Abstract
Despite growing knowledge of the adverse effects of cigarette smoking on general health, smoking is one of the most widely prevalent addictions around the world. Globally, about 1.1 billion smokers and over 8 million people die each year because of cigarette smoking. Smoking acts as a source for a variety of oral and systemic diseases. Various periodontal issues such as increased pocket depth, loss of alveolar bone, tooth mobility, oral lesions, ulcerations, halitosis, and stained teeth are more common among smokers. This systematic review was conducted according to the guidelines from PRISMA, and research articles were retrieved from the Web database sources on 31 May 2021. The quality of research articles was ensured by the type of evidence from combined schema incorporating as schema-13 evidence type description, Cochrane health promotion and public health field (CHPPHF), and the health gains notation framework-14 screening question for quality assessment of qualitative and quantitative studies. Smokers have been found to have bleeding on probing, periodontal pockets, and clinical attachment loss compared to nonsmokers. Oral and respiratory cancers are among the most lethal known diseases caused by cigarette smoking and other commonly occurring sequelae such as stained teeth, periodontal diseases, etc.
1. Introduction
Oral diseases appear to be a global problem that should be addressed as a matter of global health concern. Oral health issues include various behavioral and social features such as habits, oral health knowledge, practices, availability, modifiable risk factors, and accessibility to oral health treatments [1]. Health is considered as a significant factor in making life valuable [2]. In general, lifestyles and behavioral patterns are continuously changing, making people more susceptible to oral disorders. Common preventable risk factors for oral diseases include consuming great amounts of sugary food and alcohol and smoking excessively [3]. Back in 2015, untreated oral disorders crippled over half of the world’s population (age-standardized prevalence: 48.0 percent), affecting 3.5 million individuals worldwide [4,5].
Oral and orofacial problems can affect children and adolescents, affecting physical functioning and psychosocial well-being [6]. One of the scientific theories used to influence human health-related behaviors is the knowledge, attitude, and practices (KAP) theory. According to the KAP theory, healthy knowledge is the foundation for developing an optimistic and healthy lifestyle, attitudes are the motivating factor behind changing behavior, and the goal is to promote oral health [7]. This is why oral health professionals play a critical role in disease prevention and diagnosis through screening and raising awareness [8]. Recently, a shift of focus in health care has been noticed, signaling a transition from biological to a more complete and broader biopsychosocial concept of health [9].
The oral cavity is a speculum for a person’s current health issues. Some of the modifiable risk factors for poor oral hygiene include cigarette smoking, betel quid chewing, and alcohol consumption. Despite the fact that there is growing knowledge of the adverse effects of cigarette smoking on general health, smoking is one of the widely prevalent addictions around the world [10]. Globally, about 1.1 billion smokers and over 8 million people die each year due to cigarette smoking [11]. Smoking acts as a source for a variety of diseases, including cardiovascular diseases (CVD), chronic obstructive pulmonary diseases (COPD), cancer, and periodontal disease (POD), as one of the five top risk factors for the global burden of the disease [12,13,14]. According to the alcohol and drugs survey, 15% of people currently smoke cigarettes, with 17% of men and 13% of women. Teenagers aged 15–19 years have been found to smoke at an estimated rate of 8%, with 10% of males and 6% of females being current smokers. The frequency was 16 percent among people aged 20–24 years and 25 years and older [15].
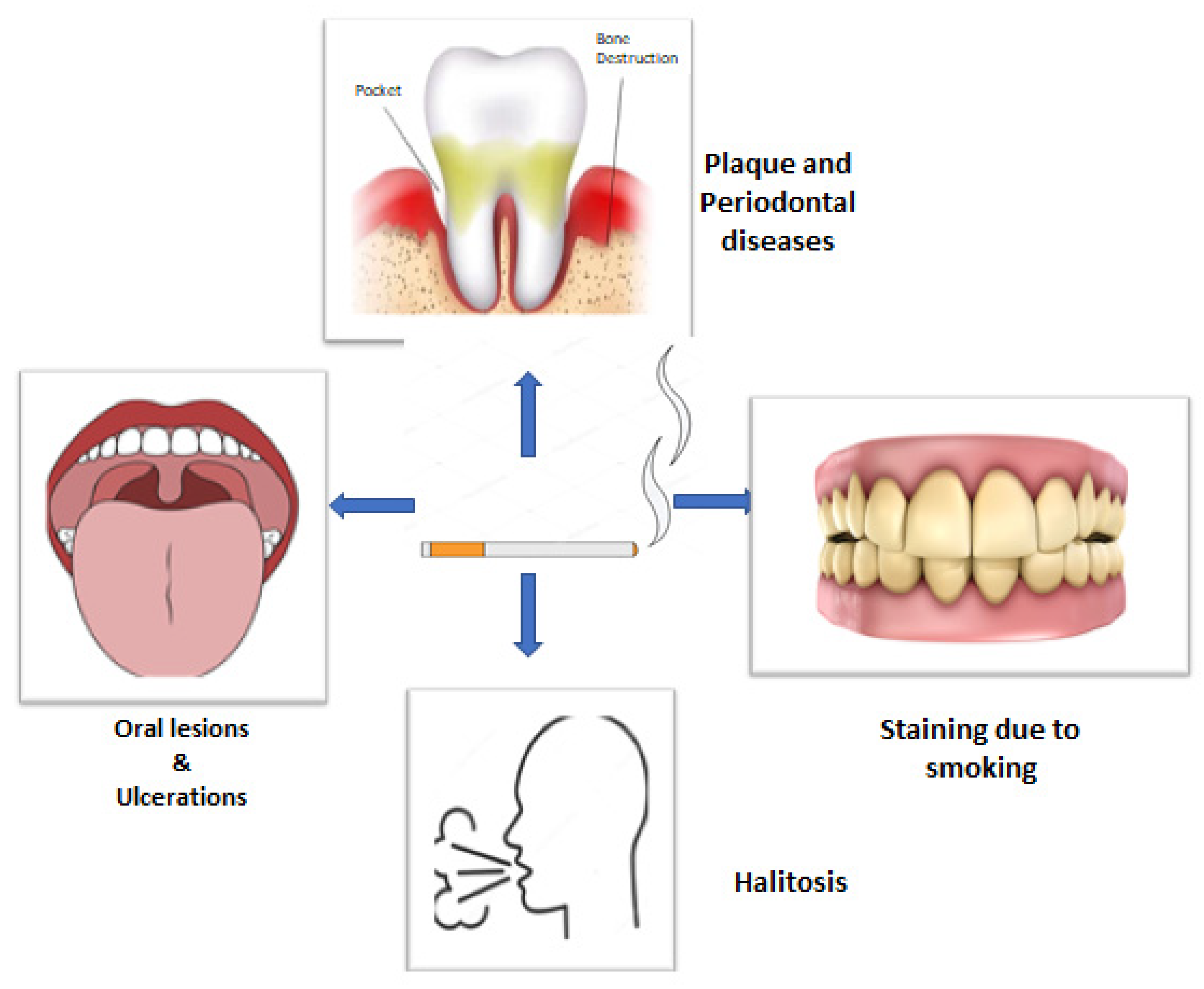
Tobacco smoking has numerous and well-documented negative consequences. The oral cavity is the first to get exposed to cigarette smoke, wherein the soft and hard tissues come in direct contact, making it the first area of confrontation [16]. Tobacco smoking, particularly in the form of cigarettes, has been proved to be a significant risk factor for periodontitis (Figure 1) [17]. Other than plaque, smoking has been identified as an important risk factor for POD. It also affects the prevalence of POD, severity, progression, and treatment response. According to epidemiological research, smokers have a much higher risk of POD than nonsmokers, and the increased risk is proportionate to the duration and rate of smoking [18,19]. Various gingival and periodontal issues such as gingivitis, increased pocket depth, loss of alveolar bone, tooth mobility, oral lesions, ulcerations, halitosis, and stained teeth are more common among smokers [20].
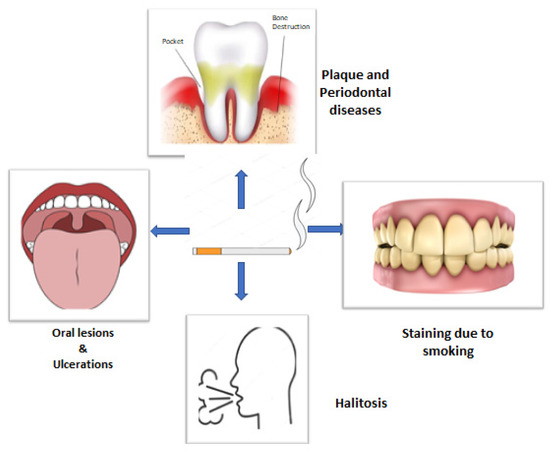
Figure 1.
Diagrammatic presentation of possible effects of smoking on oral health.
According to a meta-analysis, exposure to cigarette smoke in the environment relates to a considerably increased risk of lung cancer [21]. Cigarette smoking has also been linked to several other oral cancers. Kumar, A. et al. presented a clinic-pathological investigation that showed that 29.4% of people with established oral cancer cases chewed only tobacco, 25.5% only smoked, 42.2% chewed both types of tobacco (smoke and nonsmokers), and 2.9% did not chew tobacco. 83.3% of those who solely chewed tobacco had oral cavity malignancies, with 6.7% having malignancies of the oropharynx and hypopharynx. Of those who only smoked tobacco, 69.2% of individuals had the disease [22]. This predicts that there is a high chance of developing cancer regardless of how you use tobacco (smoking, chewing, etc.).
Rationale: The most critical risk factor associated with the onset of various gingival and periodontal diseases is tobacco smoking. It reduces the quality of life of patients and poses a risk to oral health. It has been demonstrated that oral health among smokers is compromised in comparison to nonsmokers. Thus, this study is aimed at reviewing the literature to evaluate the effect of smoking on oral health.
Objectives: In this systematic review, we aim to examine the effects of cigarette smoking on oral health, present the major oral diseases caused by cigarette smoking, and determine if there is any possibility of bacterial or fungal infections among smokers.
2. Material and Methods
2.1. Protocol and Registration
This systematic review was conducted according to the guidelines from PRISMA (http://www.prisma-statement.org accessed on 4 October 2021). Research articles were retrieved from the Web database sources on 31 May 2021. The study has been registered on PROSPERO with registration number CRD42021273462. Initially, articles were assessed from MDPI, PubMed, Scopus, and Web of Science (WOS).
2.2. Research Questions
The research questions of this systematic review are as follows: What are the effects of cigarette smoking on oral health? What are the major oral diseases caused by cigarette smoking? Is there any possibility of bacterial or fungal infections among smokers?
2.3. Data Sources
Relevant articles for inclusion in the review were found through a search of electronic databases. Keywords included “smoking and or oral health”, “cigarette smoking”, “Smoking effects and or oral health”, and “smoking and or tobacco use”.
2.4. Search Strategies
Electronic databases were searched for articles according to the selected keywords such as smoking, cigarette smoking, and tobacco use following the MeSH strategy, which evaluates the effects of cigarette smoking on oral health, published between 2011 to 2021. The number of articles retrieved from each database is shown in Table 1.

Table 1.
Search strategy for study-related articles.
2.5. Study Selection and Criteria for Eligibility of Articles
A detailed search of research articles from Web sources was performed to find out the studies that examined the effects of cigarette smoking on oral health and were published between 2011 to 2021. Two researchers assessed possibly relevant research papers against the previously specified inclusion and exclusion criteria to validate the selection approach, as shown in Table 2.

Table 2.
Criteria for inclusion and exclusion of research studies.
2.6. Quality Assessment
The quality of research articles was ensured by the type of evidence from combined schema incorporating as schema-13 evidence type description, Cochrane health promotion and public health field (CHPPHF), and the health gains notation framework-14 screening question for quality assessment of qualitative and quantitative studies.
The CHPPHF quality assessment tool was utilized to assess the quality of articles. This instrument scored the criteria for allocation bias, selection bias, intervention integrity, blinding, withdrawals and dropouts, confounding, data collecting procedures, and statistical analysis for internal and external validity.
Qualitative research was evaluated and scored for quality using questions adapted from the CHPPHF’s Critical Appraisal Skills Programme. There were 19 Type V evidence articles among 3696 articles that have not been further rated for quality. The studies were categorized as weak, moderate, or strong evidence based on their quality.
The quality of published evidence was categorized as I, II-1, II-2, II-3, and III. The articles that had at least evidence from the one proper RCT were categorized as “I”. Articles with data from well-designed controlled trials that were not randomized were classed as “II-1”. Evidence from well-designed case-control analytic investigations or cohort, ideally by many centers or research groups, was graded as “II-2”. This included evidence from time or place comparisons with or without the intervention. “II-3” was used to describe dramatic results from uncontrolled studies. While renowned experts’ judgments were based on clinical experience, descriptive research or expert committee reports were designated as “III.”
A, B, C, D, and E were the categorized grades based on recommendations. Research having sufficient evidence to support the suggestion that the condition is included in a periodic health examination (PHE) were categorized as “A”. Reports with sufficient evidence to recommend that the condition be specially examined in a PHE were categorized as “B”. Reports that were given the “C” grade had too little evidence to support the inclusion or exclusion of a disease from a PHE; recommendations may be made on other reasons. Reports with sufficient evidence to indicate that a condition is expressly omitted from consideration in a PHE were categorized as “D”. When there was adequate information found to support the suggestion that the condition is expressly excluded from PHE, it was considered as “E”.
2.7. Data Extraction
Two researchers (S.A. and N.A.) performed the independent sampling and extraction of required data from the papers included in the current analysis after reading the entire text. The authors of the study, year of publication, study duration, business, group of references, country, number of samples, type of samples, and smoking effects on oral health were all extracted and reported in Table 3.
3. Results
3.1. Study Selection Results
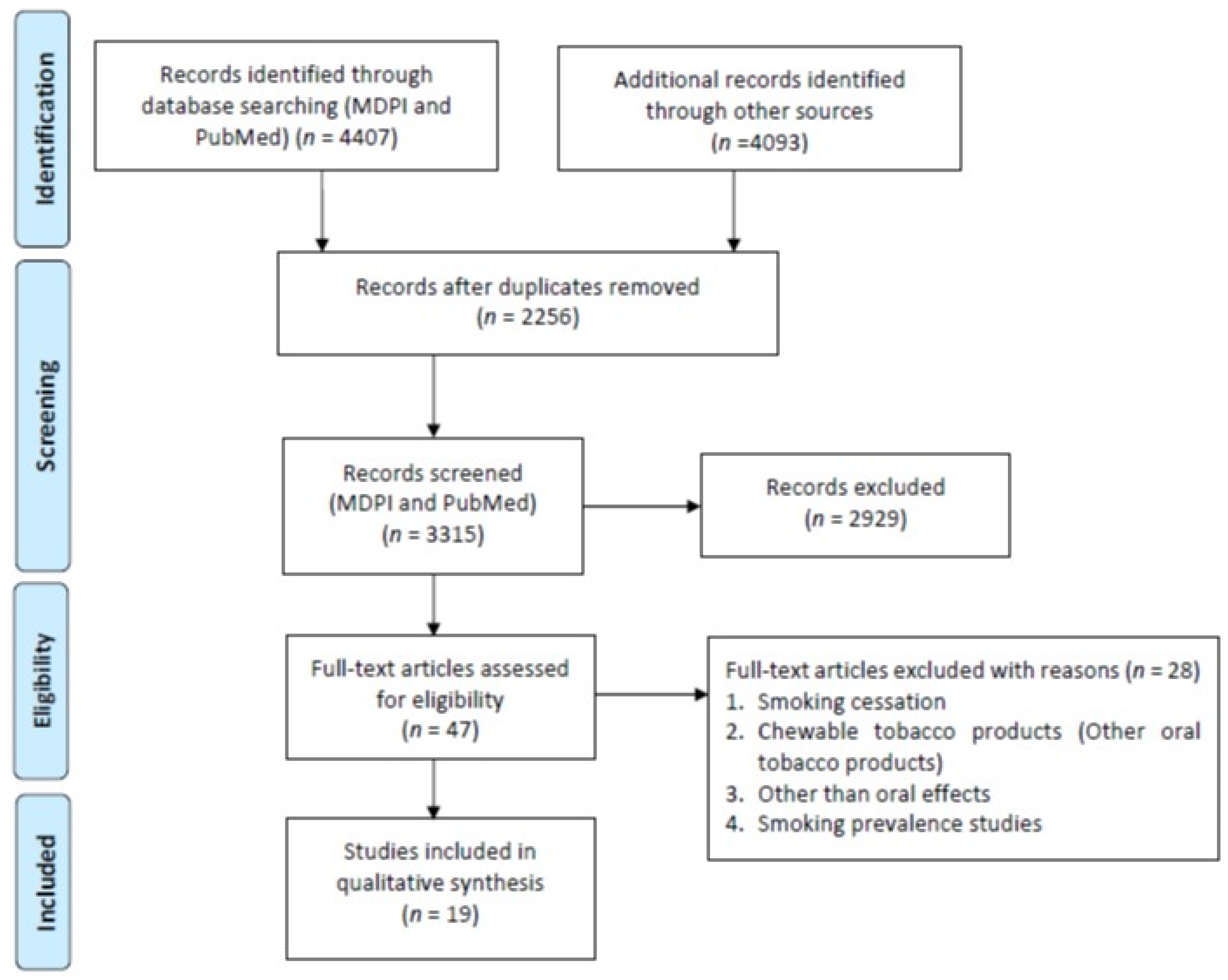
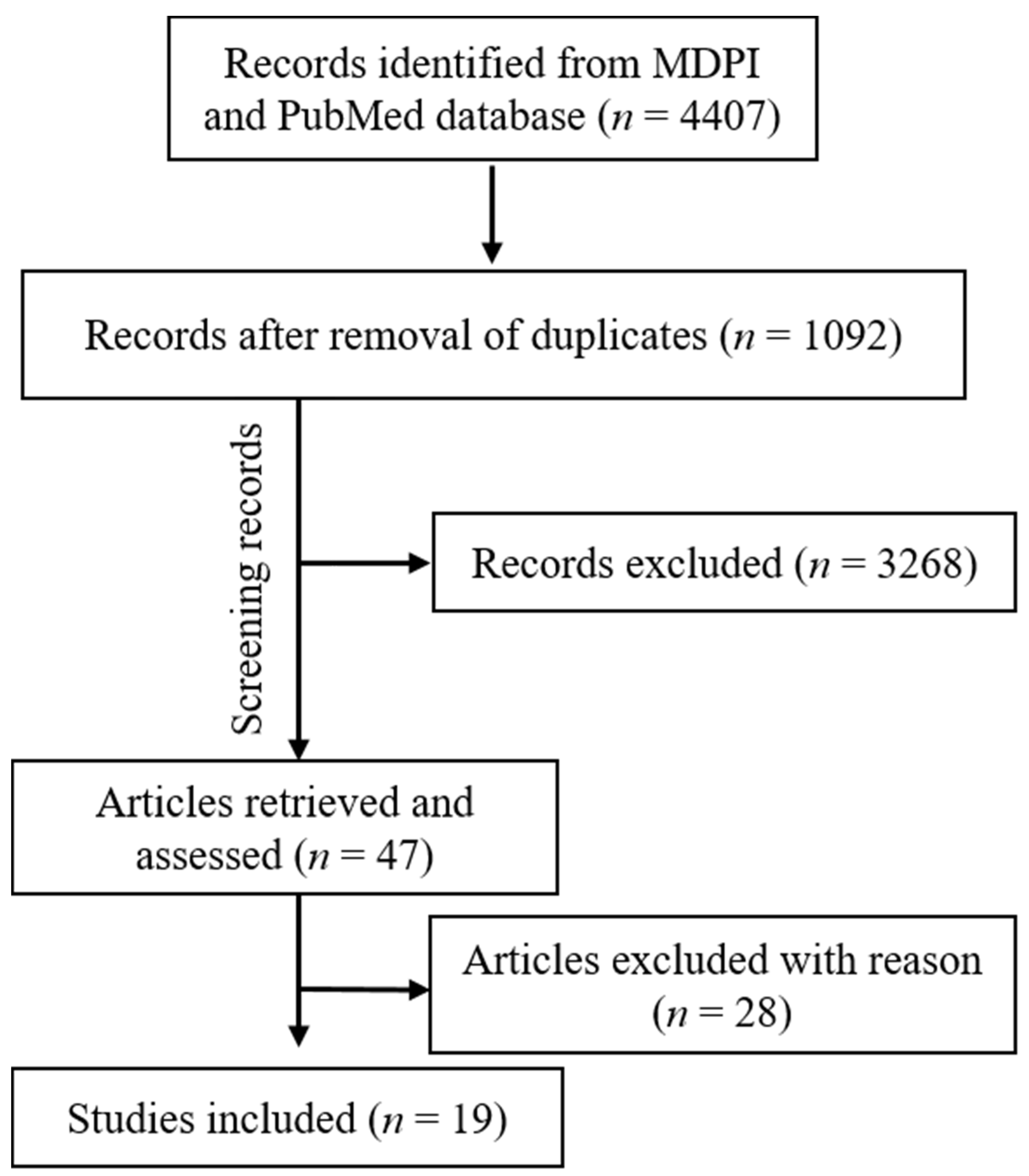
A total of 3696 studies were retrieved from the PubMed, Scopus (2271), WOS (1822), and MDPI (711) according to the inclusion/exclusion criteria as listed in Table 2. Data were extracted from 19 studies that purely met the eligibility criteria. Figure 2 and Figure 3 shows the results of the total studies evaluated. Initially, a total of 3696 articles were screened from PubMed, 711 from MDPI and 4093 form other databases (Scopus and WOS) as per the search criteria described in Table 1. After identifying duplicate articles, 972 were excluded from PubMed, 657 from Scopus, 507 from WOS, and 120 from MDPI. After removal of duplicates, the remaining 3315 articles were screened by reading the title and abstracts, after which 2929 articles were excluded. The remaining 47 articles were then fully read and assessed for the inclusion/exclusion criteria and quality assessment. After reading the full text, 28 studies were excluded due to reasons including cigarette cessation studies with no oral effects, prevalence of cigarette smoking among different population reporting no oral effects, questionnaire-based studies, and studies with chewable tobacco or other oral tobacco products. The finally selected articles were finalized to proceed further for data extraction. Based on the quality assessment of the research studies, these 19 articles were screened for the present study. A meta-analysis of the studies included in this study was not performed due to the methodological heterogeneity of the findings.
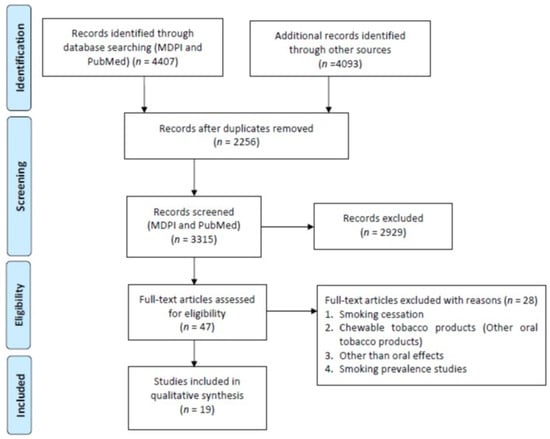
Figure 2.
Flowchart of articles retrieved from different Web sources.
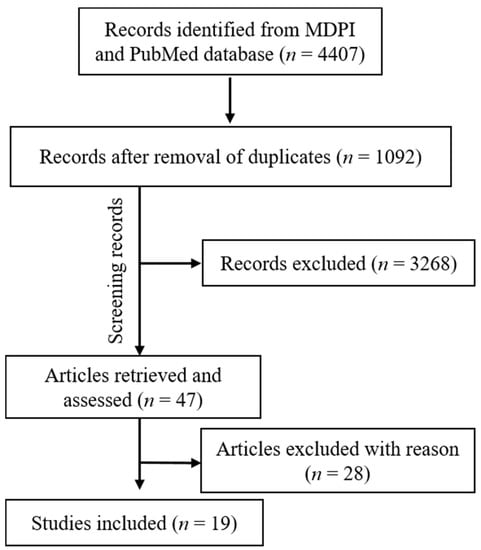
Figure 3.
Articles included in the current study (MDPI and PubMed).
From the total retrieved articles (MDPI, PubMed, Scopus, and Web of Sciences), only MDPI and PubMed articles were further included in this study.
3.2. Study Features
A total of 19 studies were included in this systematic review. The studies were conducted in different countries; many researchers cited different time durations. The number of citations for each of the study was observed from Google Scholar. Each of the included studies was published in reputed and indexed journals. Table 3 summarizes the sample type, total sample size, adopted methodology, results, and the conclusion of each study. Different types of samples were collected from the patients to observe the effects of smoking on oral health, including biopsies, blood, buckle cells, teeth, saliva, etc. In the included studies, a total of 26,236 samples were observed.

Table 3.
Methodology, outcomes, and conclusion of articles included in the current study.
3.3. Quality Assessment of Research Articles
All of the articles were shortlisted and screened based on the inclusion and exclusion criteria, titles, and abstract. Full texts were read one by one after the screening process and assessed for the quality of material using the CHPPHF recommendations. CHPPHF assessed articles for internal and external validity and rated the criteria for allocation biases, selection biases, intervention integrity, blinding, withdrawals and dropouts, confounding, data collection methods, and statistical analysis. No statistical assessment of publishing bias was carried out for the included studies as there were limited experimental techniques. A total of eight RCTs were included in the present systematic review.
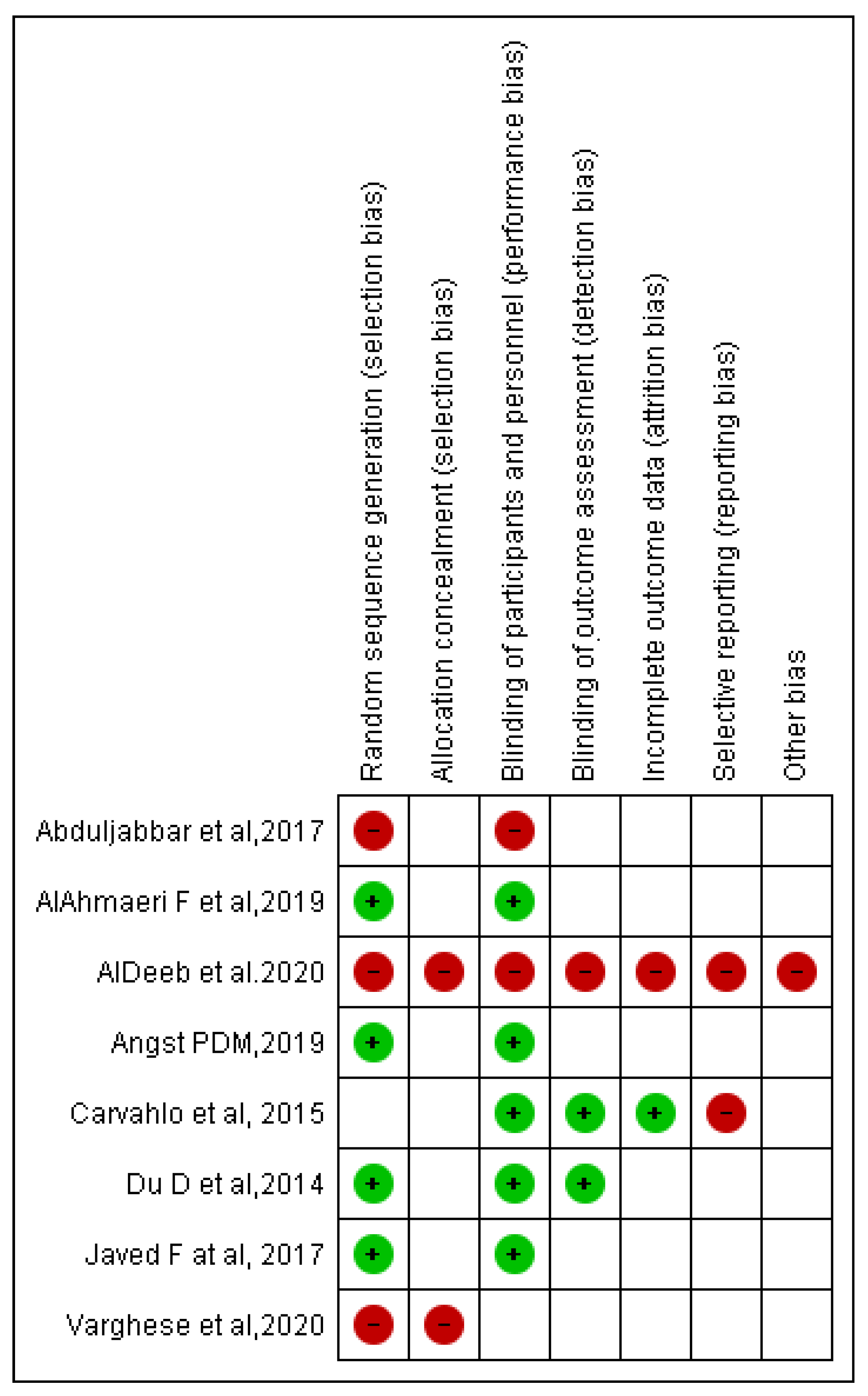
Bias assessment was conducted according to the Cochrane tool of bias risk assessment. Overall, four included RCTs were at higher risk of bias, four were at lower risk of bias, and many items according to Cochrane tool of bias risk assessment were unclear in eight included RCTs. Selection bias was observed in two of the eight included RCTs, as was performance bias in two studies, detection bias in one study, attrition bias in one study, reporting bias in two studies, and other concerns in one study (Figure 4).
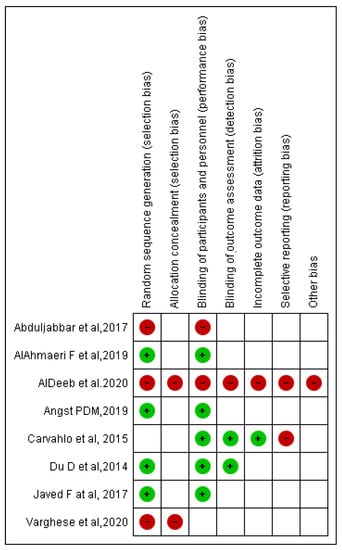
Figure 4.
Risk of bias summary: review authors judgment about each risk of bias item for each included RCT.
4. Discussion
Cigarette smoking has been linked to a variety of health problems. When comparing current smokers to nonsmokers, the rate of mortality from any cause was two to three times higher [42,43]. In many smoking-related studies, the duration of smoking, a quantity of cigarettes smoked per day, brand of cigarettes smoked, cigarette type, and topographical factors related to smoking all are linked to the severity of tobacco consumption [19]. Because of tobacco use, other lifestyle risk factors, and poor dental care usage, smokers are at a higher risk for many oral diseases. As many oral health problems go unrecognized and untreated, the lack of regular dental care becomes particularly problematic [8,44].
Oral diseases are one of the most frequent chronic diseases, and they are significant public health issues due to their prevalence, effect on people and society, and treatment costs [33,45]. Oral disease determinants are well understood. Oral hygiene, smoking, drinking, hazardous behaviors, and stress are all risk factors for various chronic diseases, and efficient public health interventions to prevent oral diseases [7]. Smoking is one of the most common risk factors for oral diseases [3].
Dental caries and periodontal disease and the probable consequences of both (tooth loss) are serious dental public health issues that affect people all over the world [6,21,46]. Individuals’ quality of life and general health are negatively impacted by poor oral health and untreated oral illnesses [9]. A significant positive association between tobacco smoking and higher risk for periodontitis has been found in prospective longitudinal studies.
The elevations in interleukin (IL)-1 and IL-6 associated with smoking levels upregulate bone resorption through the increase in the ratio between the receptor activator of nuclear factor-κβ ligand (RANKL) and its inhibitor osteoprotegerin (OPG). In addition, higher concentrations of elastase and matrix metalloproteinase (MMP)-8 and MMP-9 with proteolytic activity and decreased levels of protease inhibitors, such as alpha-2-macroglobulin and α-1-antitrypsin, may compromise periodontal healing.
If smoking were eliminated in this population, the risk of periodontitis would be reduced by approximately 14% as calculated using the population attributable risk fraction. In underdeveloped countries, the burden of oral diseases is significantly higher [4,47,48]. Among smokers and nonsmokers, in the treatment of chronic periodontitis (CP), Al-Ahmari et al. (2019) checked the effectiveness of scaling & root planning (SRP) with and without the adjunct antimicrobial photodynamic therapy (aPDT). Bleeding on probing (BOP), plaque index (PI), clinical attachment loss (CAL), and probing pocket depth (PD) 4 mm were all assessed at baseline, one month, and three months of follow-up. Smokers and nonsmokers had similar BOP, PI, PD, and clinical AL at the start of the study. PD, PI, and clinical AL were shown to be greater in smokers than nonsmokers after a one-month and three-month follow-up. At the one-month and three-month follow-ups, all nonsmokers’ BOP, PI, clinical AL, and PD were equivalent [34]. In similar research, Al-Bayaty et al. (2013) conducted a study to observe the effects of cigarette smoking on gingival bleeding, to measure the serum haptoglobin, cotinine, and alpha 1-antitrypsin concentrations in Malaysian smokers. BOP levels were determined to be low, whereas PI values were high. Smokers had considerably more significant levels of serum haptoglobin, cotinine, and alpha 1-antitrypsin than nonsmokers. There was a strong connection between PI and smoking duration (years) and blood cotinine levels [24].
Even though tobacco use has decreased in many high-income countries such as the United States and the United Kingdom, it is growing in many low- and middle-income countries [11,35]. According to the World Health Organization (WHO), there are more than 1.1 billion smokers throughout the world, with more than 80% of them residing in low- and middle-income countries [49]. After nonsurgical periodontal treatment, Varghese et al. (2020) studied the salivary 8-hydroxyguanosine (8-OHdG) levels in smokers and nonsmokers with CP. Clinical periodontal markers (PI, GI, PD, and CLI) were assessed at the start of the study. SRP was performed on patients with CPs (CP smokers) and CPns (CP nonsmokers) [40]. In a three-month follow-up period, all of the clinical measures and salivary collections were repeated. At the baseline period, the PI, GI, PD, and CAL values in the CPs and CPns groups were significantly higher as compared to the CHns and CHs groups. At baseline, salivary levels of 8-OHdG were found significantly higher in the CPs group than the other groups. All of the clinical measures in the CP group improved by the follow-up interval at the third month. However, the salivary levels of 8-OHdG in the CP smoker category were still higher values than the CPns [40]. Haswell et al. (2014) analyzed the biomarkers of biological effect (BOBE) and demonstrated the difference between smokers, nonsmokers, and ex-smokers. The levels of biomarkers were compared, and it was seen that there were 27 possible biomarkers evaluated in all, 14 of which were substantially different between smokers and nonsmokers, and 12 of which were able to discriminate between smokers and former smokers, indicating the possibility of reversibility [26].
The maxillary antrum, submandibular region, salivary glands, and tongue are commonly affected by cervicofacial actinomycosis [35]. The mandible is affected in about half of the cases, with the chin (15%), cheek (15%), and submaxillary ramus and angle (15%). The paranasal sinuses, tongue, larynx, middle ear, thyroid gland, and lachrymal pathways are all nonodontogenic orofacial regions that may also be get affected by cervicofacial actinomycosis [22]. Abduljabbar et al. (2017) worked on a project and wanted to see how effective aPDT was at preventing oral fungus colonization in smokers and nonsmokers suffering from denture stomatitis (DS). Among smokers, a statistically significant decrease in the mean fungal CFU/mL was seen at the 3-month follow-up compared to their respective baseline values of CFU/mL. When compared to their individual baseline values, nonsmokers’ mean levels were lower. After a 3-month follow-up, smokers’ fungal CFU/mL levels were statistically substantially higher than nonsmokers [30].
Study Limitations
In this systematic review, we have searched the data from a limited number of significant Web sources with specific final publication periods. The articles which have been published in any other sources may be overlooked. We have included articles that were published in the English language; as a result, articles which were published in other languages may also be overlooked.
5. Conclusions
Cigarette smoking has well-known hazardous effects on oral health and throughout the respiratory tract. Because of the impairment in oral health, it can also lead to problems in other parts of the body, such as the gastrointestinal tract system. Oral and respiratory cancers are among the most lethal known diseases caused by cigarette smoking, which can also cause plaque, dental caries, and other periodontal diseases. There is also an increased risk for bacterial and fungal infections in the oral cavity. This conclusion is based on a limited number of research studies.
Author Contributions
Project administration and supervision, M.I.K.; conceptualization, M.I.K. and A.M.; methodology, N.A. and C.Y.Y.; formal analysis, N.A. and C.Y.Y.; data curation, N.A.; validation, M.I.K., S.N.B., and P.M.; writing the original draft, N.A., S.A., A.M., and M.I.K.; writing—review and editing, C.M.M., P.T., S.A., N.A., A.M., G.A.S., and M.I.K.; funding acquisition, G.A.S. and P.M. The final manuscript has been read and approved by all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
Any external funding was not received for this systematic review.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Any type of data used in this systematic review can be assessed upon the request email to the corresponding author.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Swe, K.K.; Soe, A.K.; Aung, S.H.; Soe, H.Z. Effectiveness of oral health education on 8- to 10-year-old school children in rural areas of the Magway Region, Myanmar. BMC Oral Health 2021, 21, 2. [Google Scholar] [CrossRef]
- Zhu, S.; Häussling, V.; Aspera-Werz, R.H.; Chen, T.; Braun, B.; Weng, W.; Histing, T.; Nussler, A.K. Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model. Int. J. Mol. Sci. 2021, 22, 53. [Google Scholar] [CrossRef]
- Sharma, S.; Trivedi, H.; Sharma, V.K.; Gupta, N. Behavioral Factors and Periodontal Disease. Eur. J. Pharm. Med. Res. 2016, 3, 207–213. [Google Scholar]
- Peres, M.A.; Antunes, J.L.F.; Watt, R.G. The Contribution of Epidemiology to Oral Health Research. In Oral Epidemiology; Springer: Cham, Switzerland, 2021; pp. 3–22. [Google Scholar]
- Zou, Y.; Wang, D.-H.; Sakano, N.; Sato, Y.; Iwanaga, S.; Taketa, K.; Kubo, M.; Takemoto, K.; Masatomi, C.; Inoue, K.; et al. Associations of Serum Retinol, α-Tocopherol, and γ-Tocopherol with Biomarkers among Healthy Japanese Men. Int. J. Environ. Res. Public Health 2014, 11, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhao, L.; Ju, N.; Hua, T.; Zhang, S.; Liao, S. Relationship between oral health-related knowledge, attitudes, practice, self-rated oral health and oral health-related quality of life among Chinese college students: A structural equation modeling approach. BMC Oral Health 2021, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Zaitsu, T.; Ohara, S.; Wright, C.; Kawaguchi, Y. Factors influencing perceived oral health of Japanese middle-aged adults. Asia Pac. J. Public Health 2015, 27, NP2296–NP2304. [Google Scholar] [CrossRef]
- Shah, F.Y.; Sehrawat, P.; Bin, A. Smoking and its ramifications relating to oral mucosa. Int. J. Appl. Dent. Sci. 2020, 6, 742–744. [Google Scholar] [CrossRef]
- Omara, M.; Stamm, T.; Bekes, K. Four-dimensional oral health-related quality of life impact in children: A systematic review. J. Oral Rehabil. 2021, 48, 293–304. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Almasri, A.; Wisithphrom, K.; Windsor, L.J.; Olson, B. Nicotine and lipopolysaccharide affect cytokine expression from gingival fibroblasts. J. Periodontol. 2007, 78, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.R.F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923. [Google Scholar]
- Zhang, X.; Oluyomi, A.; Woodard, L.; Raza, S.A.; Adel Fahmideh, M.; El-Mubasher, O.; Byun, J.; Han, Y.; Amos, C.I.; Badr, H. Individual-Level Determinants of Lifestyle Behavioral Changes during COVID-19 Lockdown in the United States: Results of an Online Survey. Int. J. Environ. Res. Public Health 2021, 18, 4364. [Google Scholar] [CrossRef]
- Yun, C.; Katchko, K.M.; Schallmo, M.S.; Jeong, S.; Yun, J.; Chen, C.H.; Weiner, J.A.; Park, C.; George, A.; Stupp, S.I.; et al. Aryl Hydrocarbon Receptor Antagonists Mitigate the Effects of Dioxin on Critical Cellular Functions in Differentiating Human Osteoblast-Like Cells. Int. J. Mol. Sci. 2018, 19, 225. [Google Scholar] [CrossRef]
- Health Canada. Canadian Tobacco, Alcohol and Drugs Survey (CTADS): Summary of Results for 2017; Government of Canada: Ottawa, ON, Canada, 2017.
- Zgliczynska, M.; Szymusik, I.; Sierocinska, A.; Bajaka, A.; Rowniak, M.; Sochacki-Wojcicka, N.; Wielgos, M.; Kosinska-Kaczynska, K. Contraceptive Behaviors in Polish Women Aged 18–35—A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 2723. [Google Scholar] [CrossRef]
- Do, L.G.; Slade, G.D.; Roberts-Thomson, K.F.; Sanders, A.E. Smoking-attributable periodontal disease in the Australian adult population. J. Clin. Periodontol. 2008, 35, 398–404. [Google Scholar] [CrossRef]
- Bergstrom, J. Smoking rate and periodontal disease prevalence: 40-year trends in Sweden 1970–2010. J. Clin. Periodontol. 2014, 41, 952–957. [Google Scholar] [CrossRef]
- Eke, P.I.; Wei, L.; Thornton-Evans, G.O.; Borrell, L.N.; Borgnakke, W.S.; Dye, B.; Genco, R.J. Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J. Periodontol. 2016, 87, 1174–1185. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, F.; Xu, G.; Li, N.; Li, J.; He, Y.; Yu, J. Conventional Cigarette and E-Cigarette Smoking among School Personnel in Shanghai, China: Prevalence and Determinants. Int. J. Environ. Res. Public Health 2019, 16, 3197. [Google Scholar] [CrossRef]
- Sheng, L.; Tu, J.-W.; Tian, J.-H.; Chen, H.-J.; Pan, C.-L.; Zhou, R.-Z. A meta-analysis of the relationship between environmental tobacco smoke and lung cancer risk of nonsmoker in China. Medicine 2018, 97, e11389. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; Ahlawat, B.; Sharma, S. Site specific effect of tobacco addiction in upper aerodigestive tract tumors: A retrospective clinicopathological study. Sci. World J. 2014, 2014, 460194. [Google Scholar] [CrossRef] [PubMed]
- Yuki, D.; Kikuchi, A.; Miura, N.; Kakehi, A.; Onozawa, M. Good relationship between saliva cotinine kinetics and plasma cotinine kinetics after smoking one cigarette. Regul. Toxicol. Pharmacol. 2013, 67, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayaty, F.H.; Baharuddin, N.; Abdulla, M.A.; Ali, H.M.; Arkilla, M.B.; ALBayaty, M.F. The influence of cigarette smoking on gingival bleeding and serum concentrations of haptoglobin and alpha 1-antitrypsin. BioMed Res. Int. 2013, 2013, 684154. [Google Scholar] [CrossRef]
- Medina-Solís, C.E.; Pontigo-Loyola, A.P.; Perez-Campos, E.; Cruz, P.H.; Avila-Burgos, L.; Mendoza-Rodríguez, M.; Maupomé, G. National Survey of Oral/Dental Conditions Related to Tobacco and Alcohol Use in Mexican Adults. Int. J. Environ. Res. Public Heal. 2014, 11, 3169–3184. [Google Scholar] [CrossRef] [PubMed]
- Haswell, L.E.; Papadopoulou, E.; Newland, N.; Shepperd, C.J.; Lowe, F.J. A cross-sectional analysis of candidate biomarkers of biological effect in smokers, never-smokers and ex-smokers. Biomarkers 2014, 19, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Nides, M.; Borders, J.; Selmani, A.; Waverczak, W. Comparison of nicotine oral soluble film and nicotine lozenge on efficacy in relief of smoking cue-provoked acute craving after a single dose of treatment in low dependence smokers. Psychopharmacology 2014, 231, 4383–4391. [Google Scholar] [CrossRef]
- Lee, C.P.; Chiang, S.L.; Lee, C.H.; Tsai, Y.S.; Wang, Z.H.; Hua, C.H.; Chen, Y.C.; Tsai, E.M.; Ko, Y.C. AURKA Phe31Ile polymorphism interacted with use of alcohol, betel quid, and cigarettes at multiplicative risk of oral cancer occurrence. Clin. Oral Investig. 2015, 19, 1825–1832. [Google Scholar] [CrossRef]
- De Carvalho, L.D.; Gondo, R.; Lopes, G.C. One-year clinical evaluation of resin composite restorations of noncarious cervical lesions in smokers. J. Adhes. Dent. 2015, 17, 405–411. [Google Scholar]
- Abduljabbar, T.; Al-Askar, M.; Baig, M.K.; AlSowygh, Z.H.; Kellesarian, S.V.; Vohra, F. Efficacy of photodynamic therapy in the inactivation of oral fungal colonization among cigarette smokers and non-smokers with denture stomatitis. Photodiagn. Photodyn. Ther. 2017, 18, 50–53. [Google Scholar] [CrossRef]
- Javed, F.; BinShabaib, M.S.; Alharthi, S.S.; Qadri, T. Role of mechanical curettage with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implant mucositis in cigarette smokers: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2017, 18, 331–334. [Google Scholar] [CrossRef]
- Rodríguez-Rabassa, M.; López, P.; Rodríguez-Santiago, R.E.; Cases, A.; Felici, M.; Sánchez, R.; Yamamura, Y.; Rivera-Amill, V. Cigarette Smoking Modulation of Saliva Microbial Composition and Cytokine Levels. Int. J. Environ. Res. Public Health 2018, 15, 2479. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.R.; Krakauer, C.; Anderson, M.L.; Nelson, J.; Bush, T.; Catz, S.L.; McClure, J.B. Factors associated with future dental care utilization among low-income smokers overdue for dental visits. BMC Oral Health 2018, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- AlAhmari, F.; Ahmed, H.B.; Al-Kheraif, A.A.; Javed, F.; Akram, Z. Effectiveness of scaling and root planning with and without adjunct antimicrobial photodynamic therapy in the treatment of chronic periodontitis among cigarette-smokers and never-smokers: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2019, 25, 247–252. [Google Scholar] [CrossRef]
- Angst, P.D.M.; Finger Stadler, A.; Mendez, M.; Oppermann, R.V.; van der Velden, U.; Gomes, S.C. Supportive periodontal therapy in moderate-to-severe periodontitis patients: A two-year randomized clinical trial. J. Clin. Periodontol. 2019, 46, 1083–1093. [Google Scholar] [CrossRef]
- Al Deeb, M.; Alresayes, S.; Mokeem, S.A.; Alhenaki, A.M.; AlHelal, A.; Shafqat, S.S.; Vohra, F.; Abduljabbar, T. Clinical and immunological peri-implant parameters among cigarette and electronic smoking patients treated with photochemotherapy: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2020, 31, 101800. [Google Scholar] [CrossRef]
- Julkunen-Iivari, A.; Heikkinen, A.M.; Räisänen, I.T.; Ruokonen, H.; Meurman, J.H.; Toppila-Salmi, S.; Söder, P.; Söder, B. Tobacco Products, Periodontal Health and Education Level: Cohort Study from Sweden. Dent. J. 2020, 8, 90. [Google Scholar] [CrossRef]
- Javed, F.; Al-Zawawi, A.S.; Allemailem, K.S.; Almatroudi, A.; Mehmood, A.; Divakar, D.D.; Al-Kheraif, A.A. Periodontal Conditions and Whole Salivary IL-17A and-23 Levels among Young Adult Cannabis sativa (Marijuana)-Smokers, Heavy Cigarette-Smokers and Non-Smokers. Int. J. Environ. Res. Public Health 2020, 17, 7435. [Google Scholar] [CrossRef]
- Nettore, I.C.; Maione, L.; Desiderio, S.; De Nisco, E.; Franchini, F.; Palatucci, G.; Ungaro, P.; Cantone, E.; Macchia, P.E.; Colao, A. Influences of Age, Sex and Smoking Habit on Flavor Recognition in Healthy Population. Int. J. Environ. Res. Public Health 2020, 17, 959. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Bhat, V.; Chianeh, Y.R.; Kamath, V.; Husain, N.A.-H.; Özcan, M. Salivary 8-hydroxyguanosine levels in smokers and non-smokers with chronic periodontitis. Odontology 2020, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wychowański, P.; Starzyńska, A.; Jereczek-Fossa, B.A.; Iwanicka-Grzegorek, E.; Kosewski, P.; Adamska, P.; Woliński, J. The Effects of Smoking Cigarettes on Immediate Dental Implant. Stability—A Prospective Case Series Study. Appl. Sci. 2021, 11, 27. [Google Scholar] [CrossRef]
- Hamrah, M.S.; Harun-Or-Rashid, M.; Hirosawa, T.; Sakamoto, J.; Hashemi, H.; Emamian, M.H.; Shariati, M.; Fotouhi, A. Smoking and associated factors among the population aged 40-64 in Shahroud, Iran. Asian Pac. J. Cancer Prev. 2013, 14, 1919–1923. [Google Scholar] [CrossRef]
- Hatsukami, D.K.; Jensen, J.; Anderson, A.; Broadbent, B.; Allen, S.; Zhang, Y.; Severson, H. Oral tobacco products: Preference and effects among smokers. Drug Alcohol Depend. 2011, 118, 230–236. [Google Scholar] [CrossRef][Green Version]
- Ramalingam, A.; Santhanathas, T.; Shaukat Ali, S.; Zainalabidin, S. Resveratrol Supplementation Protects against Nicotine-Induced Kidney Injury. Int. J. Environ. Res. Public Health 2019, 16, 4445. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Flórez, L.; Fernández-Lucas, J.; Clemente-Suárez, V.J. Cultural Differences in Stress-Related Psychological, Nutrition, Physical Activity and Oral Health Factors of Professors. Nutrients 2020, 12, 3644. [Google Scholar] [CrossRef]
- Wackowski, O.A.; Manderski, M.T.B.; Lewis, M.J.; Delnevo, C.D. The impact of smokeless tobacco risk information on smokers’ risk perceptions and use intentions: A news media experiment. Health Commun. 2019, 34, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Radaeli, A.; Nardin, M.; Azzolina, D.; Malerba, M. Determinants of Smoking Status in a Sample of Outpatients Afferent to a Tertiary Referral Hospital. Int. J. Environ. Res. Public Health 2019, 16, 4136. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Manigrasso, M.; Cammalleri, V.; Biondi Zoccai, G.; Frati, G.; Avino, P.; Vitali, M. Impact of Electronic Alternatives to Tobacco Cigarettes on Indoor Air Particular Matter Levels. Int. J. Environ. Res. Public Health 2020, 17, 2947. [Google Scholar] [CrossRef]
- Jeon, H.G.; Kim, G.; Jeong, H.S.; So, W.-Y. Association between Cigarette Smoking and Physical Fitness Level of Korean Adults and the Elderly. Healthcare 2021, 9, 185. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).