Boosting Numerical Cognition in Children and Adolescents with Mathematical Learning Disabilities by a Brain-Based Intervention: A Study Protocol for a Randomized, Sham-Controlled Clinical Trial
Abstract
:1. Introduction
1.1. Neurocognitive Features of MLD
1.2. Current and New Treatment Perspectives in MLD
1.3. Research Objectives
- (1)
- Examining the long-term safety and feasibility of a multisession tRNS protocol in the pediatric population (e.g., 10 sessions);
- (2)
- Determining the effects of the tRNS setup (over bilateral dlPFCs vs. bilateral PPCs) in improving long-lasting arithmetic learning and performance and neuropsychological and psychological measures compared with sham tRNS;
- (3)
- Testing whether and the extent to which arithmetic improvements after tRNS are related to neuropsychological changes;
- (4)
- Understanding whether and the extent to which changes in spontaneous EEG after tRNS are linked to arithmetic improvements;
- (5)
- Assessing whether and the extent to which improvements in arithmetic performance correlate with changes in the psychological function of children and their parents’ stress.
2. Materials and Methods
2.1. Ethical Committee
2.2. Study Setting and Participants
2.3. Design, Randomization, and Blinding
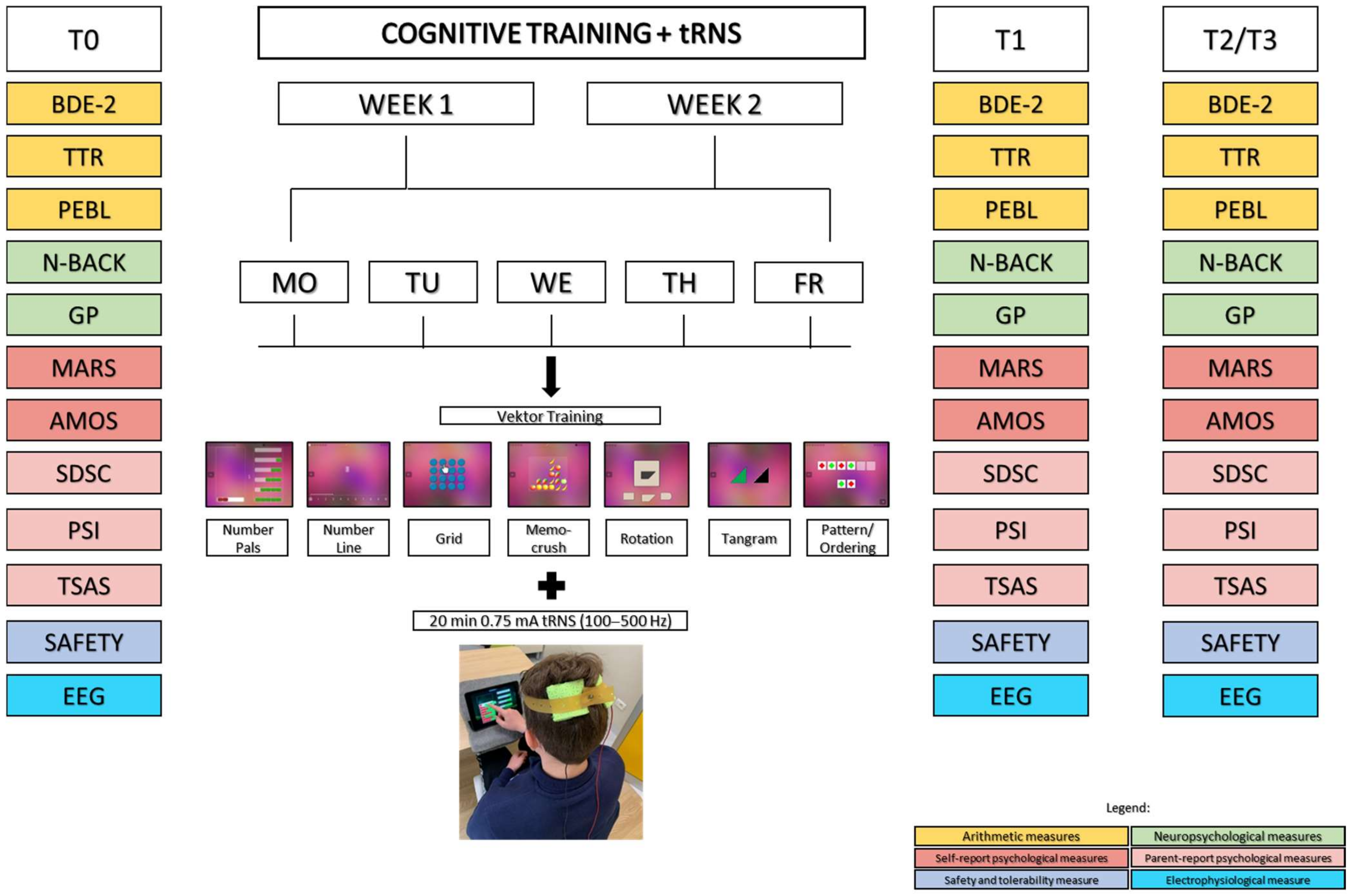
2.4. Interventions
2.4.1. Cognitive Training
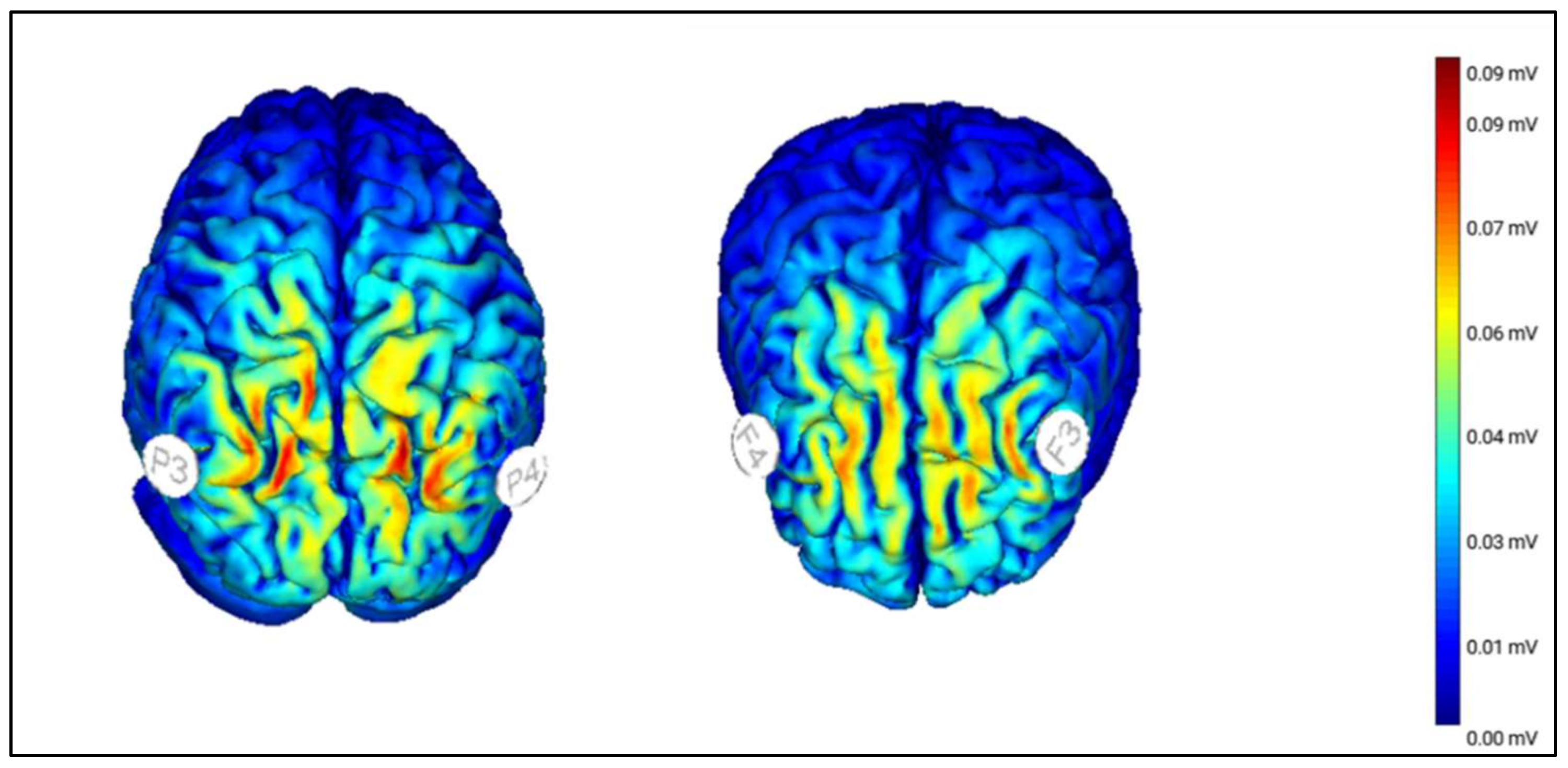
2.4.2. Transcranial Random Noise Stimulation
2.5. Outcome Measures
2.5.1. Arithmetic Measures
2.5.2. Neuropsychological Measures
2.5.3. Self-Administered Psychological Questionnaires
2.5.4. Parental Psychological Questionnaires
2.5.5. Electrophysiological Measures (EEG)
2.5.6. Safety and Tolerability
2.6. Sample Size Considerations
2.7. Safety Considerations
- (1)
- Transcranial random noise stimulation. Considering the paucity of safety data on tRNS, this technique is considered safe, as supported by a recent study in juvenile mice [61]. Previous studies [90,91] have shown that tRNS with the current intensity that we use here is less likely to be perceivable. Compared with tDCS, tRNS has the advantage of having higher cutaneous perception thresholds and lower response rates [90]. Adverse effects will be registered throughout the study. The experimenter will also follow participants for adverse effects after the end of the study.
- (2)
- Cognitive training/assessment. There is a risk that participants will find the tasks to be challenging, fatiguing, or boring. Should this occur, participants can take a break at any time or can discontinue the testing. Research staff will explain what to do and how to perform the tasks during study visits.
2.8. Protection of Risks
2.9. Missed Sessions and Early Termination of Participation
2.10. Study Monitoring and Data Management
3. Data Analysis
3.1. EEG Preprocessing
3.2. Statistical Analysis
3.3. Hypothesis and Expected Results
- (1)
- The Parietal, Frontal, and Sham groups will not differ in blindness or safety measures;
- (2)
- The Parietal and Frontal groups will experience significant improvements in the primary outcome (Number Line subtest accuracy) and arithmetic and neuropsychological measures at T1 compared with Sham group and that such improvements will persist at T3. The Parietal and Frontal groups will also significantly improve their training accuracy across the 10 days versus the Sham group. We do not have a directional hypothesis on the differences between the 2 active groups (Parietal and Frontal);
- (3)
- The Parietal and Frontal groups will significantly improve their psychological measures at least at T3 compared with the Sham group;
- (4)
- In the Parietal and Frontal Groups, arithmetic changes will correlate strongly and positively with changes in neuropsychological and spectral power EEG measures at least at T1 and with changes in psychological measures at least at T3.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Castaldi, E.; Piazza, M.; Iuculano, T. Learning Disabilities: Developmental Dyscalculia. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 174, pp. 61–75. [Google Scholar] [CrossRef]
- Ministero dell’Istruzione, dell’Università e della Ricerca, Notizie Stampa e Comunicazione. Available online: https://www.miur.gov.it/web/guest/-/scuola-pubblicati-i-dati-sugli-alunni-con-disturbi-specifici-dell-apprendimento/ (accessed on 14 June 2019).
- Parsons, S.; Bynner, J. Does Numeracy Matter More; NRDC: London, UK, 2006; pp. 1–44. [Google Scholar]
- Trzesniewski, K.H.; Donnellan, M.B.; Moffitt, T.E.; Robins, R.W.; Poulton, R.; Caspi, A. Low Self-Esteem during Adolescence Predicts Poor Health, Criminal Behavior, and Limited Economic Prospects during Adulthood. Dev. Psychol. 2006, 42, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, S.J.; Bates, T.C. Enduring Links From Childhood Mathematics and Reading Achievement to Adult Socioeconomic Status. Psychol. Sci. 2013, 24, 1301–1308. [Google Scholar] [CrossRef]
- Eispino, M.; Pereda, J.; Recon, J.; Perculeza, E.; Umali, C. Mathematics anxiety and its impact on the course and career choice of grade 11 students. Int. J. Educ. Psychol. Couns. 2017, 2, 99–119. [Google Scholar]
- Duncan, G.J.; Dowsett, C.J.; Claessens, A.; Magnuson, K.; Huston, A.C.; Klebanov, P.; Pagani, L.S.; Feinstein, L.; Engel, M.; Brooks-Gunn, J.; et al. School Readiness and Later Achievement. Dev. Psychol. 2007, 43, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen Kadosh, R.; Dowker, A.; Heine, A.; Kaufmann, L.; Kucian, K. Interventions for Improving Numerical Abilities: Present and Future. Trends Neurosci. Educ. 2013, 2, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Peters, E.; Västfjäll, D.; Slovic, P.; Mertz, C.K.; Mazzocco, K.; Dickert, S. Numeracy and Decision Making. Psychol. Sci. 2006, 17, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Reyna, V.F.; Nelson, W.L.; Han, P.K.; Dieckmann, N.F. How Numeracy Influences Risk Comprehension and Medical Decision Making. Psychol. Bull. 2009, 135, 943–973. [Google Scholar] [CrossRef] [Green Version]
- Reyna, V.F.; Brainerd, C.J. The Importance of Mathematics in Health and Human Judgment: Numeracy, Risk Communication, and Medical Decision Making. Learn. Individ. Differ. 2007, 17, 147–159. [Google Scholar] [CrossRef]
- Haberstroh, S.; Schulte-Körne, G. The Diagnosis and Treatment of Dyscalculia. Dtsch. Arztebl. Int. 2019, 116, 107. [Google Scholar] [CrossRef]
- Krause, B.; Cohen Kadosh, R. Can Transcranial Electrical Stimulation Improve Learning Difficulties in Atypical Brain Development? A Future Possibility for Cognitive Training. Dev. Cogn. Neurosci. 2013, 6, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, L.; von Aster, M. The Diagnosis and Management of Dyscalculia. Dtsch. Arztebl. Int. 2012, 109, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinsten, O.; Henik, A. Developmental Dyscalculia: Heterogeneity Might Not Mean Different Mechanisms. Trends Cogn. Sci. 2009, 13, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mazzocco, M.M.M.; Feigenson, L.; Halberda, J. Impaired Acuity of the Approximate Number System Underlies Mathematical Learning Disability (Dyscalculia): Impaired Numerical Acuity Contributes to MLD. Child. Dev. 2011, 82, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.L.; Siegler, R.S. Numerical Magnitude Representations Influence Arithmetic Learning. Child. Dev. 2008, 79, 1016–1031. [Google Scholar] [CrossRef]
- Dehaene, S.; Izard, V.; Spelke, E.; Pica, P. Log or Linear? Distinct Intuitions of the Number Scale in Western and Amazonian Indigene Cultures. Science 2008, 320, 1217–1220. [Google Scholar] [CrossRef] [Green Version]
- Rugani, R.; Vallortigara, G.; Priftis, K.; Regolin, L. Experimental Evidence From Newborn Chicks Enriches Our Knowledge on Human Spatial-Numerical Associations. Cogn. Sci. 2017, 41, 2275–2279. [Google Scholar] [CrossRef]
- Skorupski, P.; MaBouDi, H.; Galpayage Dona, H.S.; Chittka, L. Counting Insects. Phil. Trans. R. Soc. B 2018, 373, 20160513. [Google Scholar] [CrossRef]
- Agrillo, C.; Bisazza, A. Understanding the Origin of Number Sense: A Review of Fish Studies. Phil. Trans. R. Soc. B 2018, 373, 20160511. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, B. Foundational Numerical Capacities and the Origins of Dyscalculia. Trends Cogn. Sci. 2010, 14, 534–541. [Google Scholar] [CrossRef]
- Looi, C.Y.; Cohen Kadosh, R. Brain Stimulation, Mathematical, and Numerical Training. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 227, pp. 353–388. [Google Scholar] [CrossRef]
- Barth, H.; Beckmann, L.; Spelke, E.S. Nonsymbolic, Approximate Arithmetic in Children: Abstract Addition Prior to Instruction. Dev. Psychol. 2008, 44, 1466–1477. [Google Scholar] [CrossRef] [Green Version]
- Piazza, M. Neurocognitive Start-up Tools for Symbolic Number Representations. Trends Cogn. Sci. 2010, 14, 542–551. [Google Scholar] [CrossRef]
- DeWind, N.K.; Brannon, E.M. Malleability of the Approximate Number System: Effects of Feedback and Training. Front. Hum. Neurosci. 2012, 6, 68. [Google Scholar] [CrossRef] [Green Version]
- Feigenson, L.; Libertus, M.E.; Halberda, J. Links between the Intuitive Sense of Number and Formal Mathematics Ability. Child. Dev. Perspect 2013, 7, 74–79. [Google Scholar] [CrossRef]
- Geary, D.C. Consequences, Characteristics, and Causes of Mathematical Learning Disabilities and Persistent Low Achievement in Mathematics. J. Dev. Behav. Pediatrics 2011, 32, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Passolunghi, M.C.; Lanfranchi, S. Domain-Specific and Domain-General Precursors of Mathematical Achievement: A Longitudinal Study from Kindergarten to First Grade: Cognitive Precursors of Mathematical Achievement. Br. J. Educ. Psychol. 2012, 82, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Skagerlund, K.; Träff, U. Processing of Space, Time, and Number Contributes to Mathematical Abilities above and beyond Domain-General Cognitive Abilities. J. Exp. Child. Psychol. 2016, 143, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Lauer, J.E.; Lourenco, S.F. Spatial Processing in Infancy Predicts Both Spatial and Mathematical Aptitude in Childhood. Psychol. Sci. 2016, 27, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Verdine, B.N.; Golinkoff, R.M.; Hirsh-Pasek, K.; Newcombe, N.S.I. Spatial skills, their development, and their links to mathematics: Spatial skills, their development, and their links to mathematics. Monogr. Soc. Res. Child. 2017, 82, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Judd, N.; Klingberg, T. Training Spatial Cognition Enhances Mathematical Learning in a Randomized Study of 17,000 Children. Available online: https://www.nature.com/articles/s41562-021-01118-4 (accessed on 5 October 2021).
- Raghubar, K.P.; Barnes, M.A.; Hecht, S.A. Working Memory and Mathematics: A Review of Developmental, Individual Difference, and Cognitive Approaches. Learn. Individ. Differ. 2010, 20, 110–122. [Google Scholar] [CrossRef]
- Gilmore, C.; Attridge, N.; Clayton, S.; Cragg, L.; Johnson, S.; Marlow, N.; Simms, V.; Inglis, M. Individual Differences in Inhibitory Control, Not Non-Verbal Number Acuity, Correlate with Mathematics Achievement. PLoS ONE 2013, 8, e67374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Geary, D.C. Developmental Gains in Visuospatial Memory Predict Gains in Mathematics Achievement. PLoS ONE 2013, 8, e70160. [Google Scholar] [CrossRef]
- Szucs, D.; Devine, A.; Soltesz, F.; Nobes, A.; Gabriel, F. Developmental Dyscalculia Is Related to Visuo-Spatial Memory and Inhibition Impairment. Cortex 2013, 49, 2674–2688. [Google Scholar] [CrossRef] [Green Version]
- Abreu-Mendoza, R.A.; Chamorro, Y.; Garcia-Barrera, M.A.; Matute, E. The Contributions of Executive Functions to Mathematical Learning Difficulties and Mathematical Talent during Adolescence. PLoS ONE 2018, 13, e0209267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, L.; De Smedt, B. Arithmetic in the Developing Brain: A Review of Brain Imaging Studies. Dev. Cogn. Neurosci. 2018, 30, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smedt, B.; Noël, M.-P.; Gilmore, C.; Ansari, D. How Do Symbolic and Non-Symbolic Numerical Magnitude Processing Skills Relate to Individual Differences in Children’s Mathematical Skills? A Review of Evidence from Brain and Behavior. Trends Neurosci. Educ. 2013, 2, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Faye, A.; Jacquin-Courtois, S.; Reynaud, E.; Lesourd, M.; Besnard, J.; Osiurak, F. Numerical Cognition: A Meta-Analysis of Neuroimaging, Transcranial Magnetic Stimulation and Brain-Damaged Patients Studies. NeuroImage Clin. 2019, 24, 102053. [Google Scholar] [CrossRef] [PubMed]
- Berteletti, I.; Lucangeli, D.; Zorzi, M. Representation of Numerical and Non-Numerical Order in Children. Cognition 2012, 124, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.B.; DeYoung, C.G.; Gray, J.R.; Brown, J.; Mackintosh, N. Associative Learning Predicts Intelligence above and beyond Working Memory and Processing Speed. Intelligence 2009, 37, 374–382. [Google Scholar] [CrossRef]
- Kucian, K.; Loenneker, T.; Dietrich, T.; Dosch, M.; Martin, E.; von Aster, M. Impaired neural networks for approximate calculation in dyscalculic children: A functional MRI study. Behav. Brain Funct. 2006, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Kucian, K.; von Aster, M. Developmental Dyscalculia. Eur. J. Pediatr. 2015, 174, 1–13. [Google Scholar] [CrossRef]
- Gersten, R.; Chard, D.J.; Jayanthi, M.; Baker, S.K.; Morphy, P.; Flojo, J. Mathematics Instruction for Students With Learning Disabilities: A Meta-Analysis of Instructional Components. Rev. Educ. Res. 2009, 79, 1202–1242. [Google Scholar] [CrossRef]
- Montague, M. Effective instruction in mathematics for students with learning difficulties. In Multiple Perspectives on Difficulties in Learning, Literacy and Numeracy; Wyatt-Smith, C., Elkins, J., Gunn, S., Eds.; Springer Science and Business: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Holopainen, L.; Hakkarainen, A. Longitudinal Effects of Reading and/or Mathematical Difficulties: The Role of Special Education in Graduation From Upper Secondary Education. J. Learn. Disabil. 2019, 52, 456–467. [Google Scholar] [CrossRef]
- Iuculano, T.; Rosenberg-Lee, M.; Richardson, J.; Tenison, C.; Fuchs, L.; Supekar, K.; Menon, V. Cognitive Tutoring Induces Widespread Neuroplasticity and Remediates Brain Function in Children with Mathematical Learning Disabilities. Nat. Commun. 2015, 6, 8453. [Google Scholar] [CrossRef] [Green Version]
- Kroesbergen, E.H.; Van Luit, J.E.H. Mathematics Interventions for Children with Special Educational Needs: A Meta-Analysis. Remedial Spec. Educ. 2003, 24, 97–114. [Google Scholar] [CrossRef]
- Ise, E.; Schulte-Körne, G. Symptomatik, Diagnostik und Behandlung der Rechenstörung. Z. Kinder-Jugendpsychiatr. Psychother. 2013, 41, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Chodura, S.; Kuhn, J.-T.; Holling, H. Interventions for children with mathematical difficulties: A meta-analysis. Z. Psychol. 2015, 223, 129–144. [Google Scholar] [CrossRef]
- Kroeger, L.A.; Brown, R.D.; O’Brien, B.A. Connecting Neuroscience, Cognitive, and Educational Theories and Research to Practice: A Review of Mathematics Intervention Programs. Early Educ Dev. 2012, 23, 37–58. [Google Scholar] [CrossRef]
- Elmasry, J.; Loo, C.; Martin, D. A Systematic Review of Transcranial Electrical Stimulation Combined with Cognitive Training. Restor. Neurol. Neurosci. 2015, 33, 263–278. [Google Scholar] [CrossRef]
- Paulus, W. Transcranial Electrical Stimulation (TES—TDCS; TRNS, TACS) Methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef]
- McDonnell, M.D.; Abbott, D. What Is Stochastic Resonance? Definitions, Misconceptions, Debates, and Its Relevance to Biology. PLoS Comput. Biol. 2009, 5, e1000348. [Google Scholar] [CrossRef] [Green Version]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef]
- Reed, T.; Cohen Kadosh, R. Transcranial Electrical Stimulation (TES) Mechanisms and Its Effects on Cortical Excitability and Connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
- Pavan, A.; Ghin, F.; Contillo, A.; Milesi, C.; Campana, G.; Mather, G. Modulatory Mechanisms Underlying High-Frequency Transcranial Random Noise Stimulation (Hf-TRNS): A Combined Stochastic Resonance and Equivalent Noise Approach. Brain Stimul. 2019, 12, 967–977. [Google Scholar] [CrossRef]
- Sánchez-León, C.A.; Sánchez-López, Á.; Gómez-Climent, M.A.; Cordones, I.; Kadosh, R.C.; Márquez-Ruiz, J. Impact of Chronic Transcranial Random-Noise Stimulation (TRNS) on Prefrontal Cortex Excitation-Inhibition Balance in Juvenile Mice. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.09.04.282889v1.full (accessed on 5 October 2021).
- Snowball, A.; Tachtsidis, I.; Popescu, T.; Thompson, J.; Delazer, M.; Zamarian, L.; Zhu, T.; Cohen Kadosh, R. Long-Term Enhancement of Brain Function and Cognition Using Cognitive Training and Brain Stimulation. Curr. Biol. 2013, 23, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelletti, M.; Gessaroli, E.; Hithersay, R.; Mitolo, M.; Didino, D.; Kanai, R.; Cohen Kadosh, R.; Walsh, V. Transfer of Cognitive Training across Magnitude Dimensions Achieved with Concurrent Brain Stimulation of the Parietal Lobe. J. Neurosci. 2013, 33, 14899–14907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, T.; Krause, B.; Terhune, D.B.; Twose, O.; Page, T.; Humphreys, G.; Cohen Kadosh, R. Transcranial Random Noise Stimulation Mitigates Increased Difficulty in an Arithmetic Learning Task. Neuropsychologia 2016, 81, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Pasqualotto, A. Transcranial Random Noise Stimulation Benefits Arithmetic Skills. Neurobiol. Learn. Mem. 2016, 133, 7–12. [Google Scholar] [CrossRef]
- Looi, C.Y.; Lim, J.; Sella, F.; Lolliot, S.; Duta, M.; Avramenko, A.A.; Cohen Kadosh, R. Transcranial Random Noise Stimulation and Cognitive Training to Improve Learning and Cognition of the Atypically Developing Brain: A Pilot Study. Sci. Rep. 2017, 7, 4633. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Brunoni, A.R.; Charvet, L.E.; Clark, V.P.; Cohen, L.G.; Deng, Z.-D.; Dmochowski, J.; Edwards, D.J.; Frohlich, F.; Kappenman, E.S.; et al. Rigor and Reproducibility in Research with Transcranial Electrical Stimulation: An NIMH-Sponsored Workshop. Brain Stimul. 2018, 11, 465–480. [Google Scholar] [CrossRef] [Green Version]
- SPIRIT. SPIRIT 2013 Checklist: Recommended Items to Address in a Clinical Trial Protocol and Related Documents. 1–5 (Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT), Cananda). 2013. Available online: https://www.spirit-statement.org (accessed on 15 June 2019).
- Istituto Superiore di Sanità, Consensus Conference, Disturbi Specifici Dell’apprendimento, Sistema Nazionale per le Linee Guida, Ministero della Salute. 2011. Available online: http://www.lineeguidadsa.it/download_documentiDSA/Raccomandazioni_CC_DSA (accessed on 5 October 2021).
- Biancardi, A.; Bachmann, C.; Nicoletti, C.B. Batteria Discalculia Evolutiva-2—BDE 2. Test. per la Diagnosi dei Disturbi Dell’elaborazione Numerica e del Calcolo in età Evolutiva—8–13 Anni; Erikson: Trento, Italy, 2016. [Google Scholar]
- Nemmi, F.; Helander, E.; Helenius, O.; Almeida, R.; Hassler, M.; Räsänen, P.; Klingberg, T. Behavior and Neuroimaging at Baseline Predict Individual Response to Combined Mathematical and Working Memory Training in Children. Dev. Cogn. Neurosci. 2016, 20, 43–51. [Google Scholar] [CrossRef]
- Bieck, S.M.; Artemenko, C.; Moeller, K.; Klein, E. Low to No Effect: Application of TRNS during Two-Digit Addition. Front. Neurosci. 2018, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Berger, I.; Dakwar-Kawar, O.; Grossman, E.S.; Nahum, M.; Cohen Kadosh, R. Scaffolding the Attention-Deficit/Hyperactivity Disorder Brain Using Transcranial Direct Current and Random Noise Stimulation: A Randomized Controlled Trial. Clin. Neurophysiol. 2021, 132, 699–707. [Google Scholar] [CrossRef]
- Costanzo, F.; Varuzza, C.; Rossi, S.; Sdoia, S.; Varvara, P.; Oliveri, M.; Menghini, D. Evidence for reading improvement following tDCS treatment in children and adolescents with dyslexia. Restor. Neurol. Neurosci. 2016, 34, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, F.; Rossi, S.; Varuzza, C.; Varvara, P.; Vicari, S.; Menghini, D. Long-Lasting Improvement Following TDCS Treatment Combined with a Training for Reading in Children and Adolescents with Dyslexia. Neuropsychologia 2019, 130, 38–43. [Google Scholar] [CrossRef]
- Lazzaro, G.; Costanzo, F.; Varuzza, C.; Rossi, S.; De Matteis, M.E.; Vicari, S.; Menghini, D. Individual Differences Modulate the Effects of TDCS on Reading in Children and Adolescents with Dyslexia. Sci. Stud. Read. 2020, 1–16. [Google Scholar] [CrossRef]
- Lazzaro, G.; Bertoni, S.; Menghini, D.; Costanzo, F.; Franceschini, S.; Varuzza, C.; Ronconi, L.; Battisti, A.; Gori, S.; Facoetti, A.; et al. Beyond Reading Modulation: Temporo-Parietal TDCS Alters Visuo-Spatial Attention and Motion Perception in Dyslexia. Brain Sci. 2021, 11, 263. [Google Scholar] [CrossRef]
- De Vos, T. Tempo Test Rekenen (TTR). Arithmetic Number Fact Test; Nijmegen: Berkhout, The Netherlands, 1992. [Google Scholar]
- Mueller, S.T.; Piper, B.J. The Psychology Experiment Building Language (PEBL) and PEBL Test Battery. J. Neurosci. Methods 2014, 222, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, S.T. The PEBL Manual, Version 0.13; Lulu Press: Morrisville, NC, USA, 2012; ISBN 978-0557658176. Available online: http://www.lulu.com/shop/shane-t-mueller/the-pebl-manual/paperback/product-20595443.html (accessed on 5 October 2021).
- Perez, W.A.; Masline, P.J.; Ramsey, E.G.; Urban, K.E. Unified Tri-Services Cognitive Performance Assessment Battery: Review and methodology (AAMRL-TR-887-007); Armstrong Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base: Dayton, OH, USA, 1987. [Google Scholar]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Urgesi, C.; Campanella, F.; Fabbro, F. Contributo alla Taratura Italiana (NEPSY–II. Italian Standardization); Giunti OS Organizzazioni Speciali: Firenze, Italy, 2011. [Google Scholar]
- Cornoldi, C.; Saccani, M. Ansia per la Matematica: La Scala MARS-R per la Valutazione e L’Intervento Metacognitivo. Difficoltà in Matematica; Erikson: Trento, Italy, 2005; pp. 133–152. [Google Scholar]
- Cornoldi, C.; De Beni, R.; Zamperlin, C.; Meneghetti, C. AMOS S-15. Abilità e Motivazione allo Studio: Prove di Valutazione per Ragazzi Dagli 8 ai 15 Anni; Erickson: Trento, Italy, 2005. [Google Scholar]
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC) Construct Ion and Validation of an Instrument to Evaluate Sleep Disturbances in Childhood and Adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Laghi, F.; Serantoni, G.; Di Blasio, P.; Camisasca, E. Adattamento Italiano (a cura di), Parenting Stress Index: Manuale, 4th ed.; IRIS PubliCatt: Firenze, Italy, 2016; Available online: https://publicatt.unicatt.it/handle/10807/96058?mode=full.766#.YW0d-BwRWUk (accessed on 5 October 2021).
- Kaufman, J.; Birmaher, B.; Rao, U.; Ryan, U. Kiddie-Sads Present and Lifetime Version Diagnostic and Statistical Manual of Mental Disorders 5—K-SADS-DSM 5; Erikson: Trento, Italy, 2016. [Google Scholar]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A Systematic Review on Reporting and Assessment of Adverse Effects Associated with Transcranial Direct Current Stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef]
- Ambrus, G.G.; Paulus, W.; Antal, A. Cutaneous Perception Thresholds of Electrical Stimulation Methods: Comparison of TDCS and TRNS. Clin. Neurophysiol. 2010, 121, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What Do You Feel If I Apply Transcranial Electric Stimulation? Safety, Sensations and Secondary Induced Effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Seltman, H.J. Mixed Models. A Flexible Approach to Correlated Data. Experimental Design and Analysis; Carnegie Mellon University: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version; R Core Team: Vienna, Austria, 2021; Volume 3, pp. 31–104. [Google Scholar]
- Luke, S.G. Evaluating Significance in Linear Mixed-Effects Models in R. Behav. Res. 2017, 49, 1494–1502. [Google Scholar] [CrossRef]
- Antal, A.; Herrmann, C.S. Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural. Plast. 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Monte-Silva, K.; Kuo, M.-F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-Like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Rufener, K.S.; Krauel, K.; Meyer, M.; Heinze, H.-J.; Zaehle, T. Transcranial Electrical Stimulation Improves Phoneme Processing in Developmental Dyslexia. Brain Stimul. 2019, 12, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Splittgerber, M.; Suwelack, J.H.; Kadish, N.E.; Moliadze, V. The Effects of 1 MA TACS and TRNS on Children/Adolescents and Adults: Investigating Age and Sensitivity to Sham Stimulation. Neural. Plast. 2020, 1–14. [Google Scholar] [CrossRef]
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial Magnetic and Direct Current Stimulation in Children. Curr. Neurol. Neurosci. Rep. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Finisguerra, A.; Borgatti, R.; Urgesi, C. Non-Invasive Brain Stimulation for the Rehabilitation of Children and Adolescents With Neurodevelopmental Disorders: A Systematic Review. Front. Psychol. 2019, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, O.W.; Hoy, K.E.; Wong, D.; Bailey, N.W.; Fitzgerald, P.B.; Segrave, R.A. Transcranial Random Noise Stimulation Is More Effective than Transcranial Direct Current Stimulation for Enhancing Working Memory in Healthy Individuals: Behavioural and Electrophysiological Evidence. Brain Stimul. 2020, 13, 1370–1380. [Google Scholar] [CrossRef]
- Abe, T.; Miyaguchi, S.; Otsuru, N.; Onishi, H. The Effect of Transcranial Random Noise Stimulation on Corticospinal Excitability and Motor Performance. Neurosci. Lett. 2019, 705, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Arnao, V.; Riolo, M.; Carduccio, F.; Tuttolomondo, A.; D’Amelio, M.; Brighina, F.; Gangitano, M.; Salemi, G.; Ragonese, P.; Aridon, P. Effects of Transcranial Random Noise Stimulation Combined with Graded Repetitive Arm Supplementary Program (GRASP) on Motor Rehabilitation of the Upper Limb in Sub-Acute Ischemic Stroke Patients: A Randomized Pilot Study. J. Neural. Transm. 2019, 126, 1701–1706. [Google Scholar] [CrossRef]
- Ghin, F.; Pavan, A.; Contillo, A.; Mather, G. The Effects of High-Frequency Transcranial Random Noise Stimulation (Hf-TRNS) on Global Motion Processing: An Equivalent Noise Approach. Brain Stimul. 2018, 11, 1263–1275. [Google Scholar] [CrossRef] [Green Version]
- van der Groen, O.; Wenderoth, N. Transcranial Random Noise Stimulation of Visual Cortex: Stochastic Resonance Enhances Central Mechanisms of Perception. J. Neurosci. 2016, 36, 5289–5298. [Google Scholar] [CrossRef] [Green Version]
- Brevet-Aeby, C.; Mondino, M.; Poulet, E.; Brunelin, J. Three Repeated Sessions of Transcranial Random Noise Stimulation (TRNS) Leads to Long-Term Effects on Reaction Time in the Go/No Go Task. Neurophysiol. Clin. 2019, 49, 27–32. [Google Scholar] [CrossRef]
- Dondé, C.; Brevet-Aeby, C.; Poulet, E.; Mondino, M.; Brunelin, J. Potential Impact of Bifrontal Transcranial Random Noise Stimulation (TRNS) on the Semantic Stroop Effect and Its Resting-State EEG Correlates. Neurophysiol. Clin. 2019, 49, 243–248. [Google Scholar] [CrossRef]
- Peña, J.; Sampedro, A.; Ibarretxe-Bilbao, N.; Zubiaurre-Elorza, L.; Ojeda, N. Improvement in Creativity after Transcranial Random Noise Stimulation (TRNS) over the Left Dorsolateral Prefrontal Cortex. Sci. Rep. 2019, 9, 7116. [Google Scholar] [CrossRef] [Green Version]
- Minhas, P.; Bikson, M.; Woods, A.J.; Rosen, A.R.; Kessler, S.K. Transcranial Direct Current Stimulation in Pediatric Brain: A Computational Modeling Study. Available online: https://ieeexplore.ieee.org/document/6346067 (accessed on 5 October 2021).
- Kessler, S.K.; Minhas, P.; Woods, A.J.; Rosen, A.; Gorman, C.; Bikson, M. Dosage Considerations for Transcranial Direct Current Stimulation in Children: A Computational Modeling Study. PLoS ONE 2013, 8, e76112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the Electric Field during Transcranial Direct Current Stimulation. NeuroImage 2015, 109, 140–150. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzaro, G.; Battisti, A.; Varuzza, C.; Celestini, L.; Pani, P.; Costanzo, F.; Vicari, S.; Kadosh, R.C.; Menghini, D. Boosting Numerical Cognition in Children and Adolescents with Mathematical Learning Disabilities by a Brain-Based Intervention: A Study Protocol for a Randomized, Sham-Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 10969. https://doi.org/10.3390/ijerph182010969
Lazzaro G, Battisti A, Varuzza C, Celestini L, Pani P, Costanzo F, Vicari S, Kadosh RC, Menghini D. Boosting Numerical Cognition in Children and Adolescents with Mathematical Learning Disabilities by a Brain-Based Intervention: A Study Protocol for a Randomized, Sham-Controlled Clinical Trial. International Journal of Environmental Research and Public Health. 2021; 18(20):10969. https://doi.org/10.3390/ijerph182010969
Chicago/Turabian StyleLazzaro, Giulia, Andrea Battisti, Cristiana Varuzza, Laura Celestini, Pierpaolo Pani, Floriana Costanzo, Stefano Vicari, Roi Cohen Kadosh, and Deny Menghini. 2021. "Boosting Numerical Cognition in Children and Adolescents with Mathematical Learning Disabilities by a Brain-Based Intervention: A Study Protocol for a Randomized, Sham-Controlled Clinical Trial" International Journal of Environmental Research and Public Health 18, no. 20: 10969. https://doi.org/10.3390/ijerph182010969
APA StyleLazzaro, G., Battisti, A., Varuzza, C., Celestini, L., Pani, P., Costanzo, F., Vicari, S., Kadosh, R. C., & Menghini, D. (2021). Boosting Numerical Cognition in Children and Adolescents with Mathematical Learning Disabilities by a Brain-Based Intervention: A Study Protocol for a Randomized, Sham-Controlled Clinical Trial. International Journal of Environmental Research and Public Health, 18(20), 10969. https://doi.org/10.3390/ijerph182010969






