Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review
Abstract
:1. Introduction
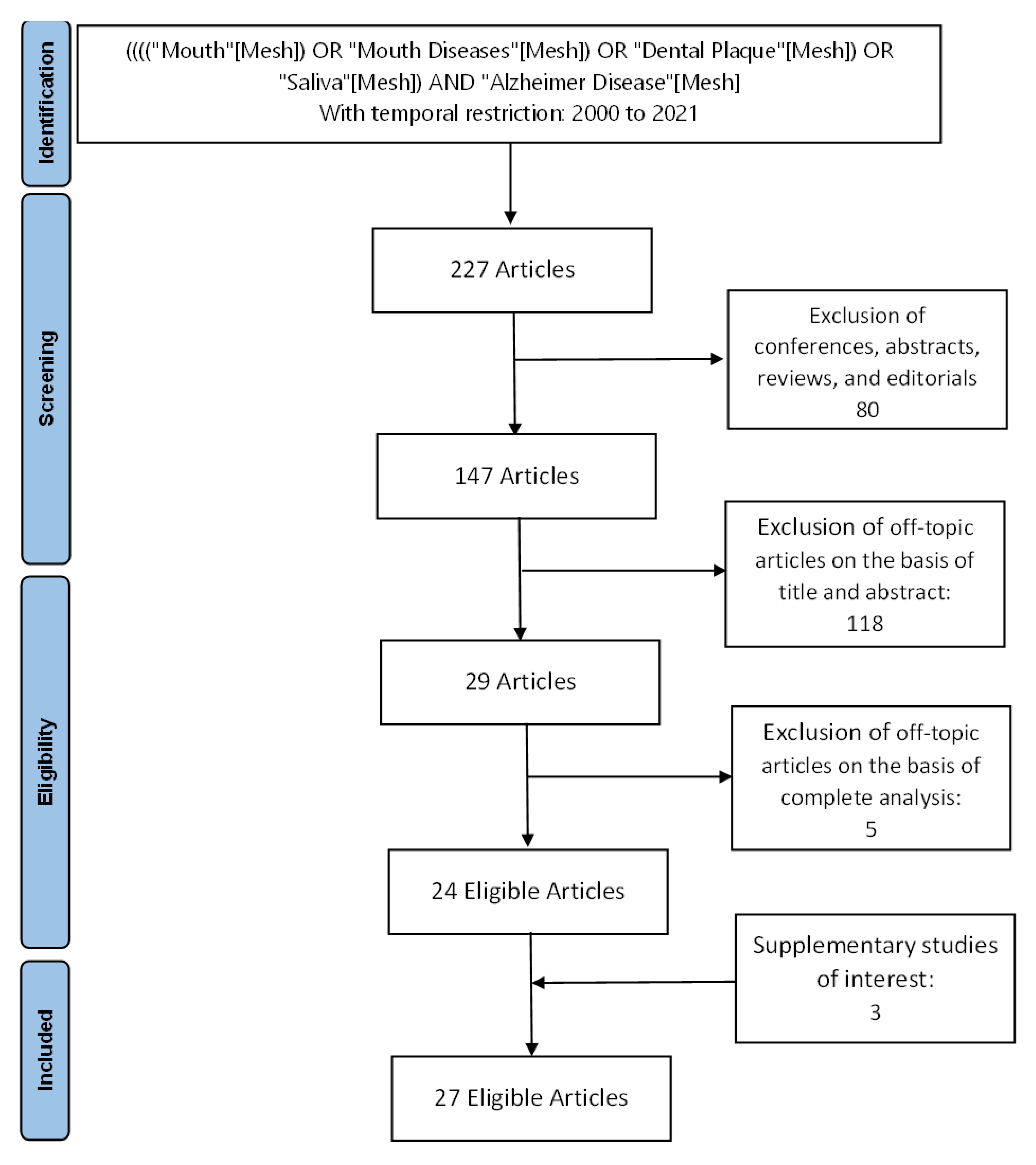
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. Study Selection
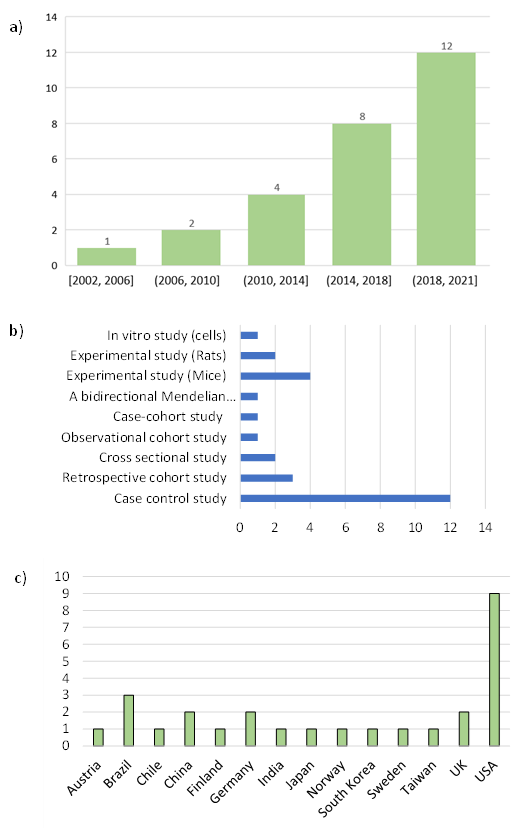
3.2. Study Characteristics
3.3. Clinical Oral Perturbations and Alzheimer’s Disease
3.4. Oral Dysbiosis and Alzheimer’s Disease
3.5. Pathophysiological Evidence between the Microbiome and Alzheimer’s Disease
3.6. Oral Microbiota and Immuno-Inflammation in Alzheimer Disease
3.7. Alzheimer’s Disease Risk Factor and Therapeutic Perspectives in Relation to the Oral Microbiome
4. Discussion
4.1. Oral Microbiome and Alzheimer’s Disease
4.2. Possible Mechanisms of the Relationship between the Oral Microbiome and Alzheimer’s Disease
4.3. Inflammation, Overnutrition and Lipotoxicity
4.4. Oral Microbiota Modulation and Alzheimer’s Disease Therapy
5. Limitations and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 1, 63–75. [Google Scholar] [CrossRef]
- Adav, S.S.; Sze, S.K. Insight of brain degenerative protein modifications in the pathology of neurodegeneration and dementia by proteomic profiling. Mol. Brain. 2016, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leandro, P.; Gomes, C.M. Protein misfolding in conformational disorders: Rescue of folding defects and chemical chaperoning. Mini Rev. Med. Chem. 2008, 8, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.; Ha-Duong, T. Exploring the Alzheimer amyloid-β peptide conformational ensemble: A review of molecular dynamics approaches. Peptides 2015, 69, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ellisdon, A.M.; Bottomley, S.P. The role of protein misfolding in the pathogenesis of human diseases. IUBMB Life 2004, 56, 119–123. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Swerdlow, R.H. Amyloid precursor protein processing and bioenergetics. Brain Res. Bull. 2017, 133, 71–79. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Alonso, A.C.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 1994, 91, 5562–5566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, A.C.; Grundke-Iqbal, I.; Iqbal, K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 1996, 2, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, J.T.; Tian, Y.; Tan, L. Epidemiology and etiology of Alzheimer’s disease: From genetic to non-genetic factors. Curr. Alzheimer Res. 2013, 10, 852–867. [Google Scholar] [CrossRef]
- Pan, C.C.; Chu, C.S.; Chen, C.L.; Chuang, Y.C.; Chen, N.C. Factors Affecting Rapid Cognitive Decline in Patients with Alzheimer’s Disease: A Longitudinal Follow-Up Study. Int. J. Environ. Res. Public Health 2021, 18, 8576. [Google Scholar] [CrossRef]
- Chantanachai, T.; Sturnieks, D.L.; Lord, S.R.; Payne, N.; Webster, L.; Taylor, M.E. Risk factors for falls in older people with cognitive impairment living in the community: Systematic review and meta-analysis. Ageing Res. Rev. 2021, 25, 101452. [Google Scholar]
- Profennoa, L.A.; Porsteinsson, A.P.; Faraonea, V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry. 2010, 67, 505–512. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; Barrios, R.; Santana, S. Association between periodontitis and amyloid β peptide in elderly people with and without cognitive impairment. J. Periodontol. 2017, 88, 1051–1058. [Google Scholar] [CrossRef]
- Kamer, A.R.; Pirraglia, E.; Tsui, W.; Rusinek, H.; Vallabhajosula, S.; Mosconi, L.; Yi, L.; McHugh, P.; Craig, R.G.; Svetcov, S.; et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging. 2015, 36, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugnani, S.; Gugnani, N. Tooth loss in Periodontitis: How valuable are the predictors? Evid. Based Dent. 2020, 21, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Pandey, A.; Deepa, D.; Asthana, A.K. C-Reactive Protein (CRP) and its Association with Periodontal Disease: A Brief Review. J. Clin. Diagn. Res. 2014, 8, 21–24. [Google Scholar]
- Cerajewska, T.L.; Davies, M.; West, N.X. Periodontitis: A potential risk factor for Alzheimer’s disease. Br. Dent. J. 2015, 218, 29–34. [Google Scholar] [CrossRef]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [Green Version]
- Paul, O.; Arora, P.; Mayer, M.; Chatterjee, S. Inflammation in Periodontal Disease: Possible Link to Vascular Disease. Front. Physiol. 2021, 11, 609614. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.; Chang, J. Association of chronic periodontitis on Alzheimer’s disease or vascular dementia. J. Am. Geriatr. Soc. 2019, 67, 1234–1239. [Google Scholar] [CrossRef] [Green Version]
- Ojeda, J.; Ávila, A.; Vidal, P.M. Gut Microbiota Interaction with the Central Nervous System throughout Life. J. Clin. Med. 2021, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Schächtle, M.A.; Rosshart, S.P. The Microbiota-Gut-Brain Axis in Health and Disease and Its Implications for Translational Research. Front. Cell Neurosci. 2021, 15, 698172. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooks, K.B.; Konsman, J.P.; O’Malley, M.A. Microbiota-gut-brain research: A critical analysis. Behav. Brain Sci. 2018, 42, 60. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, A.; Tognoni, C.M.; Yaghmoor, W.; Marghalani, A.; Stephens, D.; Ahn, J.Y.; Carreras, I.; Dedeoglu, A. Microglial response to experimental periodontitis in a murine model of Alzheimer’s disease. Sci. Rep. 2020, 10, 18561. [Google Scholar] [CrossRef]
- De Oliveira Araújo, R.; Villoria, G.E.M.; Luiz, R.R.; Esteves, J.C.; Leão, A.T.T.; Feres-Filho, E.J. Association between periodontitis and Alzheimer’s disease and its impact on the self-perceived oral health status: A case-control study. Clin. Oral Investig. 2021, 25, 555–562. [Google Scholar] [CrossRef] [PubMed]
- De Souza Rolim, T.; Fabri, G.M.C.; Nitrini, R.; Anghinah, R.; Teixeira, M.J.; de Siqueira, J.T.T.; Cestari, J.A.F.; de Siqueira, S.R.D.T. Oral Infections and Orofacial Pain in Alzheimer’s Disease: A Case-Control Study. J. Alzheimer’s Dis. 2014, 38, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Syrjälä, A.-M.H.; Ylöstalo, P.; Ruoppi, P.; Komulainen, K.; Hartikainen, S.; Sulkava, R.; Knuuttila, M. Dementia and Oral Health among Subjects Aged 75 Years or Older. Gerodontology 2012, 29, 36–42. [Google Scholar] [CrossRef]
- Holmer, J.; Eriksdotter, M.; Schultzberg, M.; Pussinen, P.J.; Buhlin, K. Association between periodontitis and risk of Alzheimer’s disease, mild cognitive impairment, and subjective cognitive decline: A case-control study. J. Clin. Periodontol. 2018, 45, 1287–1298. [Google Scholar] [CrossRef]
- Martande, S.S.; Pradeep, A.R.; Singh, S.P.; Kumari, M.; Suke, D.K.; Raju, A.P.; Naik, S.B.; Singh, P.; Guruprasad, C.N.; Chatterji, A. Periodontal Health Condition in Patients with Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other. Demen. 2014, 29, 498–502. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Hossain, S.; El-Hajj, Z.W.; Weiss, J.; Zonderman, A.B. Clinical and Bacterial Markers of Periodontitis and Their Association with Incident All-Cause and Alzheimer’s Disease Dementia in a Large National Survey. J. Alzheimers Dis. 2020, 75, 157–172. [Google Scholar] [CrossRef]
- Chen, C.K.; Wu, Y.T.; Chang, Y.C. Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Jiao, B.; Liao, X.X.; Guo, L.N.; Yuan, Z.H.; Wang, X.; Xiao, X.W.; Zhang, X.Y.; Tang, B.S.; Shen, L. Analysis of Salivary Microbiome in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Rahming, S.; Prvulovic, D. Dental health in advanced age and Alzheimer’s Disease: A possible link with bacterial toxins entering the brain? Psychiatry Res. Neuroimaging 2018, 282, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Riviere, G.R.; Riviere, K.H.; Smith, K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 2002, 17, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Watanabe, K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration, and amyloid beta production in wild type mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef] [Green Version]
- Noble, J.M.; Scarmeas, N.; Celenti, R.S.; Elkind, M.S.V.; Wright, C.B.; Schupf, N.; Papapanou, P.N. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS ONE 2014, 9, e114959. [Google Scholar] [CrossRef] [Green Version]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas Gingivalis in Alzheimer’s Disease Brains: Evidence for Disease Causation and Treatment with Small-Molecule Inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [Green Version]
- Yamada, C.; Akkaoui, J.; Ho, A.; Duarte, C.; Deth, R.; Kawai, T.; Nichols, F.; Lakshmana, M.K.; Movila, A. Potential Role of Phosphoglycerol Dihydroceramide Produced by Periodontal Pathogen Porphyromonas gingivalis in the Pathogenesis of Alzheimer’s Disease. Front. Immunol. 2020, 11, 591571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, F.; Wang, Z.; Qian, X.; Ji, Y.; Gong, L.; Ge, S.; Yan, F. Poor oral health conditions and cognitive decline: Studies in humans and rats. PLoS ONE 2020, 15, e0234659. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Zúñiga, J.; More, J.; Melgar-Rodríguez, S.; Jiménez-Unión, M.; Villalobos-Orchard, F.; Muñoz-Manríquez, C.; Monasterio, G.; Valdés, J.L.; Vernal, R.; Paula-Lima, A. Alzheimer’s Disease-Like Pathology Triggered by Porphyromonas gingivalis in Wild Type Rats Is Serotype Dependent. Front. Immunol. 2020, 11, 588036. [Google Scholar] [CrossRef]
- Hayashi, K.; Hasegawa, Y.; Takemoto, Y.; Cao, C.; Takeya, H.; Komohara, Y.; Mukasa, A.; Kim-Mitsuyama, S. Continuous intracerebroventricular injection of Porphyromonas gingivalis lipopolysaccharide induces systemic organ dysfunction in a mouse model of Alzheimer’s disease. Exp. Gerontol. 2019, 120, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leblhuber, F.; Huemer, J.; Steiner, K.; Gostner, J.M.; Fuchs, D. Knock-on effect of Continuous intracerebroventricular disease? Wien Klin. Wochenschr. 2020, 132, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry: Novel perspectives on brain disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Kamer, A.R.; Craig, R.G.; Pirraglia, E.; Dasanayake, A.P.; Norman, R.G.; Boylan, R.J.; Nehorayoff, A.; Glodzik, L.; Brys, M.; de Leon, M.J. TNF-Alpha and Antibodies to Periodontal Bacteria Discriminate between Alzheimer’s Disease Patients and Normal Subjects. J. Neuroimmunol. 2009, 216, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Cestari, J.A.; Fabri, G.M.; Kalil, J.; Nitrini, R.; Jacob-Filho, W.; De Siqueira, J.T.; Siqueira, S.R. Oral Infections and Cytokine Levels in Patients with Alzheimer’s Disease and Mild Cognitive Impairment Compared with Controls. J. Alzheimers Dis. 2016, 52, 1479–1485. [Google Scholar] [CrossRef] [Green Version]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef]
- Laugisch, O.; Johnen, A.; Maldonado, A.; Ehmke, B.; Bürgin, W.; Olsen, I.; Potempa, J.; Sculean, A.; Duning, T.; Eick, S. Periodontal Pathogens and Associated Intrathecal Antibodies in Early Stages of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 66, 105–114. [Google Scholar] [CrossRef]
- Sims, R.; Hill, M.; Williams, J. The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 2020, 23, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tabrett, A.; Horton, M.W. The influence of host genetics on the microbiome. F1000Research 2020, 9, 84. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Richmond, R.C.; Chen, Y.; Mai, X.M. Mixed evidence for the relationship between periodontitis and Alzheimer’s disease: A bidirectional Mendelian randomization study. PLoS ONE 2020, 15, e0228206. [Google Scholar] [CrossRef]
- Song, X.; Chen, J.; Hou, Z.; Xie, N. Antimicrobial therapy and the potential mechanisms in Alzheimer’s disease. Neurosci. Lett. 2021, 741, 135464. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.S.; Chu, C.H.; Young, F.Y.F. Oral Health and Care for Elderly People with Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2020, 17, 5713. [Google Scholar] [CrossRef]
- Soscia, S.J.; Kirby, J.E.; Washicosky, K.J.; Tucker, S.M.; Ingelsson, M.; Hyman, B.; Burton, M.A.; Goldstein, L.E.; Duong, S.; Tanzi, R.E.; et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS ONE 2010, 5, e9505. [Google Scholar] [CrossRef] [PubMed]
- Bulgart, H.R.; Neczypor, E.W.; Wold, L.E.; Mackos, A.R. Microbial involvement in Alzheimer disease development and progression. Mol. Neurodegener. 2020, 15, 42. [Google Scholar] [CrossRef]
- Gareau, M.G. Cognitive Function and the Microbiome. Int. Rev. Neurobiol. 2016, 131, 227–246. [Google Scholar] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. Inflammation and Its Role in Regeneration and Repair. Circ. Res. 2019, 124, 1166–1168. [Google Scholar] [CrossRef]
- Levin, B.R.; Baquero, F.; Ankomah, P.P.; Mc Call, I.C. Phagocytes, Antibiotics, and Self-Limiting Bacterial Infections. Trends Microbiol. 2017, 25, 878–892. [Google Scholar] [CrossRef]
- Levin, B.R.; Antia, R. Why we don’t get sick: The within-host population dynamics of bacterial infections. Science 2001, 292, 1112–1115. [Google Scholar] [CrossRef]
- Cunliffe, J. Intentional pathogen killing--or denial of substrate? Scand. J. Immunol. 2007, 66, 604–609. [Google Scholar] [CrossRef]
- Horliana, A.C.; Chambrone, L.; Foz, A.M.; Artese, H.P.; Rabelo Mde, S.; Pannuti, C.M.; Romito, G.A. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: A systematic review. PLoS ONE 2014, 9, e98271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, R.D. Bacterial Translocation from the Gastrointestinal Tract; Springer: Boston, MA, USA, 1999; pp. 11–50. [Google Scholar]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Maurer, P.; Hoffman, E.; Mast, H. Bacterial meningitis after tooth extraction. Br. Dent. J. 2009, 206, 69–71. [Google Scholar] [CrossRef]
- Kim, K.S. Microbial translocation of the blood-brain barrier. Int. J. Parasitol. 2006, 36, 607–614. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Exp. Biol. Med. 2018, 243, 159–165. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Harding, A.; Chukkapalli, S.; Olsen, I.; Kesavalu, L.; Crean, S. Apolipoprotein E Related Co-Morbidities and Alzheimer’s Disease. J. Alzheimers Dis. 2016, 51, 935–948. [Google Scholar] [CrossRef] [Green Version]
- Humphries, D.L.; Scott, M.E.; Vermund, S.H. Pathways Linking Nutritional Status and Infectious Disease: Causal and Conceptual Frameworks; Humana Press: Totowa, NJ, USA, 2020; pp. 3–22. [Google Scholar]
- Yu, B.; Yu, L.; Klionsky, D.J. Nutrition acquisition by human immunity, transient overnutrition and the cytokine storm in severe cases of COVID-19. Med. Hypotheses 2021, 155, 110668. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Routier, A.; Blaizot, A.; Agossa, K.; Dubar, M. What do we know about the mechanisms of action of probiotics on factors involved in the pathogenesis of periodontitis? A scoping review of in vitro studies. Arch. Oral Biol. 2021, 129, 105196. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Stultz, J.; Abernethy, A.D.; Goodson, J.M. Phototargeting human periodontal pathogens in vivo. Lasers Med. Sci. 2015, 30, 943–952. [Google Scholar] [CrossRef]
- Soukos, N.S.; Som, S.; Abernethy, A.D.; Ruggiero, K.; Dunham, J.; Lee, C.; Doukas, A.G.; Goodson, J.M. Phototargeting oral black-pigmented bacteria. Antimicrob. Agents Chemother. 2005, 49, 1391–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maitre, Y.; Mahalli, R.; Micheneau, P.; Delpierre, A.; Guerin, M.; Amador, G.; Denis, F. Pre- and Probiotics Involved in the Modulation of Oral Bacterial Species: New Therapeutic Leads in Mental Disorders? Microorganisms 2021, 9, 1450. [Google Scholar] [CrossRef]
- Godoy, J.A.; Rios, J.A.; Zolezzi, J.M.; Braidy, N.; Inestrosa, N.C. Signaling pathway cross talk in Alzheimer’s disease. Cell Commun. Signal. 2014, 12, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Brennan, L.J.; Strauss, J. Cognitive impairment in older adults and oral health considerations: Treatment and management. Dent. Clin. N. Am. 2014, 58, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Garcia-Ptacek, S.; Religa, D.; Holmer, J.; Buhlin, K.; Eriksdotter, M.; Sandborgh-Englund, G. Dental care utilization in patients with different types of dementia: A longitudinal nationwide study of 58,037 individuals. Alzheimers Dement. 2018, 14, 10–19. [Google Scholar] [CrossRef]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Matthew, P. World Alzheimer Report 2015, The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Dis. Int. 2015, 87, 84. [Google Scholar]
- Martínez-Cué, C.; Rueda, N. Signalling Pathways Implicated in Alzheimer’s Disease Neurodegeneration in Individuals with and without Down Syndrome. Int. J. Mol. Sci. 2020, 21, 6906. [Google Scholar] [CrossRef] [PubMed]
| Reference(s) | Study Design | Objectives | Evaluated Factors | Mains Results and Limitations * |
|---|---|---|---|---|
| Riviere et al., 2002 [36] | Case control study N = 38/40 | Assessment of the oral treponema presence in the human brain, the hippocampus and the trigeminal ganglia in AD patients and controls. | T. denticola, Treponema amylovorum, Treponema maltophilum, T. medium, Treponema pectinovorum, Treponema socranskii, T. vincentii | Detection of oral Treponema in brainstem, cortex and trigeminal ganglia in human subjects with Alzheimer’s disease. AD patients had more different Treponema species in the brain than controls. Treponema brain colonisation significantly more frequent in patients with Alzheimer’s disease than in controls. * Possibility of a post-mortem contamination of tissues or assays |
| Kamer et al., 2009 [47] | Case-control study N = 18/16 | Evaluation of the difference in concentration of TNF α and antibodies to periodontal bacteria in serum of AD and normal controls. Investigation of the use of biomarkers (TNF α and antibodies against periodontal bacteria) for the diagnosis of AD) | A. actinomycetemcomitans, T. forsythia, P. gingivalis (IgG). TNF-α, IL-1β and IL-6 in plasma. APOE 4ε gene expression. | Higher expression of antibodies to periodontal bacteria and TNF α in serum of AD patients than in controls. These measures could contribute to the diagnosis of AD. No significant difference in cytokine levels between carriers of APOE 4ε and non-carriers * Small sample size, study design |
| Syrjälä et al., 2010 [29] | Case control study N = 76/278 | Analysis of the association between diagnosed dementia and oral health among an elderly population aged 75 years or older. | Diagnosis of dementia as Alzheimer’s disease, vascular dementia, dementia due to other general medical (DSM-IV), dementia with Lewy bodies (McKeith Criteria). Number of teeth with dental caries and with deep periodontal pockets (≥4mm), edentulousness, oral hygiene index. | Significant probability to have carious teeth, teeth with deep periodontal pockets and poor oral and denture hygiene in patient with Alzheimer’s disease and persons with other types of dementia compared with non-demented persons. * Study design, sample size, lack of an assessment of inter-examiner and intra-examiner reliability of the oral examinations. |
| Poole et al., 2013 [49] | Case control study N = 10/10 | Assessment of the presence of the major periodontal bacteria and/or their components in the brain tissue of people with and without dementia. | P. gingivalis, T. denticola, T. forsythia | Presence of P. gingivalis- LPS in AD brain with 12 h maximum postmortem delay. No evidence of P. gingivalis LPS in brain tissue of non-AD controls with a longer post-mortem time (up to 43 h). * Study design, small sample size. |
| De Souza Rolim et al., 2014 [28] | Case control study N = 29/30 | Assessment of mandibular function, orofacial pain and oral status in patients with mild AD compared to age- and sex-matched healthy subjects. | Temporomandibular disorders McGill Pain Questionnaire, DMFT index, plaque and gingival bleeding indexes (PI, BI), PPD, cementoenamel junction (CEJ) and (CAL) | Significant higher prevalence of orofacial pain, articular abnormalities in temporomandibular joints and periodontal infections in AD patients than in healthy controls. * Study design, sample size |
| Martande et al., 2014 [31] | Case control study N = 58/60 | Comparison of periodontal health status in people with and without AD from 50 to 80 years | Plaque index (PI), gingival index (GI), PPD, CAL), percentage bleeding on probing (% BOP). Degree of cognitive impairment by Mini-Mental State Examination (MMSE) | Significant differences in mean GI, PI, PD, CAL, and %BOP between different groups (Non AD, Mild, moderate and severe AD). Significant elevation of periodontal parameters in AD patients compared to non-AD patients. Deterioration of periodontal status with AD progression * Study design, sample size, no assessment of socio-economic status, education, changes in oral hygiene regimes as dementia progresses, dietary changes and access to dental care. |
| Noble et al., 2014 [38] | Case-cohort study N = 110/109 | Study of the association between pre-morbid levels of serum IgG antibodies to selected periodontal microbiota and risk for incident AD. | P. gingivalis, T. forsythia, A. actinomycetemcomitans, T. denticola, C. rectus, Eubacterium nodatum, Actinomyces naeslundii (IgG) | The serum concentration of immunoglobulin G (IgG) against common periodontal bacteria represents a risk factor for developing AD. High concentrations of serum IgG associated to Actinomyces naeslundii was associated to an increased risk for developing incident AD. * Sample size, Possibility of reverse causality due to the lack of exclusion of patients with mild cognitive impairment (levels of antibodies to the periodontal microbiota may have been affected by cognitive impairment). |
| Kamer et al., 2015 [15] | Cross sectional study N = 38 | Evaluation of the association between periodontal disease burden with the brain amyloid load in cognitively normal patients. | CAL, PPD, Bleeding on probing (BOP). Amyloid load in AD vulnerable brain areas (prefrontal cortex, middle frontal gyrus, lateral temporal lobe, inferior parietal lobule, and posterior cingulate cortex/precuneus) | Significant association between CAL and an increased amyloid load in vulnerable AD brain areas after adjusting for confounders (age, ApoE and smoking). No significant interaction between ApoE and periodontal measures. * Sample size, Limited generalisation of results due to the homogeneity of the study population, self-selected patient. |
| Cestari et al., 2016 [48] | Case control study N = 25/19/21 | Investigation of the prevalence of oral infections and blood levels of IL-1β, TNF-α, and IL-6 in patients with AD, mild cognitive impairment (MCI), and onn demented controls. | Serum level of IL-1β, IL-6, and TNF-α. | Patients with AD had significantly higher IL-6 levels than controls. Significant association between IL-6 and TNF-α in patients with AD or MCI and periodontitis *Study design, sample size |
| Ide et al., 2016 [19] | Observational cohort study N = 60 | Evaluation of the effects of periodontitis in patients with Alzheimer’s disease over a six-month follow-up. | P. gingivalis antibodies CRP, TNFα, IL 10 Cognitive scores (sMMSE and ADAS-COG) | No significant relationship between serum baseline P. gingivalis antibody levels and rates of cognitive decline. P. gingivalis antibody levels were associated with a fall in serum IL10 levels and an increase in serum TNFα levels over a six month follow up. * Small sample size and short term follow up |
| Chen et al., 2017 [33] | Retrospective cohort study N = 27 963 | Assessment of the risk of developing AD in patients with chronic periodontitis. | Chronic periodontitis and AD diagnosis (ICM 9). | No significant higher risk of developing AD in Patients with 1 year of CP exposure than those without CP. Significant higher risk of developing AD in Patients with 10 years of CP exposure than those without CP. Higher prevalence of hyperlipidaemia, depression, traumatic brain injury and co-morbiditiess in patients with CP had a than those in the unexposed cohort. * Possible underestimation of the incidence of CP or AD, No data regarding the severity of AD and education level. |
| Holmer et al., 2018 [30] | Case control study N = 154/76 | Assessment of the possible increase risk of mild cognitive impairment (MCI), subjective cognitive decline (SCD) and Alzheimer’s disease (AD) associated with periodontal disease. Investigation of the potential associations between common biofilm-induced dental diseases (dental caries and endodonticula), their sequelae (tooth loss) and cognitive impairment. | Periodontal status (oral hygiene, PPD, bleeding on pocket probing (BoP), suppuration, tooth mobility furcation involvment, marginal alveolar bone loss (MABL). Number of teeth, dental implants present, and dental caries. | Generalized marginal alveolar bone loss is associated with early cognitive impairment and AD. Poor oral health was more prevalent among cases than among healthy controls. * Study design, sample size, possible temporal bias with reverse causality (reduced cognitive function leads to poor oral health), no APOE genotyping |
| Ilievski et al., 2018 [37] | Experimental study (mice) N = 20 | Assessment of the neuropathological effects (production of extracellular Aβ42 and neurofibrillary tangles) of repeated chronic exposure to a periodontal pathogen. | P. gingivalis/Gingipain TNFα, IL1β, and IL6 Alzheimer’s disease-related genes expression (APP, BACE 1, PSEN1, ADAM10) | P. gingivalis /gingipain were detected in the hippocampus of mice infected with Pg. Significantly higher expression of the proinflammatory cytokine IL1β, IL6 and TNFα in the hippocampus of experimental mice than controls. Significant increase in the expression of APP and BACE1 in the experimental group compared to the control group. Significant decrease in the expression of ADAM10 in the experimental group compared to the control group. Detection of Phospho-Tau protein in the experimental but not in the control mice * No direct or indirect mechanisms to explain these changes could be identified. |
| Laugisch et al., 2018 [50] | Case control study N = 20/20 | Verification of the presence of periodontal pathogens and the intrathecal generation of pathogen-specific antibodies in patients with AD and with other forms of dementia (DEM-noAD). | Antibody levels against A. actinomycetemcomitans, T. denticola, T. forsythia, P. gingivalis in CSF and serum Total tau protein (T-tau) and amyloid-β (Aβ1-42) in CSF. Monocyte chemoattractant protein-1 (MCP-1/CCL2) | Periodontal pathogens may enter the brain and stimulate a local immune response. Significant association between T-tau levels in the AD group and serum levels of anti-P. gingivalis antibodies and MCP-1/CCL-2. * Sample size |
| Maurer et al., 2018 [35] | Case control study N = 20/20 | Evaluation of the possible link between Alzheimer’s dementia and bacterial infestation of the oral cavity. | A. actinomycetemcomitans, P. gingivalis, F. nucleatum | AD-patients showed higher bacterial load in dental plaque compared to controls. * Study design, sample size |
| Choi et al., 2019 [21] | Retrospective cohort study N = 262 349 | Determination of the association between chronic periodontitis (CP), AD and vascular dementia (VD) from the Korean National Health Insurance Service (NHIS) database, using a several covariates (smoking, alcohol consumption and physical activity). | CP diagnosis (CD-10 code K05.3) associated with at least one of the CP-related treatments. Prescription of dementia-related drugs as part of a diagnosis of AD (ICD-10 codes F00, G30) or VD (ICD-10 code F01) Age, sex, household income, smoking status, alcohol consumption, physical activity, body mass index, systolic blood pressure, fasting serum glucose, total cholesterol and Charlson Comorbidity Index | Chronic periodontitis patients had elevated risk for overall dementia (aHR = 1.06; 95% CI = 1.01–1.11, p = 0.042) and AD (aHR = 1.05; 95% CI = 1.00–1.11, p = 0.042) than non-chronic periodontitis participants. * Study design, Information on CP-related clinical index limited, definition of dementia based on drug reimbursement, no evaluation of level of education or apolipoprotein E (APOE) e4 genotype. |
| Dominy et al., 2019 [39] | Experimental study (mice) N = 140 | Assessment of the prevalence of P. gingivalis (Pg) in the brains of people with Alzheimer’s disease and possible Pg-dependent mechanisms of action in neurodegeneration and Alzheimer’s pathology. | P. gingivalis and Gingipaïn load in brain tissue | P. gingivalis and gingipains load in the brain play a significative role in the pathogenesis of AD. Reduction in P. gingivalis brain bacterial load and neuroinflammation as well as a blockage of Aβ 1–42 peptide production were obtained by gingipain inhibition. * Study design, sample size |
| Hayashi et al., 2019 [43] | Experimental study (Mice) N = 80 | Evaluation of the effects of brain exposure to Porphyromonas gingivalis-derived lipopolysaccharide (Pg-LPS) on cognitive impairment and organ dysfunction in an AD mouse model. | P. gingivalis LPS Morris water maze tests | No cognitive impairment could be associated with acute or continuous brain exposure to Pg-LPS. Continuous brain exposure to Pg-LPS triggered sarcopenia and heart damage in AD model mice. * Study design |
| Liu et al., 2019 [34] | Case control study N = 39/39 | Identification of differences in oral bacterial community composition between AD patients and healthy patients. Evaluation of the association between oral bacteria and AD severity Evaluation of differences in oral bacterial flora according to APOEε4 expression. | Alpha and beta diversity of salivary microbiota APOEε4 expression | Significant decrease of richness and diversity of salivary microbiota in patients with Alzheimer’s disease than in healthy controls. No bacteria associated with the severity of AD. Significant decrease in Actinobacillus and Actinomyces levels in patients with APOEε4 Abundance of Abiotrophia and Desulfomicrobium levels in patients with APOEε4(+) * Small sample size, no collection of dental plaque bacteria |
| Beydoun et al., 2020 [32] | Retrospective cohort study N = 6650 | Evaluation of the association of immune response (IgG) to periodontal pathogens with the incidence of dementia and AD mortality in middle-aged (>45 years) and elderly (>65 years) US adults. | Periodontal pathogens Immunoglobulin G (IgG): A. actinomycetemcomitans, P. gingivalis, T. forsythia, T. denticola, C. rectus, Eubacterium nodatum, P. intermedia, Prevotella nigrescens, Prevotella melaninogenica, F. nucleatum, Parvimonas micra, Selenomonas noxia, Eikenella corrodens, Capnocylophaga ochracea, Streptococcus intermedius, Streptococcus oralis, Streptococcus mutans, Vellonella Parvula, Actinomyces naeslundii. AD Mortality and Incidence Status. Clinical Attachment loss (CAL) and probing pocket depth (PPD) | AD incidence was linked to a composite of C. rectus and P. gingivalis. AD mortality risk was increased with composite loading highly on IgG for P. gingivalis, P. intermedia, Prevotella nigrescens, F. nucleatum, C. rectus, Streptococcus intermedius, Capnocylophaga Ochracea, and P. melaninogenica. Only a marginal association between incident AD risk and PPD was detected among men and older individuals * Study design |
| De Oliveira Araújo et al., 2021 [27] | Case control study N = 50/52 | Test the association between periodontitis and AD. Assessment of the possible negative impact of periodontal status on perceived oral health-related quality of life (OHRQoL) | PPD ≥ 5 mm, and CAL ≥ 5 mm Geriatric Oral Health Assessment Index (GOHAI) questionnaire. Socio-demographic data. | AD patients had fewer teeth, greater and a superior percentage of sites with plaque, calculus, and bleeding on probing than healthy controls. Significant association between periodontitis and AD after adjusting for age, gender, income and education. Periodontitis is associated with AD, but not with patients’ OHRQoL * Study design, sample size |
| Díaz-Zúñiga et al., 2020 [42] | Experimental study (Rats) N = 30 | Evaluation of the effects of short exposure to encapsulated strains of P. gingivalis on AD brain markers, neuroinflammation and cognitive decline in young rats. | P. gingivalis K1, K2, or K4 serotypes and the K1-isogenic non-encapsulated mutant (GPA). Oasis maze task. Cytokines (IL-1b, IL-4, IL-6, IL-10, TNF-α, IFN- γ). Aβ1-42 peptide and tau phosphorylation levels in hippocampus. | Significant increase of pro-inflammatory cytokines (IL-1b, IL-6, TNF-a, IFN-γ) in the hippocampus of rats infected with P. gingivalis encapsulated serotypes K1 and K2. None of these effects were observed in rats infected with the non-encapsulated bacterial strains. K1 or K2 P. gingivalis-infected rats displayed memory deficits, increased Aβ 1–42 levels, and Tau hyperphosphorylation in the hippocampus * Sample size, study design |
| Kantarci et al., 2020 [26] | Experimental study (Mice) N = 30 | Evaluation of the impact of experimentally induced periodontal disease in a mouse model of AD on the inflammatory process in the brain and microglia function. | Alveolar bone loss Insoluble Aβ40 and Aβ442 peptide. Microglial markers in brain (Iba1). Cytokine and Chemokine in CSF (GM-CSF, IFN-γ, IL-1β, IL-6, IL-10, TNF-α, MCP 1). | Periodontal disease increases bone loss in AD-modelled (5xFAD) and control wild-type (WT) mice. Alveolar bone loss is higher in 5xFAD than in WT at baseline. No significant difference in alveolar bone loss after the induction of experimental PD between 5xFAD and WT mice The mean level of insoluble Aβ42, but not Aβ40, was significantly higher in 5xFAD mice with induced PD than in 5xFAD mice without induced PD. Decline in microglial markers (Iba1 )in the proximity of Aβ plaques in 5xFAD mice with periodontal disease compared to those without periodontal disease. Induced periodontal disease reduced IL-10 in 5xFAD mice. Higher unresolved inflammation in the brain of 5xFAD mice before and after induction of periodontal disease compared to WT controls based on the ratio of TNF-α to IL-10. * Study design, Sample size, no characterization of the bacterial flora |
| Leblhuber et al., 2020 [44] | Cross sectional study N = 20 | Evaluation of the effects of chronic low-grade immune activation by salivary periodontopathogen bacteria in AD patients | A. actinomycetemcomitans, T. denticola, T. forsythia, P. gingivalis, P. intermedia in salivary. MMSE and CDT. Serum concentrations of neopterin and of tryptophan. | Significant association between the salivary presence of P. gingivalis and lower MMSE and lower tendency to CDT. Significant lower neopterin concentrations associated with the presence of T. denticola in AD patient saliva. Significant lower kynurenine concentrations associated with the presence of T. forsythia in AD patient saliva. * Sample size, study design, no evaluation of the ApoE status |
| Sun et al., 2020 [53] | A bidirectional Mendelian randomization study N = 4924 /7301 | Examination of the potential causal relationship between AD and chronic periodontitis bidirectionally in the population of European ancestry. | Single-nucleotide polymorphisms associated with periodontitis and AD. | No evidence for a bidirectional genetic relationship between AD and periodontitis from analysis of Genome-Wide Association Studies data. * Mendelian randomisation does not assess the impact of the duration of periodontal disease on the risk of developing AD, The genetic tool used to define PD may not be suitable for detecting the causal link between PD and AD. |
| Yamada et al., 2020 [40] | In vitro study (cells) | Evaluation of the influence of phosphoglycérol dihydrocéramide (PGDHC) produced by P. gingivalis on hallmark findings in AD. | Concentrations of PGDHC, Aβ peptide, Protein Tau and senescence-associated secretory phenotype (β-galactosidase, cathepsin B, cysteine, TNF-α and IL-6 pro-inflammatory cytokines) | Exposure to PGDHC, but not to Pg-LPS, significantly enhances secretion of Aβ peptide in a dose-dependent manner from CHO-7WD101 cells in vitro 1. PGDHC also significantly Induces the Site-Specific Phosphorylation of protein Tau in a dose-dependent manner in SH-SY5Y2 cells compared to the control cells. PGDHC or Pg-LPS elevated expression of β-galactosidase, cathepsin B, cysteine, TNF-α and IL-6 pro-inflammatory cytokines in SH-SY5Y cells. * In vitro study |
| Zhang et al., 2020 [41] | Experimental study (Rats) N = 24 | Examination of the association between oral health and cognition in humans and rats. | Morris Water Maze test Concentrations of Aβ1–40 peptide, TNF-α, IL-1, IL-6 and CRP in the hippocampus and the cerebral cortex | No significant differences in the Morris Water Maze test between AD rats and AD rats with induced periodontitis. Significant elevation of Aβ1-40 concentration in the cerebral cortex in AD rats with periodontitis than in AD rats. TNF-α levels in the hippocampus of the AD with periodontitis group were significantly higher than those of the AD and the control group IL-1 levels in the cerebral cortex of the AD with periodontitis group were significantly higher than those of the AD group and the control group. IL-6 levels in the hippocampus and the cerebral cortex of the AD with periodontitis groups were significantly higher than those of the AD and the control group. * Study design, sample size, no detection of periodontal pathogens in the brain. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maitre, Y.; Mahalli, R.; Micheneau, P.; Delpierre, A.; Amador, G.; Denis, F. Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11157. https://doi.org/10.3390/ijerph182111157
Maitre Y, Mahalli R, Micheneau P, Delpierre A, Amador G, Denis F. Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(21):11157. https://doi.org/10.3390/ijerph182111157
Chicago/Turabian StyleMaitre, Yoann, Rachid Mahalli, Pierre Micheneau, Alexis Delpierre, Gilles Amador, and Frédéric Denis. 2021. "Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 21: 11157. https://doi.org/10.3390/ijerph182111157
APA StyleMaitre, Y., Mahalli, R., Micheneau, P., Delpierre, A., Amador, G., & Denis, F. (2021). Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. International Journal of Environmental Research and Public Health, 18(21), 11157. https://doi.org/10.3390/ijerph182111157






