Capillaroscopic Evidence of Microvascular Damage in Volleyball Players
Abstract
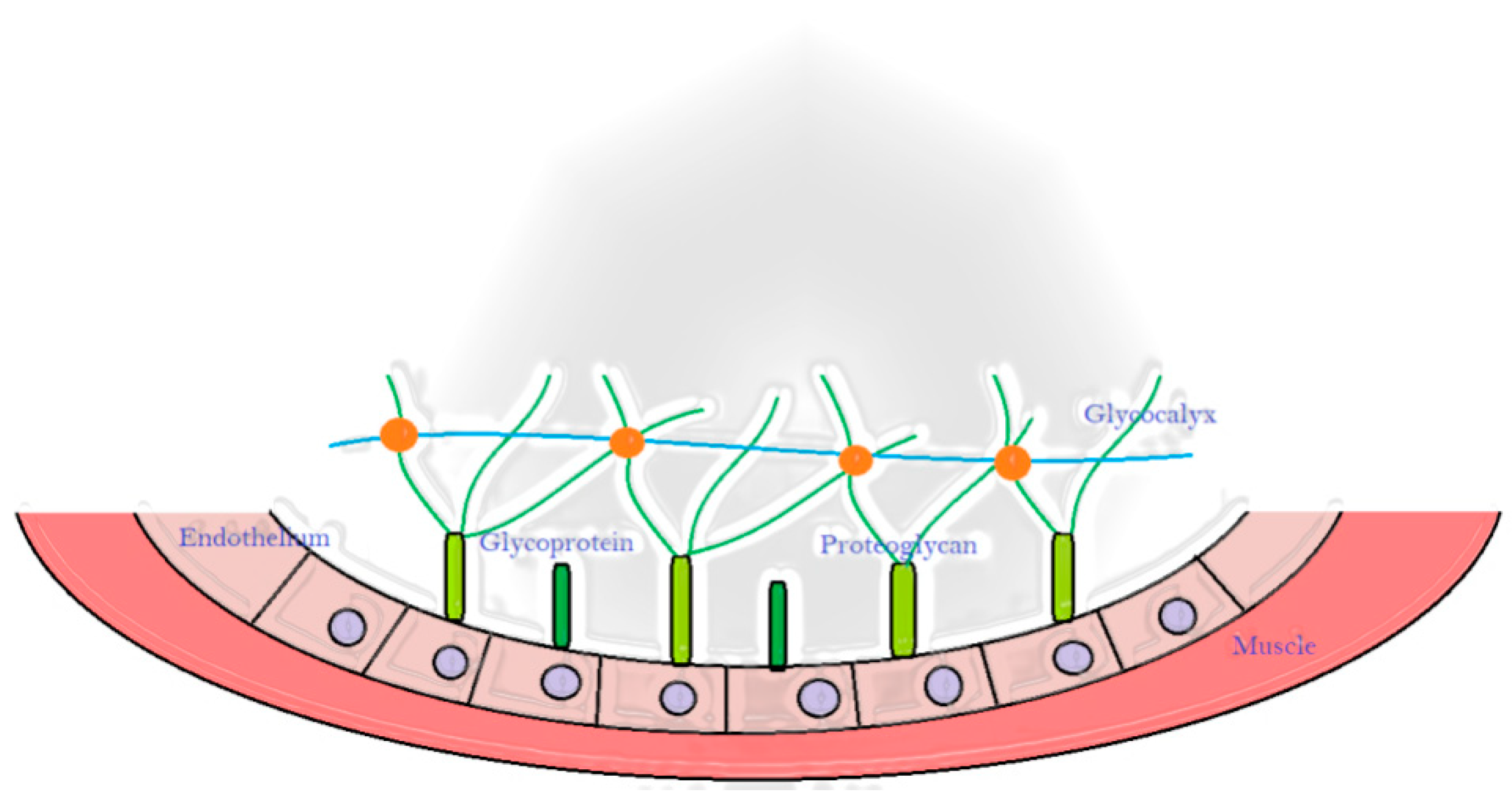
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Statistical Analysis
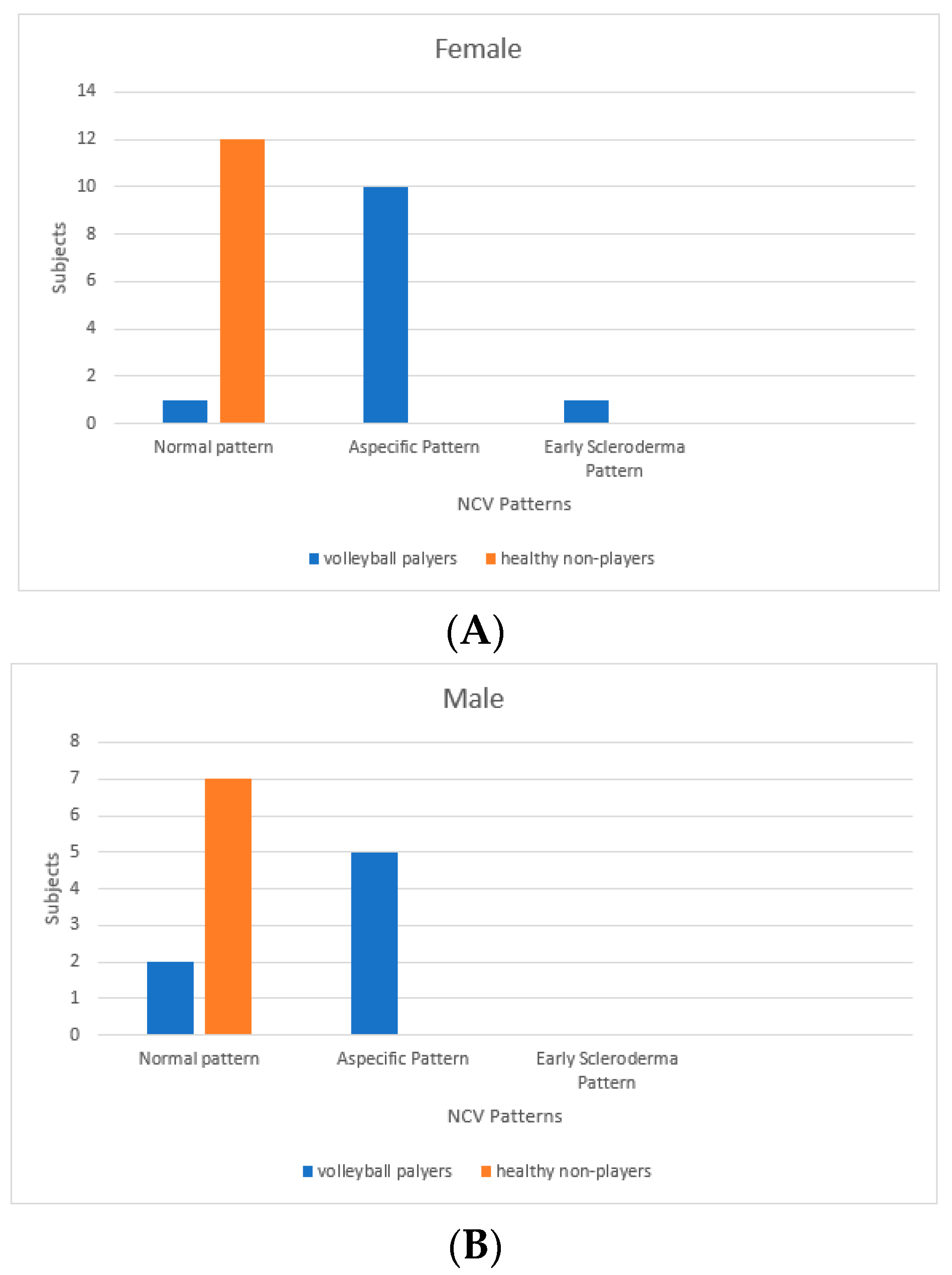
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fédération Internationale de Volleyball. Available online: http://www.fivb.com/en/thefivb/history (accessed on 27 July 2021).
- Bahr, R.; Bahr, I.A. Incidence of acute volleyball injuries: A prospective cohort study of injury mechanisms and risk factors. Scand. J. Med. Sci. Sports 1997, 7, 166–171. [Google Scholar] [CrossRef]
- Kilic, O.; Maas, M.; Verhagen, E.; Zwerver, J.; Gouttebarge, V. Incidence, aetiology and prevention of musculoskeletal injuries in volleyball: A systematic review of the literature. Eur. J. Sport Sci. 2017, 17, 765–793. [Google Scholar] [CrossRef]
- Kadi, F.; Hägg, G.; Håkansson, R.; Holmner, S.; Butler-Browne, G.S.; Thornell, L.E. Structural changes in male trapezius muscle with work-related myalgia. Acta Neuropathol. 1998, 95, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lindman, R.; Hagberg, M.; Angqvist, K.A.; Söderlund, K.; Hultman, E.; Thornell, L.E. Changes in muscle morphology in chronic trapezius myalgia. Scand. J. Work Environ. Health 1991, 17, 347–355. [Google Scholar] [CrossRef]
- Kostianen, S.; Orava, S. Blunt injury of the radial and ulnar arteries in volley ball players. A report of three cases of the antebrachial-palmar hammer syndrome. Br. J. Sports Med. 1983, 17, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N. Ischemia-reperfusion: Mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation 1999, 6, 167–178. [Google Scholar] [CrossRef]
- Seal, J.B.; Gewertz, B.L. Vascular dysfunction in ischemia-reperfusion injury. Ann. Vasc. Surg. 2005, 19, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial function: A critical determinant in atherosclerosis? Circulation 2004, 109 (Suppl. 1), II27–II33. [Google Scholar] [CrossRef]
- Patel, J.C. Functions of endothelium. Indian J. Med. Sci. 2001, 55, 165–166. [Google Scholar]
- Verhamme, P.; Hoylaerts, M.F. The pivotal role of the endothelium in haemostasis and thrombosis. Acta ClinicaBelgica 2006, 61, 213–219. [Google Scholar] [CrossRef]
- Oliver, M.G.; Specian, R.D.; Perry, M.A.; Granger, D.N. Morphologic assessment of leukocyte-endothelial cell interactions in mesenteric venules subjected to ischemia and reperfusion. Inflammation 1991, 15, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Kurose, I.; Argenbright, L.W.; Wolf, R.; Lianxi, L.; Granger, D.N. Ischemia/reperfusion-induced microvascular dysfunction: Role of oxidants and lipid mediators. Am. J. Physiol. 1997, 272, H2976–H2982. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, B.; Glasz, J.; Menger, M.D.; Messmer, K. Leukocytes contribute to hepatic ischemia/reperfusion injury via intercellular adhesion molecule-1-mediated venular adherence. Surgery 1995, 117, 195–200. [Google Scholar] [CrossRef]
- Kupinski, A.M.; Shah, D.M.; Bell, D.R. Transvascular albumin flux in rabbit hindlimb after tourniquet ischemia. Am. J. Physiol. 1993, 264, H901–H908. [Google Scholar] [CrossRef]
- Greenstein, D.; Gupta, N.K.; Martin, P.; Walker, D.R.; Kester, R.C. Impaired thermoregulation in Raynaud’s phenomenon. Angiology 1995, 46, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, G.; Xiao, B.; Lin, H.; Qu, H.; Zhang, D.; Shi, M.; Lang, L.; Yang, B.; Yan, M. Nailfold capillary morphological characteristics of hand-arm vibration syndrome: A cross-sectional study. BMJ Open 2016, 6, e012983. [Google Scholar] [CrossRef]
- Khan, F.; Belch, J.J. Skin blood flow in patients with systemic sclerosis and Raynaud’s phenomenon: Effects of oral L-arginine supplementation. J. Rheumatol. 1999, 26, 2389–2394. [Google Scholar]
- White, C.R.; Haidekker, M.A.; Stevens, H.Y.; Frangos, J.A. Extracellular signal-regulated kinase activation and endothelin-1 production in human endothelial cells exposed to vibration. J. Physiol. 2004, 555, 565–572. [Google Scholar] [CrossRef]
- Suttles, J.; Milhorn, D.M.; Miller, R.W.; Poe, J.C.; Wahl, L.M.; Stout, R.D. CD40 signaling of monocyte inflammatory cytokine synthesis through an ERK1/2-dependent pathway. A target of interleukin (il)-4 and il-10 anti-inflammatory action. J. Biol. Chem. 1999, 274, 5835–5842. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Raynaud’s phenomenon and nailfold capillaroscopic findings in anorexia nervosa. Curr. Med. Res. Opin. 2018, 34, 547–550. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Smith, V. How to perform and interpret capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2013, 27, 237–248. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Nailfold capillaroscopic findings in a semi-professional volleyball player. Clin. Hemorheol. Microcirc. 2020, 74, 281–285. [Google Scholar] [CrossRef]
- Herrick, A.L.; Berks, M.; Taylor, C.J. Quantitative nailfold capillaroscopy-update and possible next steps. Rheumatol. Oxford 2021, 60, 2054–2065. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Czirják, L.; Kumánovics, G. Patients with Systemic Sclerosis with and without Overlap Syndrome Show Similar Microvascular Abnormalities. Diagnostics 2021, 11, 1606. [Google Scholar] [CrossRef]
- Batko, B.; Maga, P.; Urbanski, K.; Ryszawa-Mrozek, N.; Schramm-Luc, A.; Koziej, M.; Mikolajczyk, T.; McGinnigle, E.; Czesnikiewicz-Guzik, M.; Ceranowicz, P.; et al. Microvascular dysfunction in ankylosing spondylitis is associated with disease activity and is improved by anti-TNF treatment. Sci. Rep. 2018, 8, 13205. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Ginaldi, L.; De Martinis, M. Microvascular Damage in a Young Female Archer Assessed by Nailfold Videocapillaroscopy: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 4218. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM 2019, 112, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Catalogna, A.; De Pietro, F.; Ginaldi, L.; De Martinis, M. Raynaud’s phenomenon in a drummer player: Microvascular disorder and nailfold video capillaroscopic findings. Int. J. Environ. Res. Public Health 2021, submitted. [Google Scholar]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Nailfold Capillaroscopic Findings in an Orthopedic Surgeon: Reversible Abnormalities after the Cessation of Radiation Exposure. Radiat. Res. 2020, 193, 236–240. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, W.J.; Yao, J.S.; Schafer, M.F.; Nuber, G.; Flinn, W.R.; Blackburn, D.; Suker, J.R. Upper extremity arterial injury in athletes. J. Vasc. Surg. 1989, 9, 317–327. [Google Scholar] [CrossRef]
- Vlychou, M.; Spanomichos, G.; Chatziioannou, A.; Georganas, M.; Zavras, G.M. Embolisation of a traumatic aneurysm of the posterior circumflex humeral artery in a volleyball player. Br. J. Sports Med. 2001, 35, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Dearing, J. Volleyball Fundamentals; OCLC 50643900; Human Kinetics: Champaign, IL, USA, 2003; ISBN 0736045082. [Google Scholar]
- Stoyneva, Z.B.; Dermendjiev, S.M. Specific features of vibration-induced disorders. Folia Med. Plovdiv 2010, 52, 27–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sirufo, M.M.; Catalogna, A.; De Pietro, F.; Ginaldi, L.; De Martinis, M. Raynaud’s phenomenon in a drummer player: Microvascular disorder and nailfold video capillaroscopic findings. EXCLI J in press. 2021. [Google Scholar]
- Gemne, G. Diagnostics of hand-arm system disorders in workers who use vibrating tools. Occup. Environ. Med. 1997, 54, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.I.; Costello, J.T.; Bailey, S.J.; Bishop, N.; Wadley, A.J.; Young-Min, S.; Gilchrist, M.; Mayes, H.; White, D.; Gorczynski, P.; et al. “Beet” the cold: Beetroot juice supplementation improves peripheral blood flow, endothelial function, and anti-inflammatory status in individuals with Raynaud’s phenomenon. J. Appl. Physiol. 2019, 127, 1478–1490. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; Oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Rubio-Gayosso, I.; Platts, S.H.; Duling, B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ Physiol. 2006, 290, H2247–H2256. [Google Scholar] [CrossRef]
- Mulivor, A.W.; Lipowsky, H.H. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1672–H1680. [Google Scholar] [CrossRef]
- Rosenbaum, Y.A.; Awan, H.M. Acute hand injuries in athletes. Phys. Sportsmed. 2017, 45, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Temprano, K.K. A Review of Raynaud’s Disease. Mo Med. 2016, 113, 123–126. [Google Scholar] [PubMed]
- Rosato, E.; Borghese, F.; Pisarri, S.; Salsano, F. The treatment with N-acetylcysteine of Raynaud’s phenomenon and ischemic ulcers therapy in sclerodermic patients: A prospective observational study of 50 patients. Clin. Rheumatol. 2009, 28, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
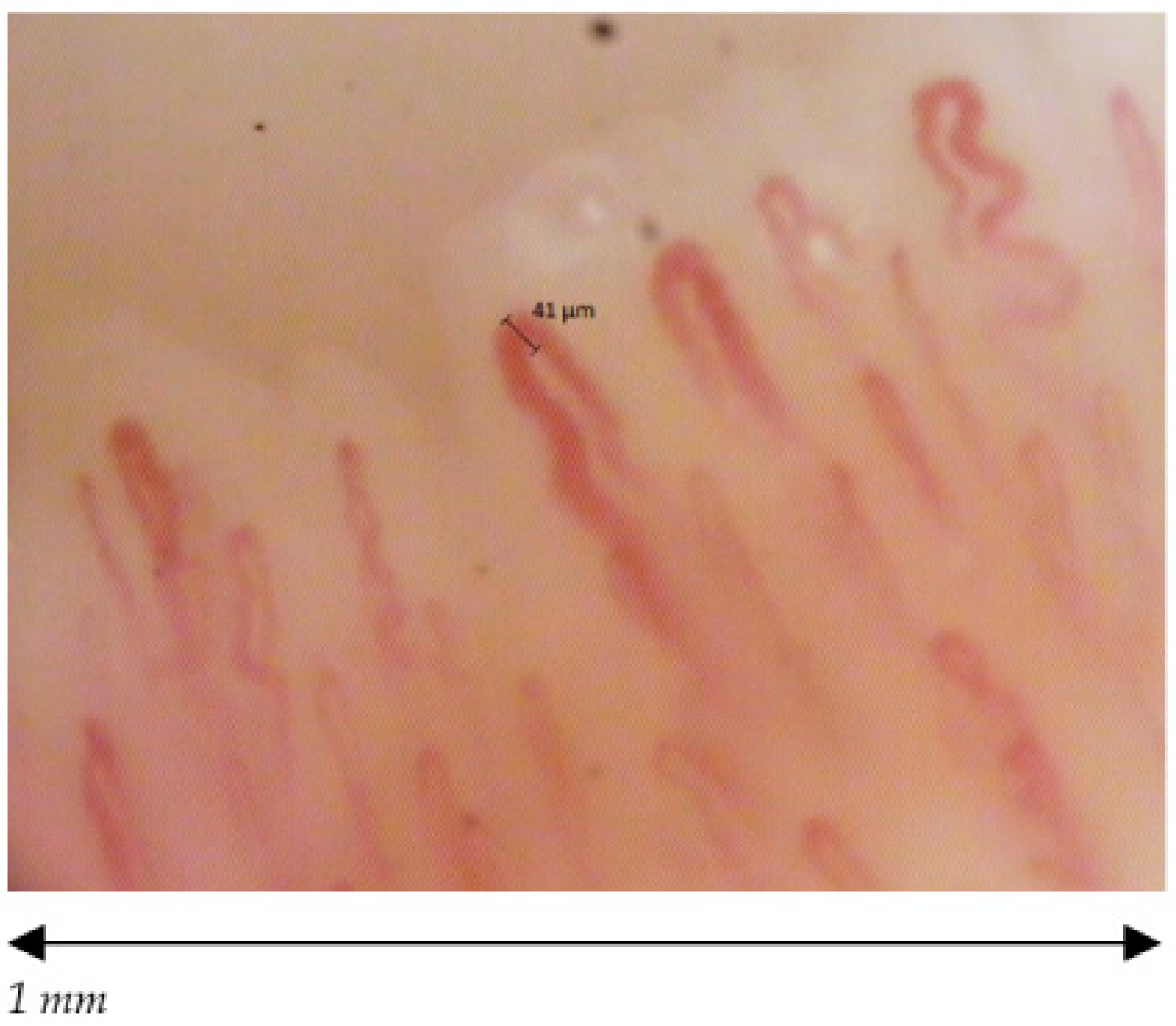
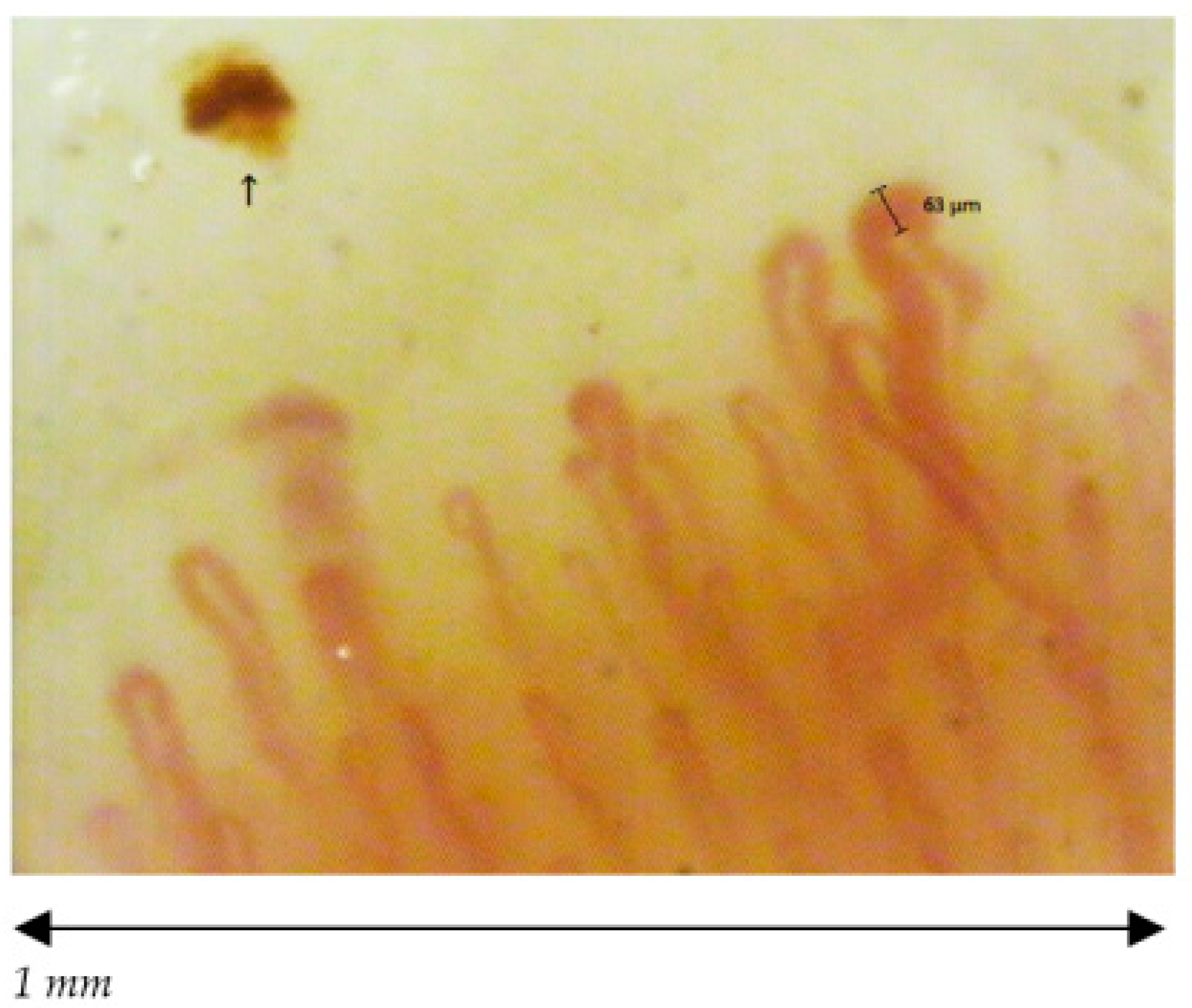
| Capillaroscopic Parameter | Normal Characteristics | Aspecific Pattern | Early Scleroderma Pattern |
|---|---|---|---|
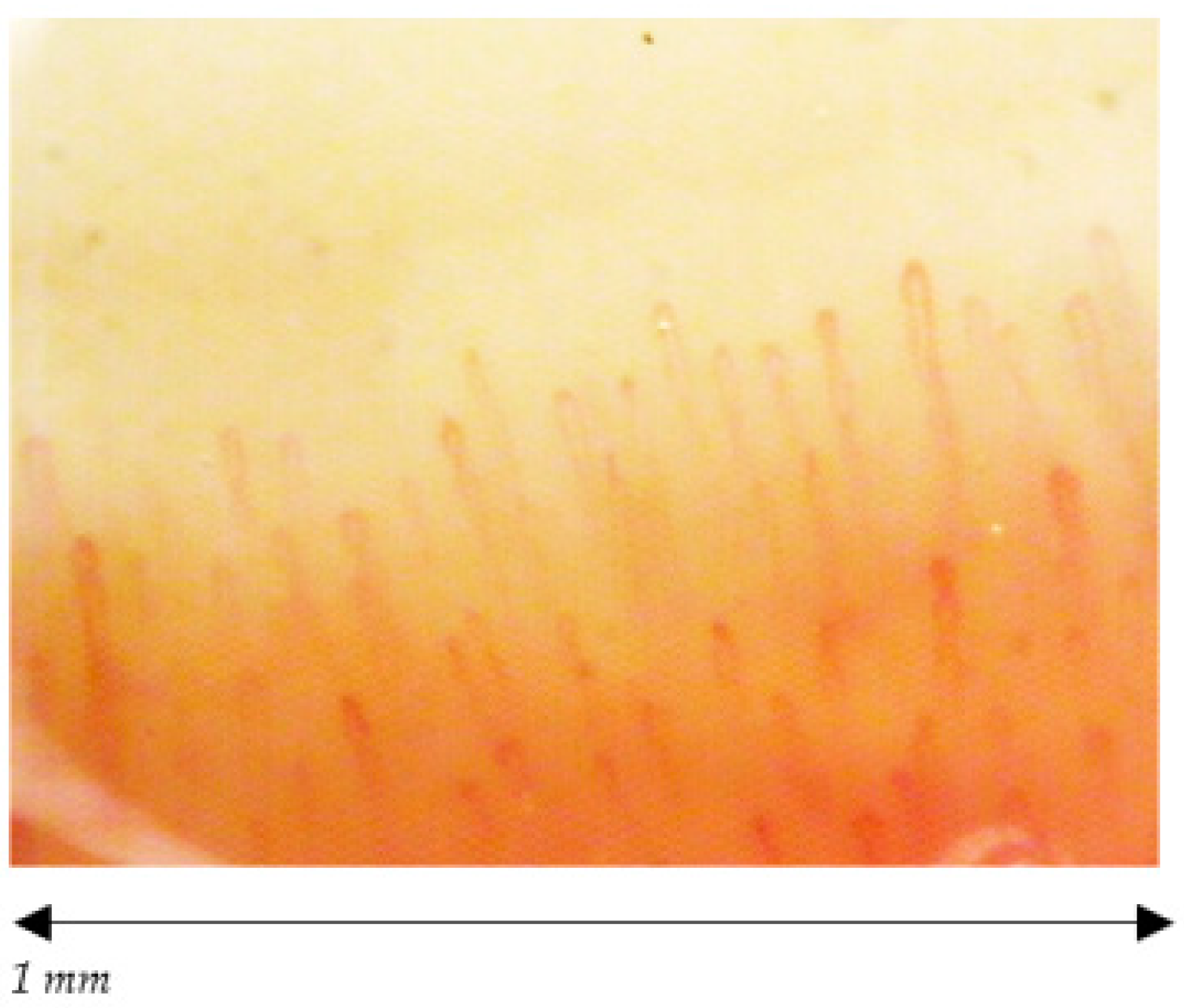
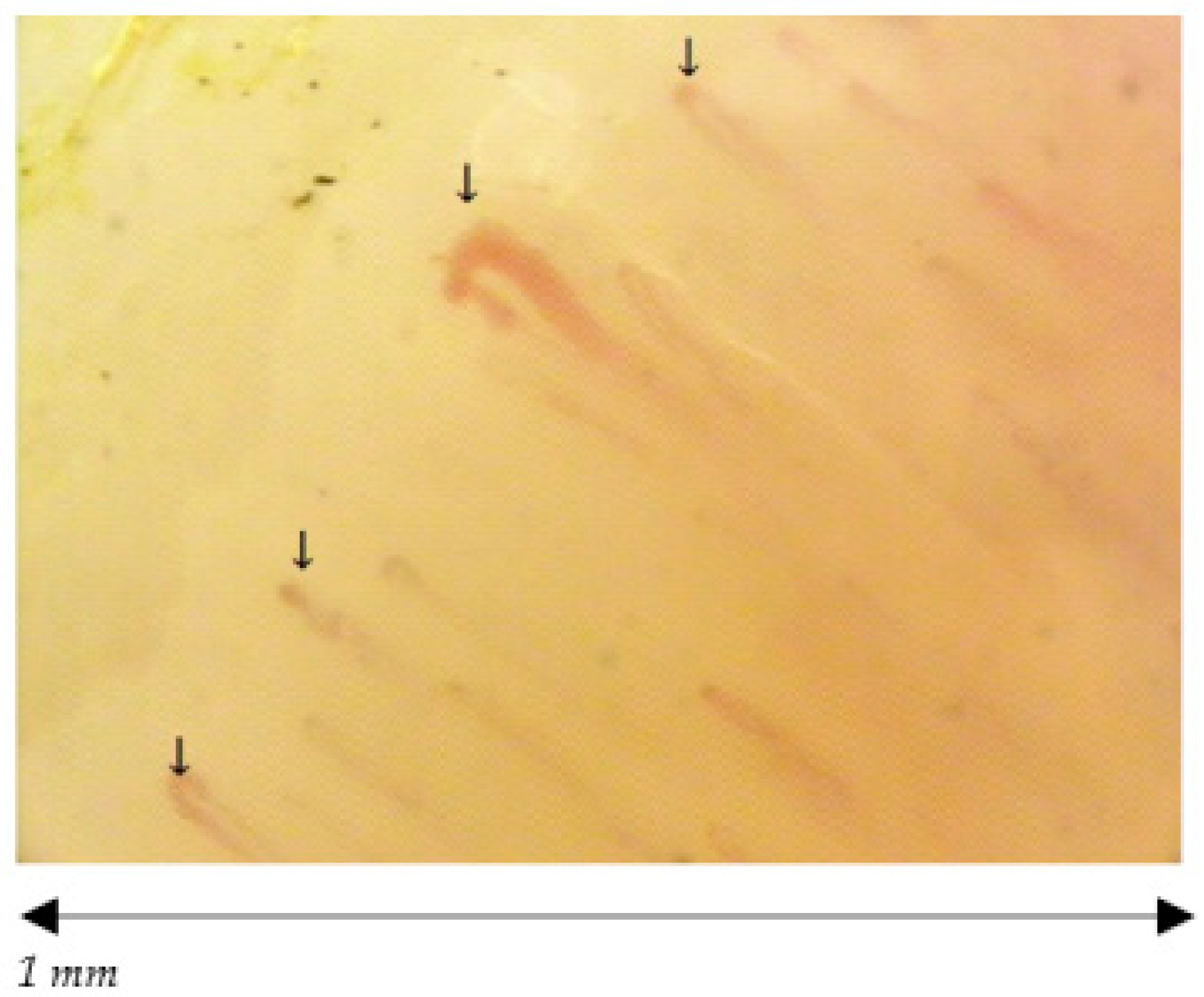
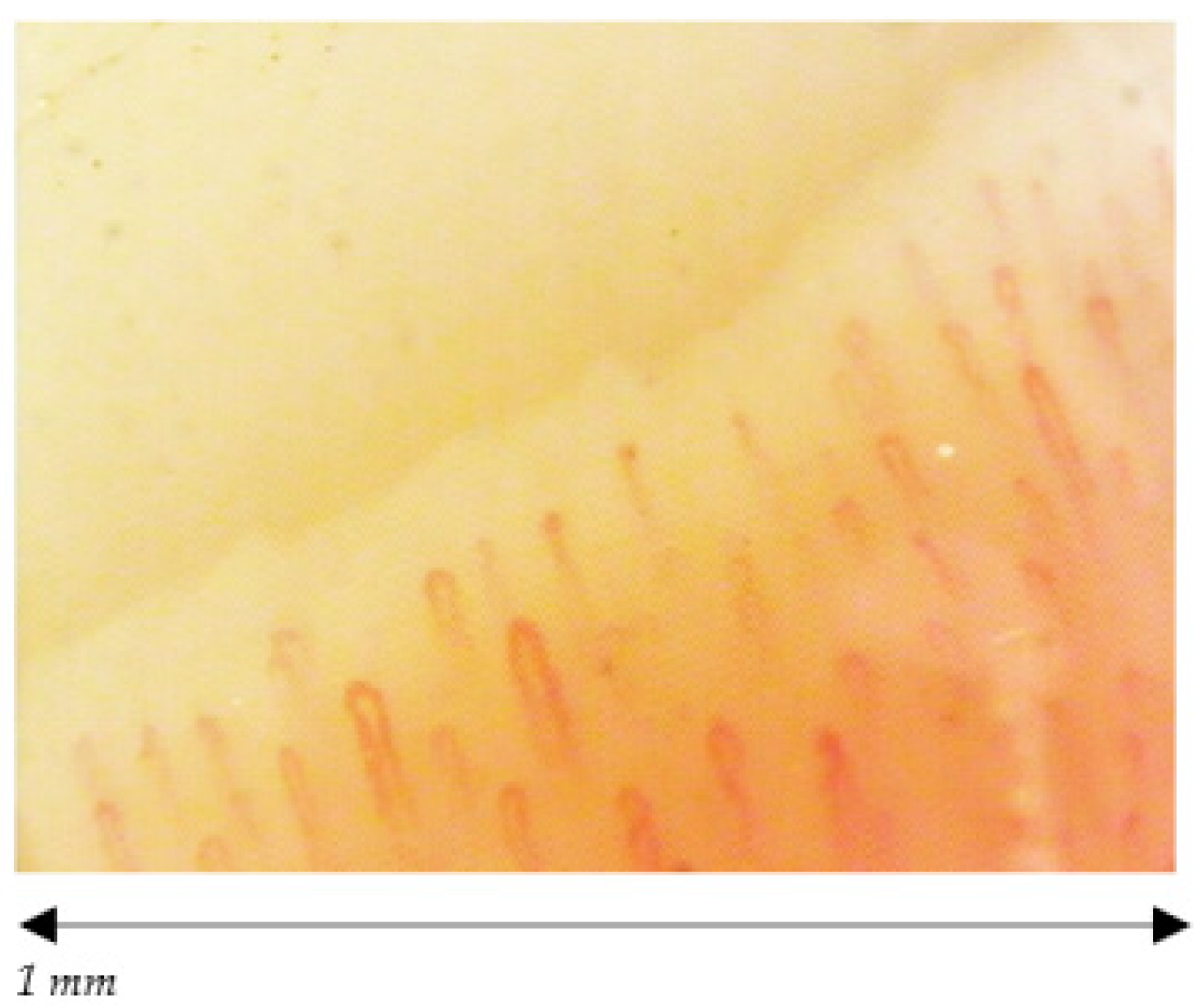
| Capillary morphology | Harpin-like (1), tortuous (2), crossing (3) shape with convex head | Not (1), (2), (3) or non-convex head | Absence of abnormal morphology |
| Capillary dimension (µm) | The diameter of arterial (or afferent) limb can vary from 6 to 19 µm (average value: 11 ± 3 µm). The diameter of the venous (or efferent) limb is generally greater, 8–20 µm (average value: 12 ± 3 µm) | Ectasia of the efferent tract of loops between 20–50 µm | few giant capillaries >50 µm |
| Capillary density | Normal range (≥7, on average between 9 and 14) | Within normal range or lowered density | Within normal range (≥7) No evident loss of capillaries |
| Capillary blood flow | Normally dynamic, no stasis or thrombosis | Granular flow | Irregular flow |
| Hemorrhages | No | Yes/No | Yes/No |
| Subject | Age | Role | Age of Playing | Training per Week | Match per Week | Capillary Density | Capillary Dimension | Abnormal Morfology | Haemorrhages | Flux | Pattern |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 25 | Middle Hitter | 10 | 5 | 1 | ↓ | 20–50 | Yes | Yes | Granular | Aspecific |
| M2 | 23 | Middle Hitter | 10 | 5 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| M3 | 25 | Libero | 18 | 5 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| M4 | 20 | Setter | 5 | 5 | 1 | ≥7 | Normal | No | No | Normal | Normal |
| M5 | 26 | Opposite Hitter | 15 | 5 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| M6 | 24 | Opposite Hitter | 11 | 5 | 1 | ≥7 | Normal | No | No | Normal | Normal |
| M7 | 24 | Opposite Hitter | 11 | 5 | 1 | ≥7 | Normal | Yes | No | Normal | Aspecific |
| M8 | 26 | ≥7 | Normal | No | No | Normal | Normal | ||||
| M9 | 22 | ≥7 | Normal | No | No | Normal | Normal | ||||
| M10 | 21 | ≥7 | Normal | No | No | Normal | Normal | ||||
| M11 | 26 | ≥7 | Normal | No | No | Normal | Normal | ||||
| M12 | 24 | ≥7 | Normal | No | No | Normal | Normal | ||||
| M13 | 25 | ≥7 | Normal | No | No | Normal | Normal | ||||
| M14 | 21 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F1 | 21 | Middle Hitter | 13 | 3 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| F2 | 26 | Libero | 10 | 3 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| F3 | 28 | Middle Hitter | 5 | 3 | 1 | ≥7 | 20–50 | Yes | Yes | Granular | Aspecific |
| F4 | 26 | Opposite Hitter | 7 | 3 | 1 | ≥7 | 20–50 | Yes | Yes | Granular | Aspecific |
| F5 | 29 | Setter | 20 | 3 | 1 | ≥7 | >50 (giant) | No | Yes | Granular | Early Scleroderma Pattern |
| F6 | 24 | Middle Hitter | 10 | 3 | 1 | ≥7 | 20–50 | Yes | Yes | Granular | Aspecific |
| F7 | 29 | Libero | 15 | 3 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| F8 | 27 | Middle Hitter | 4 | 3 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| F9 | 27 | Middle Hitter | 12 | 3 | 1 | Normal | Normal | No | No | Normal | Normal |
| F10 | 28 | Middle Hitter | 5 | 3 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| F11 | 30 | Libero | 15 | 4 | 1 | ≥7 | 20–50 | Yes | No | Granular | Aspecific |
| F12 | 32 | Opposite Hitter | 17 | 3 | 1 | ≥7 | 20–50 | Yes | No | Normal | Aspecific |
| F13 | 29 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F14 | 27 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F15 | 30 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F16 | 21 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F17 | 23 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F18 | 26 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F19 | 27 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F20 | 25 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F21 | 25 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F22 | 23 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F23 | 29 | ≥7 | Normal | No | No | Normal | Normal | ||||
| F24 | 21 | ≥7 | Normal | No | No | Normal | Normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirufo, M.M.; Catalogna, A.; Raggiunti, M.; De Pietro, F.; Galeoto, G.; Bassino, E.M.; Ginaldi, L.; De Martinis, M. Capillaroscopic Evidence of Microvascular Damage in Volleyball Players. Int. J. Environ. Res. Public Health 2021, 18, 10601. https://doi.org/10.3390/ijerph182010601
Sirufo MM, Catalogna A, Raggiunti M, De Pietro F, Galeoto G, Bassino EM, Ginaldi L, De Martinis M. Capillaroscopic Evidence of Microvascular Damage in Volleyball Players. International Journal of Environmental Research and Public Health. 2021; 18(20):10601. https://doi.org/10.3390/ijerph182010601
Chicago/Turabian StyleSirufo, Maria Maddalena, Alessandra Catalogna, Martina Raggiunti, Francesca De Pietro, Giovanni Galeoto, Enrica Maria Bassino, Lia Ginaldi, and Massimo De Martinis. 2021. "Capillaroscopic Evidence of Microvascular Damage in Volleyball Players" International Journal of Environmental Research and Public Health 18, no. 20: 10601. https://doi.org/10.3390/ijerph182010601
APA StyleSirufo, M. M., Catalogna, A., Raggiunti, M., De Pietro, F., Galeoto, G., Bassino, E. M., Ginaldi, L., & De Martinis, M. (2021). Capillaroscopic Evidence of Microvascular Damage in Volleyball Players. International Journal of Environmental Research and Public Health, 18(20), 10601. https://doi.org/10.3390/ijerph182010601









