A Delphi Study Protocol to Identify Recommendations on Physical Activity and Exercise in Patients with Diabetes and Risk of Foot Ulcerations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.2.1. Scientific Committee
2.2.2. Expert Panel (Panelists)
- Selection criteria for podiatrists and podologists:
- 2.
- Selection criteria for endocrinologists, family physicians, and vascular surgeons:
- 3.
- Selection criteria for experts in physical activity and sports sciences and physiotherapists:
2.3. Delphi Method
2.3.1. Data Collection Instrument
- Literature Review:
- 2.
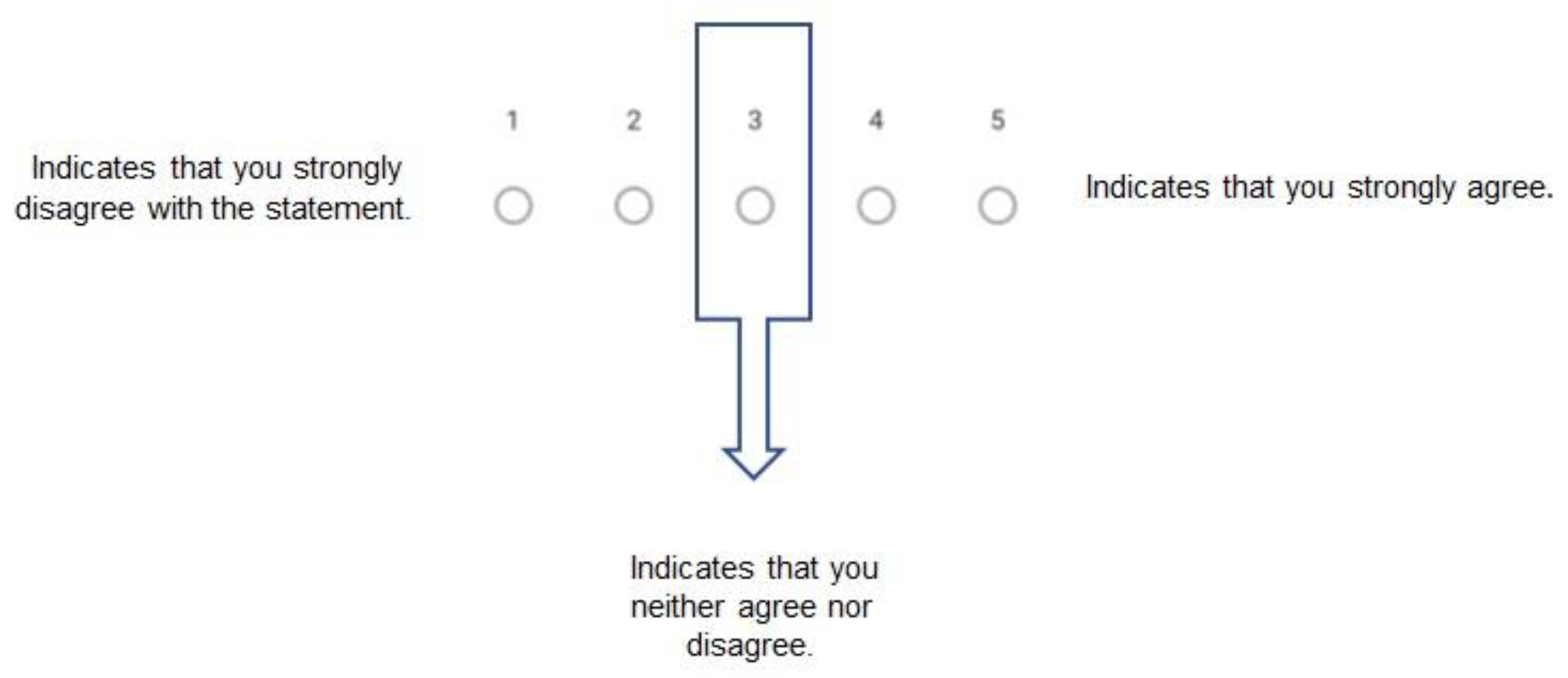
- Questionnaire Format:
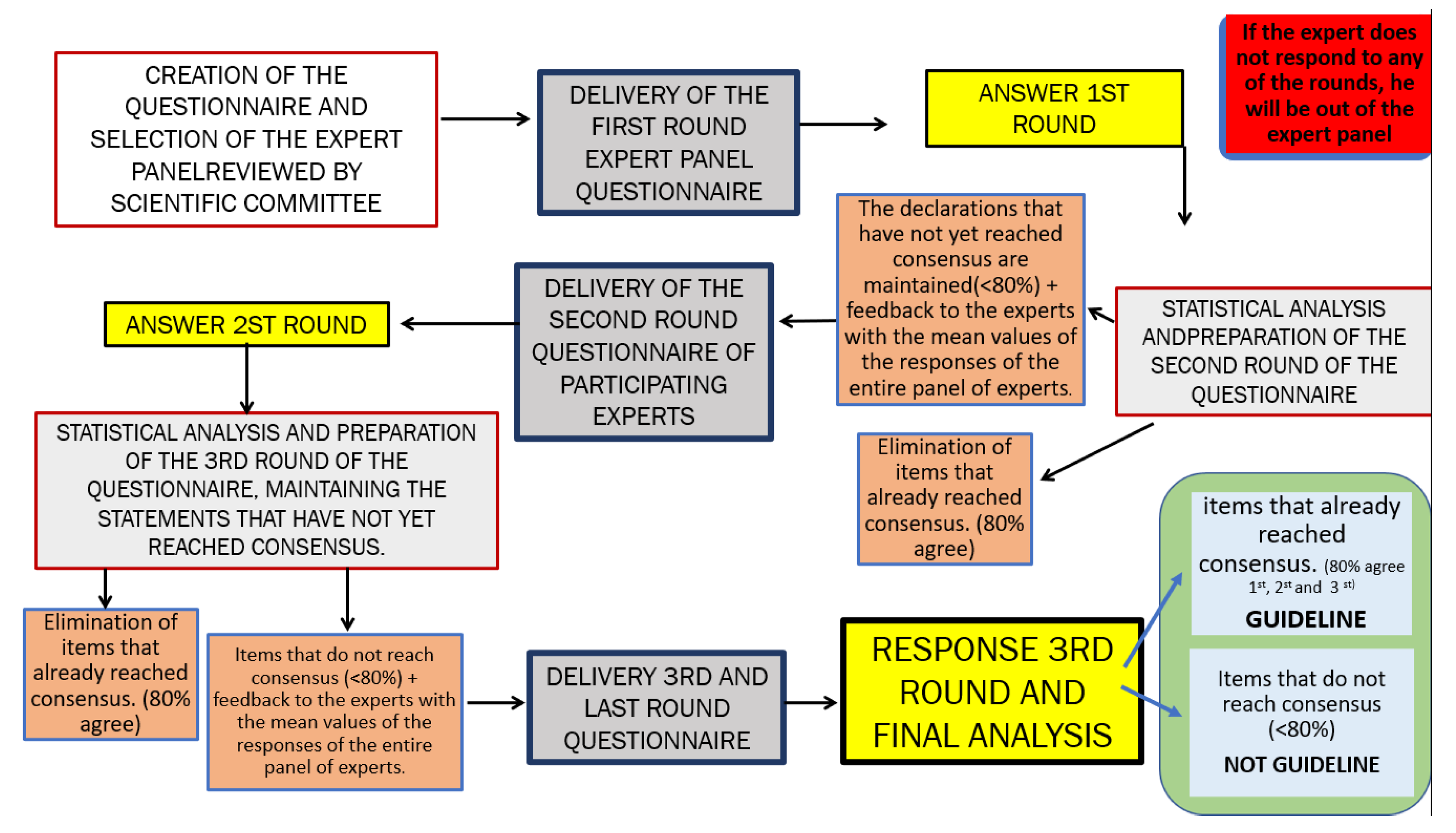
2.3.2. Delphi Process
- Study objectives.
- Methodology.
- Candidate types and criteria for their selection.
- Number of questionnaires to be completed and approximate time for each.
- Approximate process duration.
- Potential use of the collected information.
- Benefits of and objectives for participation (final report).
- First Questionnaire Round:
- 2.
- Second Questionnaire Round:
- 3.
- Third Questionnaire Round:
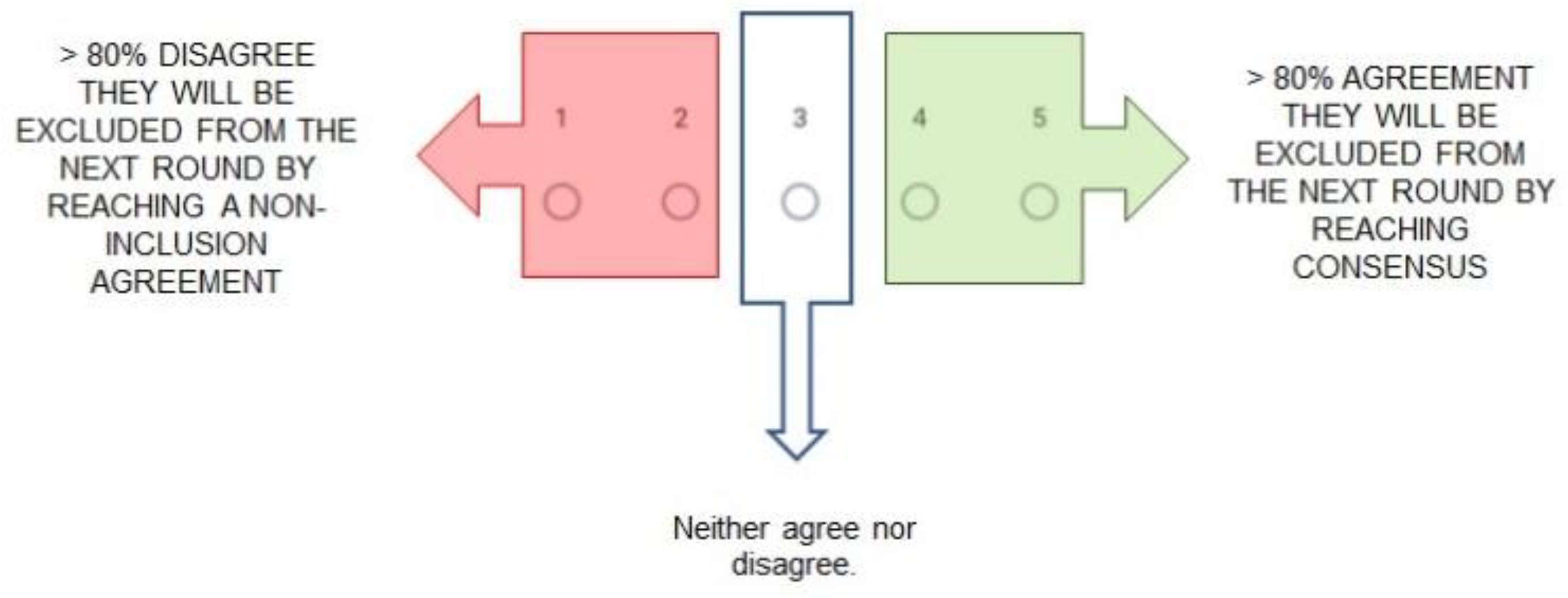
2.3.3. Defining and Reaching Consensus
2.4. Sample Size and Statistical Methods
2.4.1. Sample Size
2.4.2. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Martínez, G.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia, I.; Pérez, V.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Incidence of diabetes mellitus in Spain as results of the nation-wide cohort di@bet.es study. Sci. Rep. 2020, 10, 2765. [Google Scholar] [CrossRef] [Green Version]
- Jeon, B.J.; Choi, H.J.; Kang, J.S.; Tak, M.S.; Park, E.S. Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation. Int. Wound J. 2017, 14, 537–545. [Google Scholar] [CrossRef]
- O’Loughlin, A.; McIntosh, C.; Dinneen, S.F.; O’Brien, T. Review paper: Basic concepts to novel therapies: A review of the diabetic foot. Int. J. Low. Extrem. Wounds 2010, 9, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis†. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Panero, A.J.; Ruiz-Muñoz, M.; Cuesta-Vargas, A.I.; Gónzalez-Sánchez, M. Prevention, assessment, diagnosis and management of diabetic foot based on clinical practice guidelines: A systematic review. Medicine 2019, 98, e16877. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Yu, J.; Liu, S.; Zhang, R.; Ma, X.; Yang, Y.; Wang, P. Diagnostic Accuracy of Monofilament Tests for Detecting Diabetic Peripheral Neuropathy: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2017, 2017, 8787261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 42. [Google Scholar] [CrossRef]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes. Metab. Res. Rev. 2020, 36, e3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, S.; Pascale, R.; Vitale, M.; Esposito, S. Epidemiology of diabetic foot. Le Infez. Med. Riv. Period. Eziologia Epidemiol. Diagn. Clin. Ter. Delle Patol. Infett. 2012, 20 (Suppl. 1), 8–13. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3662275&tool=pmcentrez&rendertype=abstract (accessed on 19 October 2021).
- Matos, M.; Mendes, R.; Silva, A.B.; Sousa, N. Physical activity and exercise on diabetic foot related outcomes: A systematic review. Diabetes Res. Clin. Pract. 2018, 139, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey-Murto, S.; Varpio, L.; Wood, T.J.; Gonsalves, C.; Ufholz, L.A.; Mascioli, K.; Wang, C.; Foth, T. The Use of the Delphi and Other Consensus Group Methods in Medical Education Research: A Review. Acad. Med. 2017, 92, 1491–1498. [Google Scholar] [CrossRef]
- Sudore, R.L.; Lum, H.D.; You, J.J.; Hanson, L.C.; Meier, D.E.; Pantilat, S.Z.; Matlock, D.D.; Rietjens, J.A.; Korfage, I.J.; Ritchie, C.S.; et al. Defining Advance Care Planning for Adults: A Consensus Definition From a Multidisciplinary Delphi Panel. J. Pain Symptom Manag. 2017, 53, 821–832.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dribin, T.E.; Sampson, H.A.; Camargo, C.A.; Brousseau, D.C.; Spergel, J.M.; Neuman, M.I.; Shaker, M.; Campbell, R.L.; Michelson, K.A.; Rudders, S.A.; et al. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2020, 146, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Arriero-Marín, J.M.; Orozco-Beltrán, D.; Carratalá-Munuera, C.; López-Pineda, A.; Gil-Guillen, V.F.; Soler-Cataluña, J.J.; Chiner-Vives, E.; Nouni García, R.; Quesada, J.A. A modified Delphi consensus study to identify improvement proposals for COPD management amongst clinicians and administrators in Spain. Int. J. Clin. Pract. 2021, 75, e13934. [Google Scholar] [CrossRef] [PubMed]
- Trevelyan, E.G.; Robinson, N. Delphi methodology in health research: How to do it? Eur. J. Integr. Med. 2015, 7, 423–428. [Google Scholar] [CrossRef]
- El metodo delphi: Landeta, Jon: Libros. Available online: https://www.amazon.es/El-metodo-delphi-Jon-Landeta/dp/8434428369 (accessed on 31 January 2021).
- Jünger, S.; Payne, S.A.; Brine, J.; Radbruch, L.; Brearley, S.G. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat. Med. 2017, 31, 684–706. [Google Scholar] [CrossRef] [Green Version]
- Keeney, S.; Hasson, F.; McKenna, H.P. A critical review of the Delphi technique as a research methodology for nursing. Int. J. Nurs. Stud. 2001, 38, 195–200. [Google Scholar] [CrossRef]
- Toronto, C. Considerations when conducting e-Delphi research: A case study. Nurse Res. 2017, 25, 10–15. [Google Scholar] [CrossRef]
- Jandhyala, R. Delphi, non-RAND modified Delphi, RAND/UCLA appropriateness method and a novel group awareness and consensus methodology for consensus measurement: A systematic literature review. Curr. Med. Res. Opin. 2020, 36, 1873–1887. [Google Scholar] [CrossRef]
- Akins, R.B.; Tolson, H.; Cole, B.R. Stability of response characteristics of a Delphi panel: Application of bootstrap data expansion. BMC Med Res. Methodol. 2005, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- de Villiers, M.R.; de Villiers, P.J.T.; Kent, A.P. The Delphi technique in health sciences education research. Med. Teach. 2005, 27, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Marti-Martinez, L.M.; Gracia-Sánchez, A.; Ferrer-Torregrosa, J.; Lorca-Gutierrez, R.; Garcia-Campos, J.; Sánchez-Pérez, S.P. Description of the surgical technique for condylectomy with minimally invasive surgery to treat interdigital helomas on the lesser toes: A Delphi study. J. Foot Ankle Res. 2019, 12, 13. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; Snowling, M.J.; Thompson, P.A.; Greenhalgh, T.; Adams, C.; Archibald, L.; Baird, G.; Bauer, A.; Bellair, J.; Boyle, C.; et al. CATALISE: A multinational and multidisciplinary Delphi consensus study. Identifying language impairments in children. PLoS ONE 2016, 11, e0158753. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Pawlowski, S.D. The Delphi method as a research tool: An example, design considerations and applications. Inf. Manag. 2004, 42, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Liao, F.; An, R.; Pu, F.; Burns, S.; Shen, S.; Jan, Y.-K. Effect of Exercise on Risk Factors of Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2019, 98, 103–116. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gracia-Sánchez, A.; López-Pineda, A.; Chicharro-Luna, E.; Gil-Guillén, V.F. A Delphi Study Protocol to Identify Recommendations on Physical Activity and Exercise in Patients with Diabetes and Risk of Foot Ulcerations. Int. J. Environ. Res. Public Health 2021, 18, 10988. https://doi.org/10.3390/ijerph182010988
Gracia-Sánchez A, López-Pineda A, Chicharro-Luna E, Gil-Guillén VF. A Delphi Study Protocol to Identify Recommendations on Physical Activity and Exercise in Patients with Diabetes and Risk of Foot Ulcerations. International Journal of Environmental Research and Public Health. 2021; 18(20):10988. https://doi.org/10.3390/ijerph182010988
Chicago/Turabian StyleGracia-Sánchez, Alba, Adriana López-Pineda, Esther Chicharro-Luna, and Vicente F. Gil-Guillén. 2021. "A Delphi Study Protocol to Identify Recommendations on Physical Activity and Exercise in Patients with Diabetes and Risk of Foot Ulcerations" International Journal of Environmental Research and Public Health 18, no. 20: 10988. https://doi.org/10.3390/ijerph182010988
APA StyleGracia-Sánchez, A., López-Pineda, A., Chicharro-Luna, E., & Gil-Guillén, V. F. (2021). A Delphi Study Protocol to Identify Recommendations on Physical Activity and Exercise in Patients with Diabetes and Risk of Foot Ulcerations. International Journal of Environmental Research and Public Health, 18(20), 10988. https://doi.org/10.3390/ijerph182010988





