-matter/12742736 (accessed on 14 October 2021).
- Obasohan, D.N.; Karo, H.A.; Obasohan, P. Socioeconomic and Demographic Barriers to Assessing Ante Natal Care Services among Women of Child Bearing Age in Wushishi Local Government Area, Niger State, Nigeria. World J. Pharm. Res. 2018, 7, 1264–1271. [Google Scholar]
- Obasohan, P. Religion, Ethnicity and Contraceptive Use among Reproductive Age Women in Nigeria. Int. J. MCH AIDS (IJMA) 2014, 3, 63. [Google Scholar] [CrossRef]
- Kawo, K.N.; Asfaw, Z.G.; Yohannes, N. Multilevel Analysis of Determinants of Anemia Prevalence among Children Aged 6-59 Months in Ethiopia: Classical and Bayesian Approaches. Anemia 2018, 2018, 3087354. [Google Scholar] [CrossRef]
- Lia, F.; Taylor, C. Using Household Survey Data to Explore the Effects of Improved Housing Conditions on Malaria Infection in Children in Sub-Saharan Africa; ICF International: Rockville, MD, USA, 2016. [Google Scholar]
- Dhewantara, P.W.; Ipa, M.; Widawati, M. Individual and Contextual Factors Predicting Self-Reported Malaria among Adults in Eastern Indonesia: Findings from Indonesian Community-Based Survey. Malar. J. 2019, 18, 118. [Google Scholar] [CrossRef]
- Adedokun, S.T. Correlates of Childhood Morbidity in Nigeria: Evidence from Ordinal Analysis of Cross-Sectional Data. PLoS ONE 2020, 15, e0233259. [Google Scholar] [CrossRef] [PubMed]
- Gabr, H.M.K.M. Investigating Poverty and Labour Force Participation among Older Population in Egypt: A Multilevel Simultaneous Equations Modeling Approach. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2016. [Google Scholar]
- Rozi, S.; Mahmud, S.; Lancaster, G.; Hadden, W.; Pappas, G. Multilevel Modeling of Binary Outcomes with Three-Level Complex Health Survey Data. Open J. Epidemiol. 2016, 7, 27–43. [Google Scholar] [CrossRef][Green Version]
- Prestevez, R. How to Compute Intraclass Correlation (ICC) for THREE-Level Negative Binomial Hierarchical Model? Available online: https://stats.stackexchange.com/questions/174071/how-to-compute-intraclass-correlation-icc-for-three-Level-negative-binomial-hi (accessed on 11 May 2021).
- Leckie, G.; Browne, W.J.; Goldstein, H.; Merlo, J.; Austin, P.C. Partitioning Variation in Multilevel Models for Count Data. Psychol. Methods 2020, 25, 787–801. [Google Scholar] [CrossRef] [PubMed]
- MLwiN User Forum. VPC in Three and Four Levels Binary Response Models. Available online: https://www.cmm.bris.ac.uk/forum/viewtopic.php?t=60 (accessed on 9 June 2021).
- Heck, R.H.; Thomas, S.; Tabata, L. Multilevel Modeling of Categorical Outcomes Using IBM SPSS, 2nd ed.; Routledge: New York, NY, USA, 2014; ISBN 978-1-84872-956-8. [Google Scholar]
- Azikiwe, C.; Ifezulike, C.; Siminialayi, I.; Amazu, L.; Enye, J.; Nwakwunite, O. A Comparative Laboratory Diagnosis of Malaria: Microscopy versus Rapid Diagnostic Test Kits. Asian Pac. J. Trop. Biomed. 2012, 2, 307–310. [Google Scholar] [CrossRef]
- Zgambo, M.; Mbakaya, B.C.; Kalembo, F.W. Prevalence and Factors Associated with Malaria Parasitaemia in Children under the Age of Five Years in Malawi: A Comparison Study of the 2012 and 2014 Malaria Indicator Surveys (MISs). PLoS ONE 2017, 12, e0175537. [Google Scholar] [CrossRef]
| (a) | |||
|---|---|---|---|
| Variables (Categories) | Malaria Status (6–59 Months) | ||
| Total | No | Yes | |
| (n) | n (%) | n (%) | |
| Age of the child | 10,185 | χ2 = 148.15, p-value < 0.0001 | |
| 06–11 months | 1232 | 925 (0.75) | 307 (0.25) |
| 12–23 months | 2421 | 1686 (0.70) | 736 (0.30) |
| 24–35 months | 2159 | 1379 (0.64) | 780 (0.36) |
| 36–47 months | 2229 | 1332 (0.60) | 896 (0.40) |
| 48–59 months | 2143 | 1245 (0.58) | 898 (0.42) |
| Sex | 10,185 | χ2 = 0.551, p-value = 0.516 | |
| Male | 5216 | 3346 (0.64) | 1871 (0.36) |
| Female | 4968 | 3221 (0.65) | 1747 (0.35) |
| Perceived birth size | 10,060 | χ2 = 8.006, p-value = 0.073 | |
| Large | 923 | 631 (0.68) | 292 (0.32) |
| Average | 7915 | 5094 (0.64) | 2822 (0.36) |
| Small | 1222 | 764 (0.63) | 457 (0.37) |
| Birth order | 10,185 | χ2 = 157.98, p-value < 0.0001 | |
| 1st order | 1945 | 1373 (70) | 582 (30) |
| 2nd or 3rd order | 3483 | 2388 (69) | 1095 (31) |
| 4th–6th order | 3207 | 2007 (63) | 1200 (37) |
| 7th+ order | 1549 | 809 (52) | 740 (48) |
| Duration of breastfeeding | 10,185 | χ2 = 47.86, p-value < 0.0001 | |
| ever, but not currently | 7441 | 4662 (63) | 2780 (37) |
| never breastfed | 171 | 102 (60) | 69 (40) |
| still breastfeeding | 2572 | 1803 (70) | 769 (30) |
| Child had diarrheal in the last 2 weeks | 10,182 | χ2 = 41.25, p-value < 0.0001 | |
| No | 8832 | 5801 (66) | 3031 (34) |
| Yes | 1350 | 765 (57) | 585 (43) |
| Child had fever in the last 2 weeks | 10,182 | χ2 = 304.96, p-value < 0.0001 | |
| No | 7487 | 5201 (69) | 2285 (31) |
| Yes | 2695 | 1366 (51) | 1330 (49) |
| Child had acute respiratory infections in past 2 weeks | 10,183 | χ2 = 0.340, p-value = 0.6332 | |
| No | 9575 | 6168 | 3407 |
| Yes | 608 | 398 | 209 |
| Child took vitamin A supplements | 10,141 | χ2 = 173.345, p-value < 0.0001 | |
| No | 5309 | 3106 (58) | 2203 (42) |
| Yes | 4832 | 3432 (71) | 1399 (29) |
| Child had deworming treatment in the last 6 months | 10,133 | χ2 = 235.73, p-value < 0.0001 | |
| No | 7235 | 4330 (60) | 2905 (40) |
| Yes | 2898 | 2203 (76) | 695 (24) |
| Child took iron supplements | 10,151 | χ2 = 42.173, p-value < 0.0001 | |
| No | 8224 | 5183 (63) | 3040 (37) |
| Yes | 1927 | 1367 (71) | 561 (29) |
| Nutritional status | 10,185 | χ2 = 267.41, p-value < 0.0001 | |
| Well-nourished | 5688 | 4060 (71) | 1627 (29) |
| Poorly nourished | 4497 | 2507 (56) | 1990 (44) |
| Stunting | 10,185 | χ2 = 297.16, p-value < 0.0001 | |
| No | 6285 | 4457 (71) | 1827 (29) |
| Yes | 3900 | 2109 (54) | 1790 (46) |
| Wasting | 10,185 | χ2 = 4.03, p-value =0.1169 | |
| No | 9481 | 6138 (65) | 3343 (35) |
| Yes | 703 | 429 (61) | 274 (39) |
| Underweight | 10,185 | χ2 = 156.20, p-value < 0.0001 | |
| No | 7915 | 5355 (68) | 2560 (32) |
| Yes | 2269 | 1212 (53) | 1058 (47) |
| Overweight | 10,185 | χ2 = 6.20, p-value = 0.0337 | |
| No | 10,019 | 6445 (64) | 3573 (36) |
| Yes | 166 | 122 (74) | 44 (26) |
| Anaemia status | 10,183 | χ2 = 649.60, p-value < 0.0001 | |
| No | 3241 | 2664 (82) | 577 (18) |
| Yes | 6942 | 3902 (56) | 3040 (44) |
| (b) | |||
| Variables (Categories) Categories | Malaria Status | ||
| Total | No | Yes | |
| (n) | n(%) | n(%) | |
| Maternal age (years) group | 10,185 | χ2 = 14.59, p-value = 0.0095 | |
| 15–24 years | 2048 | 1265 (62) | 784 (38) |
| 25–34 years | 5262 | 3481 (66) | 1781 (34) |
| 35 years+ | 2874 | 1821 (63) | 1052 (37) |
| Maternal age at first birth | 10,185 | χ2 = 385.04, p-value < 0.0001 | |
| 10–19 years | 5406 | 3033 (56) | 2373 (44) |
| 20–29 years | 4369 | 3177 (73) | 1192 (27) |
| 30+ years | 409 | 357 (87) | 53 (13) |
| Mother working status | 10,185 | χ2 = 8.83, p-value = 0.038 | |
| Not working | 2978 | 1855 (62) | 1123 (38) |
| Working | 7207 | 4712 (65) | 2494 (35) |
| Mother’s educational status | 10,185 | χ2 = 864.57, p-value < 0.0001 | |
| No education | 3970 | 1951 (49) | 2018 (51) |
| Primary education | 1643 | 985 (60) | 658 (40) |
| Secondary and above | 4571 | 3631 (79) | 940 (21) |
| Marital status | 10,185 | χ2 = 1.756, p-value = 0.5012 | |
| Never in union | 171 | 110 (64) | 61 (36) |
| In union | 9733 | 6265 (64) | 3468 (36) |
| Widow/divorced/separated | 281 | 191 (68) | 89 (32) |
| Paternal educational status | 9604 | χ2 = 672.98, p-value < 0.0001 | |
| No education | 2872 | 1369 (48) | 1503 (52) |
| Primary education | 1423 | 817 (57) | 606 (43) |
| Secondary education | 5308 | 4018 (76) | 1290 (24) |
| Paternal occupation | 10,185 | χ2 = 0.294, p-value = 0.7133 | |
| Not working | 304 | 191 (63) | 112 (37) |
| Working | 9881 | 6376 (65) | 3505 (35) |
| Mother lives with a partner | 9733 | χ2 = 7.433, p-value =0.0291 | |
| Living with partner | 8862 | 5668 (64) | 3194 (36) |
| Living alone | 871 | 598 (69) | 273 (31) |
| Mother slept under mosquito net | 10,185 | χ2 = 91.02, p-value < 0.0001 | |
| No | 4671 | 3242 (69) | 1429 (31) |
| Yes | 5514 | 3325 (60) | 2188 (40) |
| Mother’s body weight status | 8690 | χ2 = 250.16, p-value < 0.0001 | |
| Underweight | 5310 | 3219 (61) | 2092 (39) |
| Healthy | 884 | 513 (58) | 371 (42) |
| Overweight and obese | 2537 | 1977 (78) | 559 (22) |
| Preceding birth interval | 8220 | χ2 = 15.683, p-value= 0.0155 | |
| 08–24 months | 2190 | 1356 (62) | 834 (38) |
| 25–35 months | 2884 | 1775 (62) | 1109 (38) |
| 36–59 months | 2351 | 1515 (64) | 836 (36) |
| 60+ months | 795 | 544 (68) | 251 (32) |
| Mother’s anaemia status | 10,053 | χ2 = 120.013, p-value < 0.0001 | |
| Normal | 4206 | 2991 (71) | 1214 (29) |
| Anaemic | 5847 | 3519 (60) | 2328 (40) |
| Number of ANC attendance | 6375 | χ2 = 185.99, p-value < 0.0001 | |
| None | 1342 | 715 (53) | 627 (47) |
| Less WHO number | 954 | 624 (65) | 329 (35) |
| Met WHO number | 4079 | 2987 (73) | 1092 (27) |
| Maternal autonomy | 10,185 | χ2 = 178.05, p-value < 0.0001 | |
| Less autonomy | 5071 | 2947 (58) | 2124 (42) |
| more autonomy | 5114 | 3620 (71) | 1494 (29) |
| Maternal ethnicity | 10,185 | χ2 = 325.93, p-value < 0.0001 | |
| Hausa/Fulani | 4067 | 2226 (55) | 1841 (45) |
| Ibos | 1650 | 1273 (77) | 377 (23) |
| Yoruba | 1490 | 1068 (72) | 421 (28) |
| Others | 2978 | 2000 (67) | 977 (33) |
| Religion status | 10,185 | χ2 = 255.02, p-value < 0.0001 | |
| Catholic | 1027 | 754 (73) | 273 (27) |
| Other Christian | 3438 | 2509 (73) | 929 (27) |
| Islam | 5655 | 3266 (58) | 2389 (42) |
| Others (traditional) | 64 | 39 (60) | 25 (40) |
| Place of delivery | 10,184 | χ2 = 455.18, p-value < 0.0001 | |
| Home | 5348 | 2953 (55) | 2394 (45) |
| Public health facility | 2977 | 2137 (72) | 840 (28) |
| Private health facility | 1660 | 1334 (80) | 326 (20) |
| Somewhere else | 200 | 142 (71) | 58 (29) |
| (c) | |||
| Variables (Categories) | Malaria Status (6–59 Months) | ||
| Total | No | Yes | |
| n | n(%) | n(%) | |
| Household wealth status | 10,185 | χ2 = 1102.49, p-value < 0.0001 | |
| Poor | 3882 | 1813 (47) | 2069 (53) |
| Middle | 2139 | 1335 (62) | 804 (38) |
| Rich | 4163 | 3419 (82) | 744 (18) |
| Household had mosquito bed net | 10,185 | χ2 = 65.709, p-value < 0.0001 | |
| No | 3111 | 2187 (70) | 924 (30) |
| Yes | 7074 | 4389 (62) | 2693 (39) |
| Household member size | 10,185 | χ2 = 159.22, p-value < 0.0001 | |
| 0–3 persons | 980 | 678 (71) | 282 (29) |
| 4–6 persons | 4835 | 3322 (69) | 1513 (31) |
| 7–9 persons | 2461 | 1521 (62) | 940 (38) |
| 10+ persons | 1908 | 1026 (54) | 881 (46) |
| Number of bedrooms in household | 10,185 | χ2 = 47.584, p-value < 0.0001 | |
| One room | 2807 | 1952 (70) | 854 (30) |
| Two rooms | 3489 | 2221 (64) | 1268 (36) |
| Three rooms | 2030 | 1239 (61) | 791 (39) |
| Four rooms | 981 | 604 (62) | 377 (38) |
| Five+ rooms | 877 | 550 (63) | 326 (37) |
| Number of children under-five in household | 10,185 | χ2 = 130.40, p-value < 0.0001 | |
| No children or one child | 2700 | 1880 (70) | 819 (30) |
| Two children | 4315 | 2848 (66) | 1468 (34) |
| Three children | 2054 | 1270 (62) | 783 (38) |
| Four children+ | 1115 | 568(51) | 547 (49) |
| Improved source of drinking water | 10,185 | χ2 = 298.76, p-value < 0.0001 | |
| Unimproved | 3078 | 1601 (52) | 1477 (48) |
| Improved | 7106 | 4966 (70) | 2140 (30) |
| Improved toilet facilities | 10,185 | χ2 = 650.37, p-value < 0.0001 | |
| Unimproved | 4607 | 2357 (51) | 2250 (49) |
| Improved | 5577 | 4210 (75) | 1367 (25) |
| Youngest child’s stool disposal | 6408 | χ2 = 0.102, p-value= 0.8208 | |
| Proper | 3606 | 2348 (65) | 1257 (35) |
| improper | 2803 | 1836 (66) | 967 (34) |
| Improved floor material type | 10,185 | χ2 = 329.83, p-value < 0.0001 | |
| Unimproved | 2877 | 1460 (51) | 1418 (49) |
| Improved | 7307 | 5107 (70) | 2200 (30) |
| Improved roofing materials | 10,185 | χ2 = 87.795, p-value < 0.0001 | |
| Unimproved | 1125 | 583 (52) | 542 (48) |
| Improved | 9060 | 5984 (66) | 3076 (34) |
| Improved wall materials | 10,184 | χ2 = 638.88, p-value < 0.0001 | |
| Unimproved | 3265 | 1535 (47) | 1730 (53) |
| Improved | 6919 | 5032 (73) | 1887 (27) |
| Sex of household head | 10,185 | χ2 = 7.815, p-value= 0.0283 | |
| Male | 9008 | 5824 (64) | 3273 (36) |
| Female | 1087 | 743 (68) | 344 (32) |
| Household head age (years) group | 10,185 | χ2 = 27.139, p-value= 0.0026 | |
| less 34 years | 2828 | 1825 (65) | 1003 (35) |
| 35–44 years | 3946 | 2648 (67) | 1298 (33) |
| 45–55 years | 2091 | 1300 (62) | 792 (38) |
| 56 years+ | 1318 | 794 (60) | 524 (40) |
| Household had electricity | 10,066 | χ2 = 590.89, p-value < 0.0001 | |
| No | 4296 | 2186 (51) | 2109 (49) |
| Yes | 5771 | 4296 (74) | 1475 (26) |
| Type of cooking fuel | 10,182 | χ2 = 384.85, p-value < 0.0001 | |
| Electricity and Gas | 1211 | 1088 (90) | 123 (19) |
| Biofuel/mass | 8971 | 5477 (61) | 3494 (39) |
| Under-five slept under bed net | 10,112 | χ2 = 104.81, p-value < 0.0001 | |
| No child | 1317 | 863 (66) | 454 (34) |
| All children | 4715 | 2965 (63) | 1750 (37) |
| Some children | 996 | 530 (53) | 466 (47) |
| No net in the house | 3083 | 2165 (70) | 918 (30) |
| (d) | |||
| Variables (Categories) | Malaria Status (6–59 Months) | ||
| Total | No | Yes | |
| n | n(%) | n(%) | |
| Proportion of low cluster wealth level | 10,185 | χ2 = 842.35, p-value < 0.0001 | |
| Low | 5323 | 4133 (78) | 1189 (22) |
| High | 4861 | 2433 (50) | 2428 (50) |
| Distance to health facility is no big problem | 10,185 | χ2 = 233.12, p-value < 0.0001 | |
| Low | 4702 | 2664 (57) | 2038 (43) |
| High | 5483 | 3903 (71) | 1579 (29) |
| Proportion of low cluster household with bed net | 10,185 | χ2 = 210.75, p-value < 0.0001 | |
| Low | 4981 | 2861 (57) | 2121 (43) |
| High | 5202 | 3705 (71) | 1497 (29) |
| (e) | |||
| Variables (Categories) | Malaria Status (6–59 Months) | ||
| Total | No | Yes | |
| n | n(%) | n(%) | |
| State Human Development Index (SHDI) | 10,185 | χ2 = 456.50, p-value < 0.0001 | |
| Lowest HDI | 2150 | 1219 (57) | 839 (43) |
| Low HDI | 2416 | 1297 (54) | 1120 (46) |
| Average HDI | 2223 | 1511 (68) | 712 (32) |
| High HDI | 2680 | 1886 (70) | 793 (30) |
| Highest HDI | 716 | 654 (91) | 62 (9) |
| Region of residence | 10,185 | χ2 = 428.79, p-value < 0.0001 | |
| North-central | 1436 | 906 (63) | 530 (37) |
| North-east | 1573 | 1034 (66) | 538 (34) |
| north-west | 2967 | 1502 (51) | 1465 (49) |
| South-east | 1328 | 826 (76) | 336 (25) |
| South-south | 1086 | 826 (76) | 260 (24) |
| South-west | 1794 | 1307 (73) | 487 (27) |
| State Multidimensional Poverty Index (SMPI) | 10,185 | χ2 = 364.70, p-value < 0.0001 | |
| Highly Deprived | 847 | 481 (57) | 366 (43) |
| Above Averagely deprived | 3093 | 1659 (54) | 1434 (46) |
| Averagely Deprived | 2318 | 1499 (65) | 819 (35) |
| Mildly deprived | 1939 | 1395 (72) | 544 (28) |
| Lowest deprived | 1987 | 1532 (77) | 455 (23) |
| Place of residence | 10,185 | χ2 = 724.32, p-value < 0.0001 | |
| Urban | 4485 | 3538 (79) | 946 (21) |
| Rural | 5700 | 3029 (53) | 2671 (47) |
| Variables | Model 2 (n = 9277) (Level-1 Factors Only) | Model 3 (n = 9277) (Added Level-2 Factors) | Model 4 (n = 9277) (Added Level-3 Factors) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Individual Level | AOR | 95% CI | p-Value | AOR | 95% CI | p-Value | AOR | 95% CI | p-Value |
| Child’s age | |||||||||
| 6–11 months | 1.00 | 1.00 | 1.00 | ||||||
| 12–23 months | 1.26 | 1.02–1.56 | 0.031 | 1.26 | 1.02–1.56 | 0.029 | 1.28 | 1.04–1.58 | 0.021 |
| 24–35 months | 1.65 | 1.26–2.16 | <0.001 | 1.65 | 1.26–2.15 | <0.001 | 1.65 | 1.26–2.16 | <0.001 |
| 36–47 months | 2.20 | 1.67–2.88 | <0.001 | 2.20 | 1.67–2.89 | <0.001 | 2.20 | 1.68–2.89 | <0.001 |
| 48–59 months | 2.69 | 2.04–3.55 | <0.001 | 2.68 | 2.03–3.54 | <0.001 | 2.66 | 2.02–3.51 | <0.001 |
| Duration of breastfeeding | |||||||||
| Ever breastfed | 1.00 | 1.00 | 1.00 | ||||||
| Never breastfed | 1.28 | 0.84–1.94 | 0.251 | 1.26 | 0.83–1.92 | 0.276 | 1.28 | 0.84–1.96 | 0.243 |
| Still breastfeeding | 0.63 | 0.51–0.76 | <0.001 | 0.62 | 0.51–0.76 | <0.001 | 0.61 | 0.50–0.75 | <0.001 |
| Anaemia status | |||||||||
| Not anaemic | 1.00 | 1.00 | 1.00 | ||||||
| Anaemic | 3.84 | 3.36–4.39 | <0.001 | 3.82 | 3.34–4.37 | <0.001 | 3.82 | 3.34–4.37 | <0.001 |
| Nutrition status | |||||||||
| Well-nourished | 1.00 | 1.00 | 1.00 | ||||||
| Poorly nourished | 1.07 | 0.95–1.2 | 0.284 | 1.06 | 0.94–1.19 | 0.378 | 1.05 | 0.94–1.19 | 0.386 |
| Fever in last 2 weeks | |||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 1.95 | 1.72–2.2 | <0.001 | 1.94 | 1.71–2.2 | <0.001 | 1.96 | 1.73–2.22 | <0.001 |
| Dewormed in last 2 weeks | |||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 0.75 | 0.65–0.87 | <0.001 | 0.75 | 0.65–0.87 | <0.001 | 0.75 | 0.65–0.87 | <0.001 |
| Maternal age (years) in group | |||||||||
| 15–24 years | 1.00 | 1.00 | 1.00 | ||||||
| 25–34 years | 1.04 | 0.89–1.22 | 0.611 | 1.05 | 0.9–1.23 | 0.532 | 1.06 | 0.91–1.24 | 0.464 |
| 35 years+ | 1.16 | 0.95–1.4 | 0.143 | 1.17 | 0.96–1.42 | 0.111 | 1.19 | 0.98–1.44 | 0.085 |
| Maternal age at first birth | |||||||||
| 10–19 years | 1.00 | 1.00 | 1.00 | ||||||
| 20–29 years | 0.82 | 0.72–0.93 | 0.003 | 0.82 | 0.72–0.94 | 0.003 | 0.81 | 0.71–0.93 | 0.002 |
| 30 years+ | 0.52 | 0.35–0.77 | 0.001 | 0.52 | 0.36–0.77 | 0.001 | 0.51 | 0.35–0.75 | 0.001 |
| Maternal education status | |||||||||
| No education | 1.00 | 1.00 | 1.00 | ||||||
| Primary | 0.82 | 0.68–0.99 | 0.038 | 0.85 | 0.7–1.03 | 0.093 | 0.86 | 0.71–1.04 | 0.128 |
| Secondary+ | 0.61 | 0.5–0.75 | <0.001 | 0.65 | 0.53–0.79 | <0.001 | 0.67 | 0.55–0.82 | <0.001 |
| Paternal education status | |||||||||
| No education | 1.00 | 1.00 | 1.00 | ||||||
| Primary | 0.87 | 0.71–1.06 | 0.157 | 0.89 | 0.73–1.08 | 0.244 | 0.90 | 0.74–1.10 | 0.304 |
| Secondary+ | 0.74 | 0.62–0.88 | 0.001 | 0.77 | 0.64–0.92 | 0.004 | 0.80 | 0.66–0.95 | 0.013 |
| Maternal anaemia status | |||||||||
| Not anaemic | 1.00 | 1.00 | 1.00 | ||||||
| Anaemic | 1.24 | 1.11–1.39 | <0.001 | 1.24 | 1.1–1.39 | <0.001 | 1.23 | 1.1–1.38 | <0.001 |
| Maternal ethnic group | |||||||||
| Hausa/Fulani/Kanuri | 1.00 | 1.00 | 1.00 | ||||||
| Ibo | 0.83 | 0.54–1.28 | 0.401 | 0.86 | 0.56–1.32 | 0.489 | 0.81 | 0.49–1.31 | 0.387 |
| Yoruba | 1.57 | 1.08–2.26 | 0.017 | 1.61 | 1.11–2.34 | 0.012 | 1.45 | 0.98–2.15 | 0.064 |
| Others | 1.36 | 1.08–1.71 | 0.010 | 1.33 | 1.05–1.68 | 0.016 | 1.29 | 1.02–1.63 | 0.037 |
| Maternal religion status | |||||||||
| Catholics | 1.00 | 1.00 | 1.00 | ||||||
| Other Christian | 0.89 | 0.7–1.14 | 0.359 | 0.91 | 0.72–1.16 | 0.460 | 0.92 | 0.72–1.17 | 0.491 |
| Islam | 0.82 | 0.6–1.11 | 0.199 | 0.85 | 0.62–1.15 | 0.288 | 0.90 | 0.66–1.23 | 0.499 |
| Traditionalists | 0.78 | 0.39–1.54 | 0.467 | 0.78 | 0.39–1.54 | 0.470 | 0.80 | 0.41–1.58 | 0.526 |
| Household wealth | |||||||||
| Low | 1.00 | 1.00 | 1.00 | ||||||
| Middle | 0.71 | 0.6–0.84 | <0.001 | 0.84 | 0.7–1.01 | 0.070 | 0.86 | 0.71–1.03 | 0.102 |
| Rich | 0.43 | 0.36–0.52 | <0.001 | 0.55 | 0.44–0.69 | <0.001 | 0.61 | 0.49–0.76 | <0.001 |
| Number of under-5 in household | |||||||||
| No children or one child | 1.00 | 1.00 | 1.00 | ||||||
| Two children | 1.03 | 0.9–1.19 | 0.667 | 1.04 | 0.90–1.20 | 0.584 | 1.04 | 0.91–1.20 | 0.556 |
| Three children | 1.12 | 0.94–1.33 | 0.222 | 1.12 | 0.94–1.34 | 0.201 | 1.11 | 0.93–1.32 | 0.249 |
| Four children+ | 1.48 | 1.18–1.85 | 0.001 | 1.47 | 1.17–1.84 | 0.001 | 1.46 | 1.16–1.83 | 0.001 |
| Household head age (years) group | |||||||||
| Less 35 years | 1.00 | 1.00 | 1.00 | ||||||
| 35–44 years | 0.85 | 0.73–0.98 | 0.031 | 0.85 | 0.73–0.99 | 0.041 | 0.86 | 0.74–1.00 | 0.050 |
| 45–55 years | 0.87 | 0.72–1.05 | 0.135 | 0.89 | 0.73–1.07 | 0.204 | 0.90 | 0.75–1.09 | 0.270 |
| 56 years+ | 1.07 | 0.86–1.32 | 0.549 | 1.10 | 0.89–1.36 | 0.386 | 1.12 | 0.90–1.38 | 0.304 |
| Under-5 slept under a bed net | |||||||||
| No child | 1.00 | 1.00 | 1.00 | ||||||
| All children | 0.89 | 0.74–1.07 | 0.217 | 0.89 | 0.74–1.07 | 0.202 | 0.88 | 0.73–1.06 | 0.176 |
| Some children | 1.15 | 0.91–1.46 | 0.244 | 1.16 | 0.91–1.47 | 0.234 | 1.15 | 0.91–1.46 | 0.239 |
| No net in household | 0.96 | 0.8–1.17 | 0.701 | 0.99 | 0.81–1.2 | 0.887 | 0.98 | 0.8–1.19 | 0.802 |
| Number of bedrooms in household | |||||||||
| One room | 1.00 | 1.00 | 1.00 | ||||||
| Two rooms | 1.03 | 0.88–1.20 | 0.705 | 1.01 | 0.87–1.18 | 0.894 | 1.00 | 0.86–1.17 | 0.989 |
| Three rooms | 1.07 | 0.89–1.28 | 0.477 | 1.04 | 0.86–1.25 | 0.692 | 1.02 | 0.84–1.22 | 0.869 |
| Four rooms | 0.91 | 0.72–1.14 | 0.392 | 0.87 | 0.69–1.1 | 0.240 | 0.84 | 0.67–1.06 | 0.148 |
| Five+ rooms | 0.82 | 0.64–1.05 | 0.122 | 0.78 | 0.61–1.01 | 0.062 | 0.76 | 0.59–0.98 | 0.037 |
| Cluster level | |||||||||
| Proportion of cluster’s household with no bed net | |||||||||
| Low | 1.00 | 1.00 | |||||||
| High | 0.92 | 0.76–1.12 | 0.410 | 0.97 | 0.80–1.17 | 0.718 | |||
| Distance to a health facility is no big problem | |||||||||
| Low | 1.00 | 1.00 | |||||||
| High | 0.72 | 0.6–0.86 | <0.001 | 0.76 | 0.64–0.90 | 0.002 | |||
| Proportion of low cluster wealth status | |||||||||
| Low | 1.00 | 1.00 | |||||||
| High | 1.41 | 1.13–1.75 | 0.002 | 1.15 | 0.92–1.43 | 0.226 | |||
| State-level | |||||||||
| Region of residence | |||||||||
| North central | 1.00 | ||||||||
| North-east | 0.48 | 0.22–1.05 | 0.065 | ||||||
| North-west | 1.46 | 0.62–3.45 | 0.387 | ||||||
| South-east | 1.07 | 0.51–2.25 | 0.854 | ||||||
| South-south | 0.50 | 0.25–0.98 | 0.045 | ||||||
| South-west | 1.44 | 0.64–3.25 | 0.378 | ||||||
| Type of place of residence | |||||||||
| Urban | 1.00 | ||||||||
| Rural | 2.12 | 1.75–2.57 | <0.001 | ||||||
| State human development index (HDI) | |||||||||
| Lowest HDI | 1.00 | ||||||||
| Low HDI | 1.32 | 0.71–2.45 | 0.374 | ||||||
| Average HDI | 1.50 | 0.68–3.33 | 0.314 | ||||||
| High HDI | 1.87 | 0.73–4.80 | 0.192 | ||||||
| Highest HDI | 1.03 | 0.35–2.98 | 0.961 | ||||||
| State Multidimensional poverty index (SMPI) | |||||||||
| Highly deprived | 1.00 | ||||||||
| Above averagely deprived | 1.75 | 0.90–3.43 | 0.101 | ||||||
| Averagely deprived | 1.51 | 0.62–3.68 | 0.362 | ||||||
| Mildly deprived | 1.20 | 0.44–3.26 | 0.721 | ||||||
| Lowest deprived | 1.29 | 0.41–4.03 | 0.665 | ||||||
| Intercept | 0.24 | 0.14–0.40 | <0.001 | 0.20 | 0.11–0.35 | <0.001 | 0.07 | 0.02–0.21 | <0.001 |
| Random effect | |||||||||
| Community-level variance | 0.73 | 0.58–0.90 | 0.74 | 0.59–0.91 | 0.67 | 0.54–0.84 | |||
| State-level variance | 0.38 | 0.22–0.65 | 0.39 | 0.23–0.68 | 0.20 | 0.11–0.36 | |||
| VPC: child-level | 0.749 | 0.74 | 0.79 | ||||||
| VPC: community-level | 0.165 | 0.167 | 0.161 | ||||||
| VPC: state-level | 0.09 | 0.089 | 0.048 | ||||||
| ICC%: community-level | 25.18 | 21–30 | 25.57 | 21–30 | 21.00 | 17–25 | |||
| ICC%: state-level | 8.65 | 5–14 | 8.90 | 5–14 | 4.82 | 3–8 | |||
| Model fit statistics | |||||||||
| Log-likelihood | −4818.16 | −4804.56 | −4763.47 | ||||||
| AIC | 9722.32 | 9701.12 | 9646.94 | ||||||
| BIC | 10,029.14 | 10,029.35 | 10,075.06 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. Individual and Contextual Factors Associated with Malaria among Children 6–59 Months in Nigeria: A Multilevel Mixed Effect Logistic Model Approach. Int. J. Environ. Res. Public Health 2021, 18, 11234. https://doi.org/10.3390/ijerph182111234
Obasohan PE, Walters SJ, Jacques R, Khatab K. Individual and Contextual Factors Associated with Malaria among Children 6–59 Months in Nigeria: A Multilevel Mixed Effect Logistic Model Approach. International Journal of Environmental Research and Public Health. 2021; 18(21):11234. https://doi.org/10.3390/ijerph182111234
Chicago/Turabian StyleObasohan, Phillips Edomwonyi, Stephen J. Walters, Richard Jacques, and Khaled Khatab. 2021. "Individual and Contextual Factors Associated with Malaria among Children 6–59 Months in Nigeria: A Multilevel Mixed Effect Logistic Model Approach" International Journal of Environmental Research and Public Health 18, no. 21: 11234. https://doi.org/10.3390/ijerph182111234
APA StyleObasohan, P. E., Walters, S. J., Jacques, R., & Khatab, K. (2021). Individual and Contextual Factors Associated with Malaria among Children 6–59 Months in Nigeria: A Multilevel Mixed Effect Logistic Model Approach. International Journal of Environmental Research and Public Health, 18(21), 11234. https://doi.org/10.3390/ijerph182111234







