Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. The Review Approach and Protocol Development
2.2. Search Strategies
2.3. Study Selection Process
2.4. Inclusion and Exclusion Criteria
2.5. The Operational Definition of TB Treatment Outcomes
Outcome Definition
- Cured: A pulmonary TB patient with bacteriologically confirmed TB at the beginning of treatment who was smear- or culture-negative in the last month of treatment and on at least one previous occasion.
- Treatment completed: A TB patient who completed treatment without evidence of failure BUT with no record to show that sputum smear or culture results in the last month of treatment and on at least one previous occasion were negative, either because tests were not done or because results are unavailable.
- Treatment failed: A TB patient whose sputum smear or culture is positive at month 5 or later during treatment.
- Died: A TB patient who dies for any reason before starting or during treatment.
- Lost to follow-up: A TB patient was not initiated on TB treatment or whose treatment was interrupted for two consecutive months or more.
- Treatment success: The sum of cured and treatment completed.
- Unsuccessful Treatment outcome: A sum of treatment failure, died and defaulter.
2.6. Data Extraction and Review Process
2.7. Methodological Quality Assessment
2.8. Statistical Analysis
3. Results
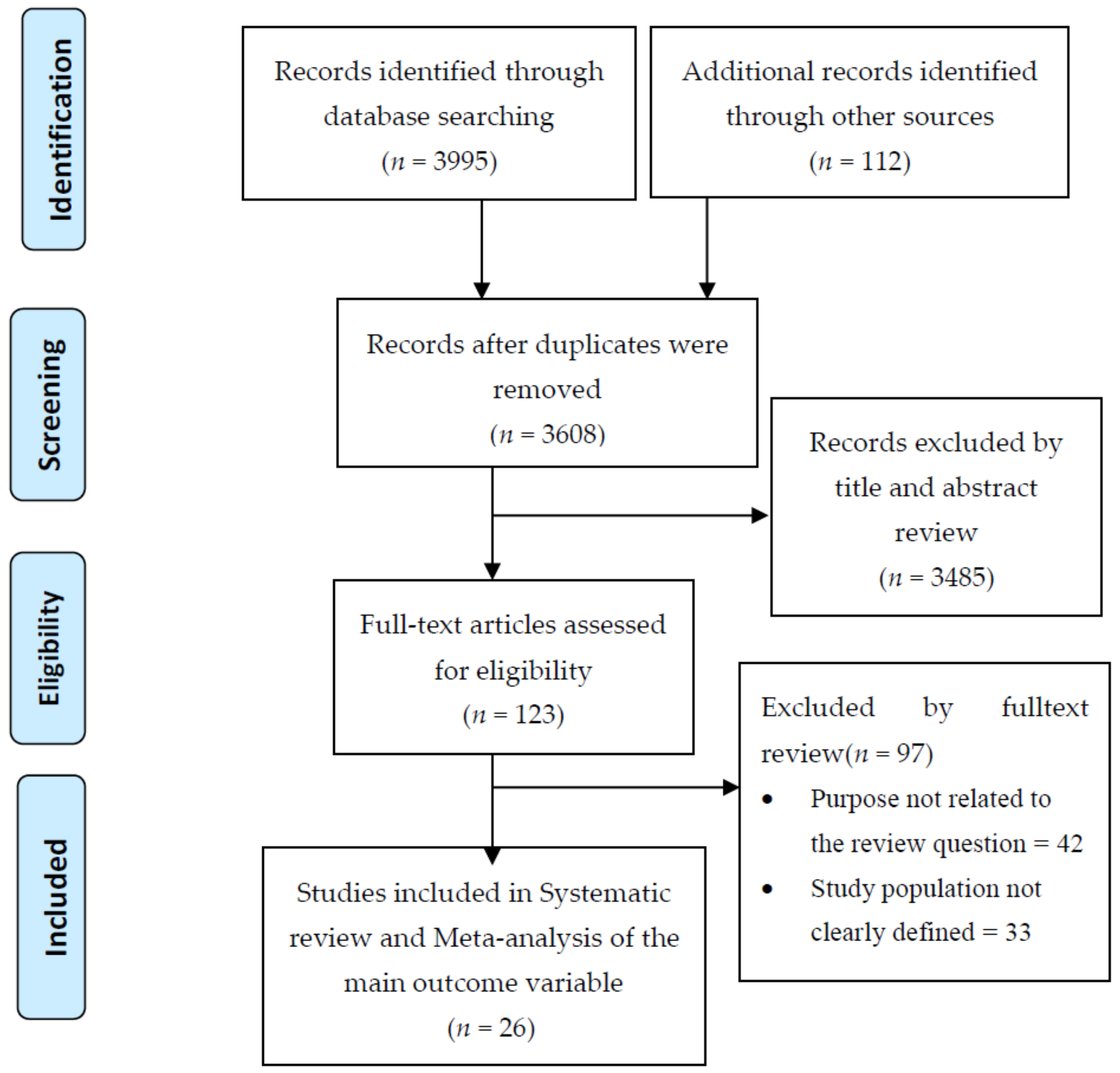
3.1. Search Results
3.2. Characteristics of Study Setting and Context
3.3. Characteristics of the Study Population
3.4. Characteristics of the Condition
3.5. Factors Associated with TB Treatment Outcome
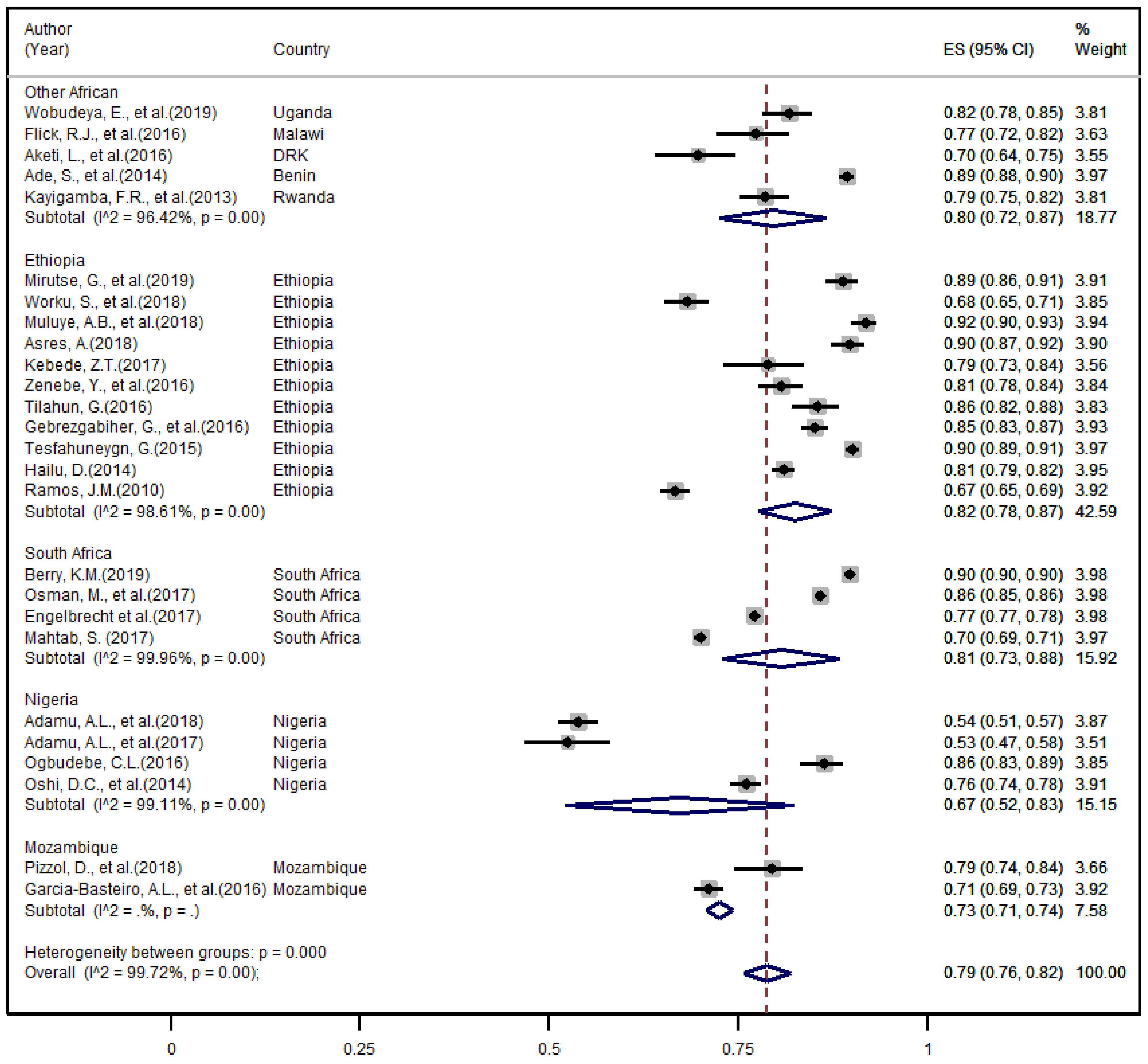
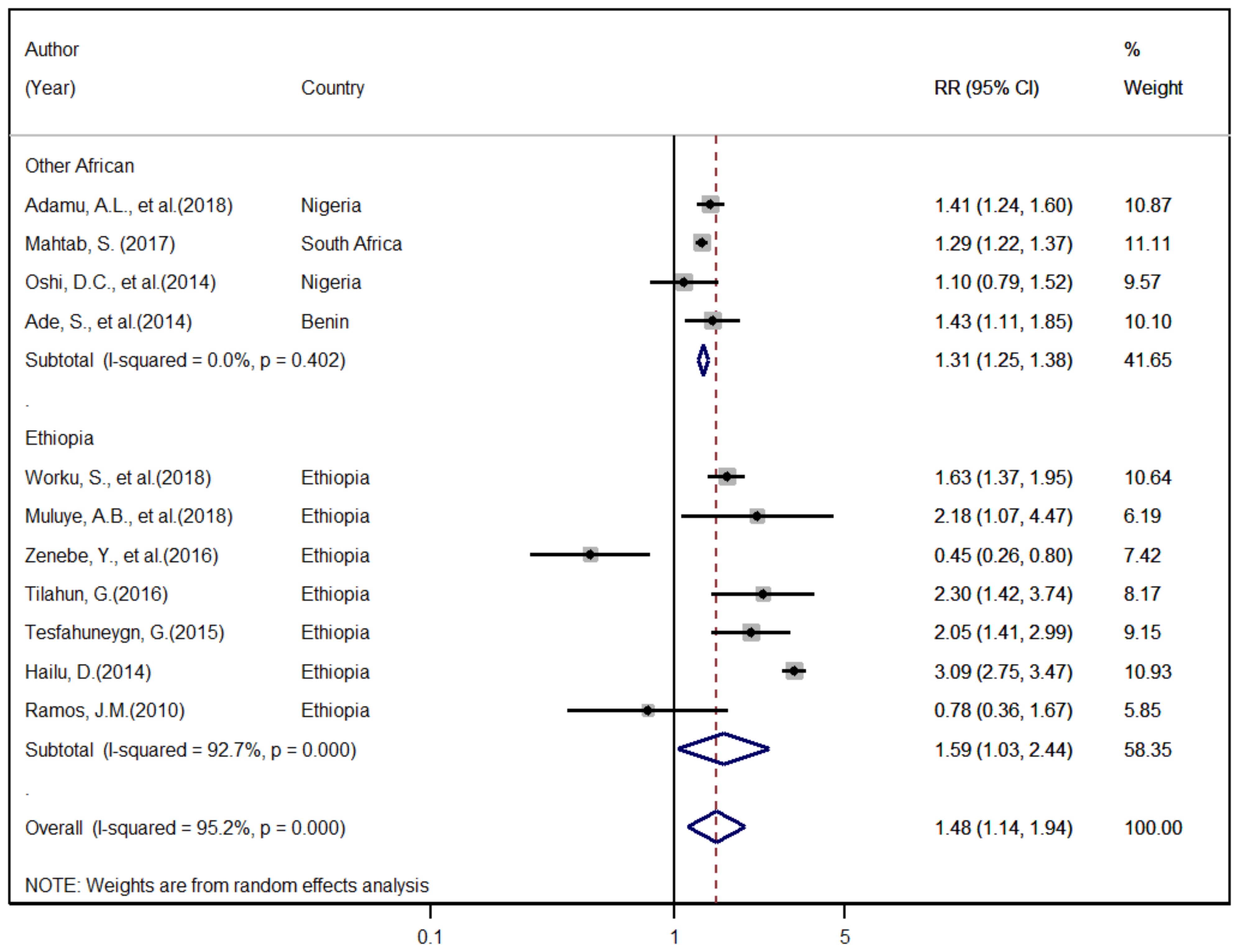
3.6. Meta-Analysis
3.6.1. The Overall TB Treatment Success Rate
3.6.2. Factors Associated with Unsuccessful TB Treatment Outcome
3.6.3. Publication Bias
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacNeil, A.; Glaziou, P.; Sismanidis, C.; Maloney, S.; Floyd, K. Global Epidemiology of Tuberculosis and Progress Toward Achieving Global Targets—2017. Morb. Mortal. Wkly. Rep. 2019, 68, 263–266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organisation. Global Tuberculosis Report 2020: Executive Summary; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- Murray, C.J.L.; Ortblad, K.F.; Guinovart, C.; Lim, S.S.; Wolock, T.M.; Roberts, D.A.; A Dansereau, E.; Graetz, N.; Barber, R.M.; Brown, J.C.; et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 1005–1070. [Google Scholar] [CrossRef]
- Soares, E.C.; Vollmer, W.M.; Cavalcante, S.C.; Pacheco, A.G.; Saraceni, V.; Silva, J.S.; Neves, G.R.; Golub, J.E.; Efron, A.R.; Durovni, B.; et al. Tuberculosis control in a socially vulnerable area: A community intervention beyond DOT in a Brazilian favela. Int. J. Tuberc. Lung Dis. 2013, 17, 1581–1586. [Google Scholar] [CrossRef]
- Chaves Torres, N.M.; Quijano Rodríguez, J.J.; Porras Andrade, P.S.; Arriaga, M.B.; Netto, E.M. Factors predictive of the success of tuberculosis treatment: A systematic review with meta-analysis. PLoS ONE 2019, 14, e0226507. [Google Scholar] [CrossRef]
- Aketi, L.; Kashongwe, Z.; Kinsiona, C.; Fueza, S.B.; Kokolomami, J.; Bolie, G.; Lumbala, P.; Diayisu, J.S. Childhood Tuberculosis in a Sub-Saharan Tertiary Facility: Epidemiology and Factors Associated with Treatment Outcome. PLoS ONE 2016, 11, e0153914. [Google Scholar] [CrossRef] [PubMed]
- Gadoev, J.; Asadov, D.; Tillashaykhov, M.; Tayler-Smith, K.; Isaakidis, P.; Dadu, A.; de Pierpaolo, C.; Sven, G.H.; Nargiza, P.; Dilrabo, U.; et al. Factors associated with unfavorable treatment outcomes in new and previously treated TB patients in Uzbekistan: A five year countrywide study. PLoS ONE 2015, 10, e0128907. [Google Scholar] [CrossRef]
- Oshi, D.C.; Oshi, S.N.; Alobu, I.; Ukwaja, K.N. Profile and treatment outcomes of tuberculosis in the elderly in southeastern Nigeria, 2011–2012. PLoS ONE 2014, 9, e111910. [Google Scholar] [CrossRef] [PubMed]
- Gebrezgabiher, G.; Romha, G.; Ejeta, E.; Asebe, G.; Zemene, E.; Ameni, G. Treatment outcome of tuberculosis patients under directly observed treatment short course and factors affecting outcome in Southern Ethiopia: A five-year retrospective study. PLoS ONE 2016, 11, e0150560. [Google Scholar] [CrossRef]
- Mok, J.; An, D.; Kim, S.; Lee, M.; Kim, C.; Son, H. Treatment outcomes and factors affecting treatment outcomes of new patients with tuberculosis in Busan, South Korea: A retrospective study of a citywide registry, 2014–2015. BMC Infect. Dis. 2018, 18, 655. [Google Scholar] [CrossRef]
- Eshetie, S.; Gizachew, M.; Alebel, A.; van Soolingen, D. Tuberculosis treatment outcomes in Ethiopia from 2003 to 2016, and impact of HIV co-infection and prior drug exposure: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0194675. [Google Scholar] [CrossRef]
- Asres, A.; Jerene, D.; Deressa, W. Delays to treatment initiation is associated with tuberculosis treatment outcomes among patients on directly observed treatment short course in Southwest Ethiopia: A follow-up study. BMC Pulm. Med. 2018, 18, 64. [Google Scholar] [CrossRef]
- Adamu, A.L.; Aliyu, M.H.; Galadanci, N.A.; Musa, B.M.; Lawan, U.M.; Bashir, U.; Abubakar, I. The impact of rural residence and HIV infection on poor tuberculosis treatment outcomes in a large urban hospital: A retrospective cohort analysis. Int. J. Equity Health 2018, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ohene, S.A.; Fordah, S.; Dela Boni, P. Childhood tuberculosis and treatment outcomes in Accra: A retrospective analysis. BMC Infect. Dis. 2019, 19, 749. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Rodrigues, L.C.; Waldman, E.A.; Prina, E.; Carvalho, C.R.R. Who are the patients with tuberculosis who are diagnosed in emergency facilities? An analysis of treatment outcomes in the state of Sao Paulo, Brazil. J. Bras. Pneumol. 2018, 44, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Waitt, C.; Squire, S. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2011, 15, 871–885. [Google Scholar] [CrossRef]
- Mbatchou Ngahane, B.H.; Dahirou, F.; Tchieche, C.; Wandji, A.; Ngnié, C.; Nana-Metchedjin, A.; Nyankiyé, E.; Endale Mangamba, M.L.; Kuaban, C. Clinical characteristics and outcomes of tuberculosis in Douala, Cameroon: A 7-year retrospective cohort study. Int. J. Tuberc. Lung Dis. 2016, 20, 1609–1614. [Google Scholar] [CrossRef]
- Schwœbel, V.; Trébucq, A.; Kashongwe, Z.; Bakayoko, A.S.; Kuaban, C.; Noeske, J.; Harouna, S.H.; Souleymane, M.B.; Piubello, A.; Ciza, F.; et al. Outcomes of a nine-month regimen for rifampicin-resistant tuberculosis up to 24 months after treatment completion in nine African countries. EClinicalMedicine 2020, 20, 100268. [Google Scholar] [CrossRef]
- Lönnroth, K.; Raviglione, M. The WHO’s new End TB Strategy in the post-2015 era of the Sustainable Development Goals. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 148–150. [Google Scholar] [CrossRef]
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- World Health Organization. Definitions and Reporting Framework for Tuberculosis—2013 Revision: Updated December 2014 and January 2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- The Joanna Briggs Institute. Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument; The Joanna Briggs Institute: Adelaide, Australia, 2007. [Google Scholar]
- Wobudeya, E.; Jaganath, D.; Sekadde, M.P.; Nsangi, B.; Haq, H.; Cattamanchi, A. Outcomes of empiric treatment for pediatric tuberculosis, Kampala, Uganda, 2010–2015. BMC Public Health 2019, 19, 446. [Google Scholar] [CrossRef]
- Pizzol, D.; Veronese, N.; Marotta, C.; Di Gennaro, F.; Moiane, J.; Chhaganlal, K.; Monno, L.; Putoto, G.; Mazzucco, W.; Saracino, A. Predictors of therapy failure in newly diagnosed pulmonary tuberculosis cases in Beira, Mozambique. BMC Res. Notes 2018, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Lee, K.; Du Preez, K.; Dunbar, R.; Hesseling, A.C.; Seddon, J.A. Excellent treatment outcomes in children treated for tuberculosis under routine operational conditions in Cape Town, South Africa. Clin. Infect. Dis. 2017, 65, 1444–1452. [Google Scholar] [CrossRef]
- Adamu, A.L.; Aliyu, M.H.; Galadanci, N.A.; Musa, B.M.; Gadanya, M.A.; Gajida, A.U.; Amole, T.G.; Bello, I.W.; Gambo, S.; Abubakar, I. Deaths during tuberculosis treatment among paediatric patients in a large tertiary hospital in Nigeria. PLoS ONE 2017, 12, e0183270. [Google Scholar] [CrossRef]
- Tilahun, G.; Gebre-Selassie, S. Treatment outcomes of childhood tuberculosis in Addis Ababa: A five-year retrospective analysis. BMC Public Health 2016, 16, 612. [Google Scholar] [CrossRef] [PubMed]
- Ogbudebe, C.L.; Izuogu, S.; Abu, C.E. Magnitude and treatment outcomes of pulmonary tuberculosis patients in a poor urban slum of Abia State, Nigeria. Int. J. Mycobacteriol. 2016, 5, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Flick, R.J.; Kim, M.H.; Simon, K.; Munthali, A.; Hosseinipour, M.C.; Rosenberg, N.E.; Kazembe, P.N.; Mpunga, J.; Ahmed, S. Burden of disease and risk factors for death among children treated for tuberculosis in Malawi. Int. J. Tuberc. Lung Dis. 2016, 20, 1046–1054. [Google Scholar] [CrossRef]
- Tesfahuneygn, G.; Medhin, G.; Legesse, M. Adherence to Anti-tuberculosis treatment and treatment outcomes among tuberculosis patients in Alamata District, northeast Ethiopia. BMC Res. Notes 2015, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Hailu, D.; Abegaz, W.E.; Belay, M. Childhood tuberculosis and its treatment outcomes in Addis Ababa: A 5-years retrospective study. BMC Pediatr. 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Ade, S.; Harries, A.D.; Trébucq, A.; Ade, G.; Agodokpessi, G.; Adjonou, C.; Azon, S.; Anagonou, S. National profile and treatment outcomes of patients with extrapulmonary tuberculosis in Benin. PLoS ONE 2014, 9, e95603. [Google Scholar] [CrossRef]
- Kayigamba, F.R.; Bakker, M.I.; Mugisha, V.; De Naeyer, L.; Gasana, M.; Cobelens, F.; van der Loeff, M.S. Adherence to tuberculosis treatment, sputum smear conversion and mortality: A retrospective cohort study in 48 Rwandan clinics. PLoS ONE 2013, 8, e73501. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.; Reyes, F.; Tesfamariam, A. Childhood and adult tuberculosis in a rural hospital in Southeast Ethiopia: A ten-year retrospective study. BMC Public Health 2010, 10, 215. [Google Scholar] [CrossRef]
- Berry, K.M.; Rodriguez, C.A.; Berhanu, R.H.; Ismail, N.; Mvusi, L.; Long, L.; Evans, D. Treatment outcomes among children, adolescents, and adults on treatment for tuberculosis in two metropolitan municipalities in Gauteng Province, South Africa. BMC Public Health 2019, 19, 973. [Google Scholar] [CrossRef] [PubMed]
- Worku, S.; Derbie, A.; Mekonnen, D.; Biadglegne, F. Treatment outcomes of tuberculosis patients under directly observed treatment short-course at Debre Tabor General Hospital, northwest Ethiopia: Nine-years retrospective study. Infect. Dis. Poverty 2018, 7, 16. [Google Scholar] [CrossRef]
- Muluye, A.B.; Kebamo, S.; Teklie, T.; Alemkere, G. Poor treatment outcomes and its determinants among tuberculosis patients in selected health facilities in East Wollega, Western Ethiopia. PLoS ONE 2018, 13, e0206227. [Google Scholar]
- Mahtab, S.; Coetzee, D. Influence of HIV and other risk factors on tuberculosis. S. Afr. Med. J. 2017, 107, 428–434. [Google Scholar] [CrossRef]
- Kebede, Z.T.; Taye, B.W.; Matebe, Y.H. Childhood tuberculosis: Management and treatment outcomes among children in Northwest Ethiopia: A cross-sectional study. Pan Afr. Med. J. 2017, 27, 25. [Google Scholar] [CrossRef]
- Engelbrecht, M.C.; Kigozi, N.G.; Chikobvu, P.; Botha, S.; van Rensburg, H.C.J. Unsuccessful TB treatment outcomes with a focus on HIV co-infected cases: A cross-sectional retrospective record review in a high-burdened province of South Africa. BMC Health Serv. Res. 2017, 17, 470. [Google Scholar] [CrossRef]
- Zenebe, Y.; Adem, Y.; Mekonnen, D.; Derbie, A.; Bereded, F.; Bantie, M.; Tulu, B.; Hailu, D.; Biadglegne, F. Profile of tuberculosis and its response to anti-TB drugs among tuberculosis patients treated under the TB control programme at Felege-Hiwot Referral Hospital, Ethiopia. BMC Public Health 2016, 16, 688. [Google Scholar] [CrossRef]
- García-Basteiro, A.L.; Respeito, D.; Augusto, O.J.; López-Varela, E.; Sacoor, C.; Sequera, V.G.; Casellas, A.; Bassat, Q.; Manhica, L.; Macete, E.; et al. Poor tuberculosis treatment outcomes in Southern Mozambique (2011–2012). BMC Infect. Dis. 2016, 16, 214. [Google Scholar]
- Mirutse, G.; Fang, M.; Kahsay, A.B.; Ma, X. Epidemiology of childhood tuberculosis and factors associated with unsuccessful treatment outcomes in Tigray, Ethiopia: A ten-year retrospective cross sectional study. BMC Public Health 2019, 19, 1367. [Google Scholar] [CrossRef]
- Izudi, J.; Semakula, D.; Sennono, R.; Tamwesigire, I.K.; Bajunirwe, F. Treatment success rate among adult pulmonary tuberculosis patients in sub-Saharan Africa: A systematic review and meta-analysis. BMJ Open 2019, 9, e029400. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Viney, K.; Gray, D.J.; McBryde, E.S.; Wagnew, M.; Clements, A.C. Mapping tuberculosis treatment outcomes in Ethiopia. BMC Infect. Dis. 2019, 19, 474. [Google Scholar] [CrossRef]
- Shimeles, E.; Enquselassie, F.; Aseffa, A.; Tilahun, M.; Mekonen, A.; Wondimagegn, G.; Hailu, T. Risk factors for tuberculosis: A case-control study in Addis Ababa, Ethiopia. PLoS ONE 2019, 14, e0214235. [Google Scholar] [CrossRef]
- Pai, M.; Memish, Z.A. Global tuberculosis control requires greater ambition and resources. J. Epidemiol. Glob. Health 2015, 5, 1–2. [Google Scholar] [CrossRef]
- Lönnroth, K.; Migliori, G.B.; Abubakar, I.; D’Ambrosio, L.; De Vries, G.; Diel, R.; Douglas, P.; Falzon, D.; Gaudreau, M.-A.; Goletti, D.; et al. Towards tuberculosis elimination: An action framework for low-incidence countries. Eur. Respir. J. 2015, 45, 928–952. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.J.; Esmaili, B.E.; Cunningham, C.K. Barriers to initiating tuberculosis treatment in sub-Saharan Africa: A systematic review focused on children and youth. Glob. Health Action 2017, 10, 1290317. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, J.; Bu, Q.; He, H.; Bai, L.; Yang, J.; Liu, Q.; Lyi, J. The Gap Between Global Tuberculosis Incidence and the First Milestone of the WHO End Tuberculosis Strategy: An Analysis Based on the Global Burden of Disease 2017 Database. Infect. Drug Resist. 2020, 13, 1281. [Google Scholar] [CrossRef] [PubMed]
- Deribew, A.; Deribe, K.; Dejene, T.; Tessema, G.A.; Melaku, Y.A.; Lakew, Y.; Amare, A.T.; Bekele, T.; Abera, S.F.; Dessalegn, M.; et al. Tuberculosis Burden in Ethiopia from 1990 to 2016: Evidence from the Global Burden of Diseases 2016 Study. Ethiop. J. Health Sci. 2018, 28, 519–528. [Google Scholar]
- Ogbuabor, D.C.; Onwujekwe, O.E. Governance of tuberculosis control programme in Nigeria. Infect. Dis. Poverty 2019, 8, 45. [Google Scholar] [CrossRef]
- Desta, K.T.; Masango, T.E.; Nkosi, Z.Z. Performance of the National Tuberculosis Control Program in the post conflict Liberia. PLoS ONE 2018, 13, e0199474. [Google Scholar] [CrossRef]
- Alipanah, N.; Jarlsberg, L.; Miller, C.; Linh, N.N.; Falzon, D.; Jaramillo, E.; Nahid, P. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018, 15, e1002595. [Google Scholar] [CrossRef]
- Harries, A.D.; Lin, Y.; Kumar, A.M.; Satyanarayana, S.; Takarinda, K.C.; Dlodlo, R.A.; Zachariah, R.; Olliaro, P. What can National TB Control Programmes in low- and middle-income countries do to end tuberculosis by 2030? F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Belay, G.M.; Wubneh, C.A. Childhood tuberculosis treatment outcome and its association with HIV co-infection in Ethiopia: A systematic review and meta-analysis. Trop. Med. Health 2020, 48, 7. [Google Scholar] [CrossRef]
- Seid, M.A.; Ayalew, M.B.; Muche, E.A.; Gebreyohannes, E.A.; Abegaz, T.M. Drug-susceptible tuberculosis treatment success and associated factors in Ethiopia from 2005 to 2017: A systematic review and meta-analysis. BMJ Open 2018, 8, e022111. [Google Scholar] [CrossRef]
- Zegeye, A.; Dessie, G.; Wagnew, F.; Gebrie, A.; Islam, S.M.S.; Tesfaye, B.; Kiross, D. Prevalence and determinants of anti-tuberculosis treatment non-adherence in Ethiopia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210422. [Google Scholar] [CrossRef]
- Castelnuovo, B. A review of compliance to anti tuberculosis treatment and risk factors for defaulting treatment in Sub Saharan Africa. Afr. Health Sci. 2010, 10, 320–324. [Google Scholar]
- Alobu, I.; Oshi, S.N.; Oshi, D.C.; Ukwaja, K.N. Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting. Asian Pac. J. Trop. Med. 2014, 7, 977–984. [Google Scholar] [CrossRef]
- Müller, A.M.; Osório, C.S.; Silva, D.R.; Sbruzzi, G.; de Tarso, P.; Dalcin, R. Interventions to improve adherence to tuberculosis treatment: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2018, 22, 731–740. [Google Scholar] [CrossRef]
- Ketema, D.B.; Muchie, K.F.; Andargie, A.A. Time to poor treatment outcome and its predictors among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara region, Ethiopia: Retrospective cohort study. BMC Public Health 2019, 19, 1481. [Google Scholar] [CrossRef] [PubMed]
- Snow, K.; Hesseling, A.C.; Naidoo, P.; Graham, S.M.; Denholm, J.; Du Preez, K. Tuberculosis in adolescents and young adults: Epidemiology and treatment outcomes in the Western Cape. Int. J. Tuberc. Lung Dis. 2017, 21, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Mamo, A.; Mama, M.; Solomon, D.; Mohammed, M. Treatment outcomes and predictors among tuberculosis patients at Madda Walabu University Goba Referral Hospital, southeast Ethiopia. Infect. Drug Resist. 2020, 13, 4763. [Google Scholar]
- Atif, M.; Anwar, Z.; Fatima, R.K.; Malik, I.; Asghar, S.; Scahill, S. Analysis of tuberculosis treatment outcomes among pulmonary tuberculosis patients in Bahawalpur, Pakistan. BMC Res. Notes 2018, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Pradipta, I.S.; van’t Boveneind-Vrubleuskaya, N.; Akkerman, O.W.; Alffenaar, J.W.C.; Hak, E. Predictors for treatment outcomes among patients with drug-susceptible tuberculosis in the Netherlands: A retrospective cohort study. Clin. Microbiol. Infect. 2019, 25, 761.e1–761.e7. [Google Scholar] [CrossRef] [PubMed]

| ID | Author (Year) | Country | Sample Size | Overall Success | Treatment Success Rate Among Different Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | % | Child | Adult | Male | Female | HIV Pos | HIV Neg | Retreatm | New TB | EPTB | PTB | ||||
| 1 | Wobudeya, E. et al., (2019) [25] | Uganda | 516 | 422 | 81.78 | 81.78 | 83.80 | 79.31 | 61.29 | 83.09 | |||||
| 2 | Mirutse, G. et al., (2019) [45] | Ethiopia | 840 | 746 | 88.81 | 88.81 | 92.14 | 87.14 | 91.23 | 90.94 | 91.43 | 84.18 | |||
| 3 | Berry, K.M. (2019) [37] | South Africa | 17007 | 15262 | 89.80 | 88.97 | |||||||||
| 4 | Worku, S. et al., (2018) [38] | Ethiopia | 985 | 672 | 68.22 | 61.62 | 68.96 | 66.67 | 69.94 | 65.13 | 69.97 | 29.41 | 66.11 | 69.97 | 67.20 |
| 5 | Muluye, A.B. et al., (2018) [39] | Ethiopia | 995 | 914 | 91.86 | 90.91 | 91.99 | 90.27 | 94.13 | 88.24 | 92.80 | 81.58 | 92.33 | 92.54 | 91.34 |
| 6 | Asres, A. (2018) [13] | Ethiopia | 699 | 627 | 89.70 | 89.70 | 89.67 | 89.74 | 75.81 | 91.05 | 89.70 | 89.93 | 89.25 | ||
| 7 | Adamu, A.L. et al., (2018) [14] | Nigeria | 1381 | 745 | 53.95 | 53.95 | 92.28 | 24.84 | 52.00 | 55.89 | 33.82 | 60.52 | 56.82 | ||
| 8 | Pizzol, D. et al., (2018) [26] | Mozambique | 301 | 239 | 79.40 | 70.23 | 86.47 | ||||||||
| 9 | Osman, M. et al., (2017) [27] | South Africa | 29,519 | 25,353 | 85.89 | 85.89 | 78.08 | 88.88 | |||||||
| 10 | Engelbrecht et al., (2017) [42] | South Africa | 66,940 | 51,668 | 77.19 | 77.19 | 74.38 | 80.04 | 75.52 | 84.70 | 77.19 | ||||
| 11 | Mahtab, S. (2017) [40] | South Africa | 12,672 | 8870 | 70.00 | 69.53 | 70.55 | 66.56 | 73.50 | 62.50 | 73.28 | 67.20 | 71.78 | ||
| 12 | Kebede, Z.T. (2017) [41] | Ethiopia | 227 | 179 | 78.85 | 78.85 | 75.00 | 85.19 | |||||||
| 13 | Adamu, A.L. et al., (2017) [28] | Nigeria | 299 | 157 | 52.51 | 52.51 | |||||||||
| 14 | Flick, R.J. et al., (2016) [31] | Malawi | 295 | 228 | 77.29 | 77.29 | |||||||||
| 15 | Aketi, L. et al., (2016) [7] | DRK | 283 | 197 | 69.61 | 69.61 | 57.14 | 72.31 | 69.81 | 70.49 | |||||
| 16 | Zenebe, Y. et al., (2016) [43] | Ethiopia | 671 | 542 | 80.77 | 87.50 | 80.26 | 84.02 | 75.58 | 61.33 | 88.03 | 91.18 | 78.13 | 76.83 | 84.55 |
| 17 | Tilahun, G. (2016) [29] | Ethiopia | 491 | 420 | 85.54 | 85.54 | 85.84 | 85.29 | 70.73 | 93.78 | 59.46 | 85.68 | 86.83 | 84.27 | |
| 18 | Garcia-Basteiro, A.L. et al., (2016) [44] | Mozambique | 1957 | 1393 | 71.18 | 71.03 | 77.22 | ||||||||
| 19 | Ogbudebe, C.L. (2016) [30] | Nigeria | 555 | 479 | 86.31 | 95.24 | 83.90 | 86.71 | 81.17 | 71.28 | 90.24 | ||||
| 20 | Gebrezgabiher, G. et al., (2016) [10] | Ethiopia | 1537 | 1310 | 85.23 | 83.17 | 83.75 | 85.99 | 84.03 | 81.92 | 85.66 | ||||
| 21 | Tesfahuneygn, G. (2015) [32] | Ethiopia | 4275 | 3853 | 90.13 | 90.13 | 89.73 | 90.70 | 84.50 | 90.54 | 78.18 | 90.44 | 92.08 | 87.54 | |
| 22 | Hailu, D. (2014) [33] | Ethiopia | 2708 | 2193 | 80.98 | 80.98 | 81.81 | 81.15 | 80.06 | 84.76 | 4.97 | 81.27 | 81.96 | 80.73 | |
| 23 | Oshi, D.C. et al., (2014) [9] | Nigeria | 1668 | 1268 | 76.02 | 76.02 | 74.45 | 78.16 | 65.79 | 78.66 | 73.28 | 76.22 | 45.74 | ||
| 24 | Ade, S. et al., (2014) [34] | Benin | 3714 | 3319 | 89.36 | 84.87 | 89.89 | 86.42 | |||||||
| 25 | Kayigamba, F.R. et al., (2013) [35] | Rwanda | 581 | 457 | 78.66 | 78.66 | |||||||||
| 26 | Ramos, J.M. (2010) [36] | Ethiopia | 2225 | 1484 | 66.70 | 66.38 | 66.97 | 81.48 | 84.45 | 87.50 | 83.35 | 83.38 | 83.23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teferi, M.Y.; El-Khatib, Z.; Boltena, M.T.; Andualem, A.T.; Asamoah, B.O.; Biru, M.; Adane, H.T. Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10678. https://doi.org/10.3390/ijerph182010678
Teferi MY, El-Khatib Z, Boltena MT, Andualem AT, Asamoah BO, Biru M, Adane HT. Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(20):10678. https://doi.org/10.3390/ijerph182010678
Chicago/Turabian StyleTeferi, Melese Yeshambaw, Ziad El-Khatib, Minyahil Tadesse Boltena, Azeb Tarekegn Andualem, Benedict Oppong Asamoah, Mulatu Biru, and Hawult Taye Adane. 2021. "Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 20: 10678. https://doi.org/10.3390/ijerph182010678
APA StyleTeferi, M. Y., El-Khatib, Z., Boltena, M. T., Andualem, A. T., Asamoah, B. O., Biru, M., & Adane, H. T. (2021). Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(20), 10678. https://doi.org/10.3390/ijerph182010678








