Burden of Mortality from Asbestos-Related Diseases in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mortality from Mesothelioma and Asbestosis
2.2. Mortality from Lung Cancer: Estimated Cases
2.2.1. Asbestos-Related Lung Cancer Deaths Estimated on the Basis of Italian Population-Based Case-Control Studies
2.2.2. Asbestos-Related Lung Cancer Cases Estimated on the Basis of Italian Occupational Cohort Studies
2.3. Mortality from Ovarian Cancer: Estimated Cases
2.4. Mortality from Asbestos-Related Diseases (ARDs): Annual Estimated Cases
3. Results
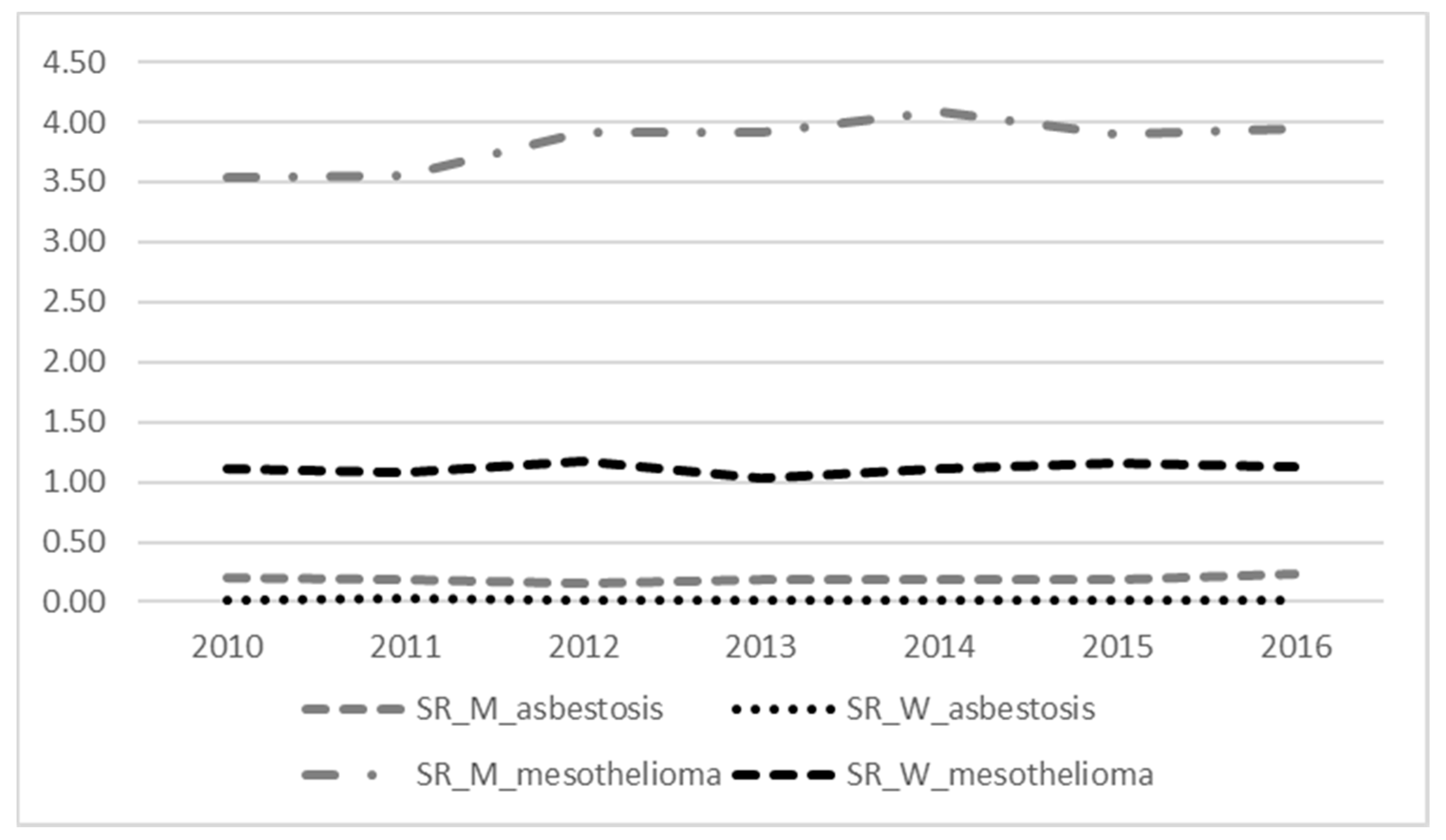
3.1. Mortality from Malignant Mesothelioma and Asbestosis
3.2. Mortality from Lung Cancer: Estimated Cases
3.3. Mortality from Ovarian Cancer: Estimated Cases
3.4. Mortality from ARDs in Italy: Annual Estimated Cases in the Period 2010–2016
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Asbestos. In Arsenic, Metals, Fibres and Dusts. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100C, pp. 219–309. [Google Scholar]
- Wolff, H.; Vehmas, T.; Oksa, P.; Rantanen, J.; Vainio, H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: Recommendations. Scand. J. Work Environ. Health 2015, 41, 5–15. [Google Scholar] [CrossRef]
- GBD 2017. Risk Factors Collaborators. Global, regional and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Diseases Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Goldberg, M.; Luce, D. The health impact of non occupational exposure to asbestos: What do we know? Eur. J. Cancer Prev. 2009, 18, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Marinaccio, A.; Binazzi, A.; Bonafede, M.; Corfiati, M.; Di Marzio, D.; Scarselli, A.; Verardo, M.; Mirabelli, D.; Gennaro, V.; Mensi, C.; et al. Malignant mesothelioma due to non-occupational asbestos exposure from Italian national surveillance system (ReNaM): Epidemiology and public health issues. Occup. Environ. Med. 2015, 72, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Takahashi, K.; Kim, R.; Jiang, Y.; Movahed, M.; Park, E.K.; Ranatnen, J. Asbestos: Use, bans and disease burden in Europe. Bull. World Health Organ. 2014, 92, 790–797. [Google Scholar] [CrossRef] [PubMed]
- International Labor Organization and World Health Organization. Outline for the Development of National Programs for Elimination of Asbestos-Related Diseases. 2007. Available online: https://www.who.int/occupational_health/publications/elim_asbestos_doc_en.pdf (accessed on 15 June 2021).
- World Health Organization. Declaration of the Sixt Ministerial Conference on Environment and Health. WHO. 2017. EURO/Ostrava2017/6. Available online: https://www.euro.who.int/__data/assets/pdf_file/0007/341944/OstravaDeclaration_SIGNED.pdf (accessed on 15 June 2021).
- Arachi, D.; Furuya, S.; David, A.; Mangwiro, A.; Chimed-Ochir, O.; Lee, K.; Tighe, P.; Takala, J.; Driscoll, T.; Takahashi, K. Development of the “National Asbestos Profile” to eliminate asbestos-related diseases in 195 countries. Int. J. Environ. Res. Public Health 2021, 18, 1804. [Google Scholar] [CrossRef] [PubMed]
- Algranti, E.; Ramos-Bonilla, J.P.; Terracini, B.; Santana, V.S.; Comba, P.; Pasetto, R.; Mazzeo, A.; Cavariani, F.; Trotta, A.; Marsili, D. Prevention of Asbestos exposure in Latin America within a global public health perspective. Ann. Glob. Health 2019, 85, 49. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Landrigan, P.J. on behalf of the Collegium Ramazzini. The global health dimensions of asbestos and asbestos-related diseases. Ann. Glob. Health 2016, 82, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, P.; Binazzi, A.; Branchi, C.; Marinaccio, A. National epidemiological surveillance systems of mesothelioma cases. Epidemiol. Prev. 2016, 40, 336–343. [Google Scholar] [CrossRef]
- Health and Safety Executive. Mesothelioma Statistics for Great Britain. 2020. Available online: https://www.hse.gov.uk/statistics/causdis/mesothelioma/mesothelioma.pdf (accessed on 15 June 2021).
- Mazurek, J.M.; Syamlal, G.; Wood, J.M.; Hendricks, S.A.; Weston, A. Malignant Mesothelioma Mortality-United States, 1999–2015. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 214–218. [Google Scholar] [CrossRef]
- López-Abente, G.; García-Gómez, M.; Menéndez-Navarro, A.; Fernández-Navarro, P.; Ramis, R.; García-Pérez, J.; Cervantes, M.; Ferreras, E.; Jiménez-Munoz, M.; Pastor-Barriuso, R. Pleural cancer mortality in Spain: Time-trends and updating of predictions up to 2020. BMC Cancer 2013, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Gogou, E.; Kerenidi, T.; Chamos, V.; Zintzaras, E.; Gourgoulianis, K.I. Mesothelioma mortality in Greece from 1983 to 2003. Int. J. Clin. Pract. 2009, 63, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Pedra, F.; Testa Tambellini, A.; de Bragança Pereira, B.; Carioca da Costa, A.C.; Albuquerque de Castro, H. Mesothelioma mortality in Brazil, 1980–2003. Int. J. Occup. Environ. Health 2008, 14, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Fazzo, L.; Minelli, G.; De Santis, M.; Bruno, C.; Zona, A.; Conti, S.; Comba, P. Epidemiological surveillance of mesothelioma mortality in Italy. Cancer Epidemiol. 2018, 55, 184–191. [Google Scholar] [CrossRef]
- Pasetto, R.; Terracini, B.; Marsili, D.; Comba, P. Occupational burden of asbestos-related cancer in Argentina, Brazil, Colombia, and Mexico. Ann. Glob. Health 2014, 80, 263–268. [Google Scholar] [CrossRef]
- Olsson, A.C.; Vermeulen, R.; Schuz, J.; Kromhout, H.; Pesch, B.; Peters, S.; Behrens, T.; Portengen, L.; Mirabelli, D.; Gustavsson, P.; et al. Exposure-response analyses of asbestos and lung cancer subtypes in a pooled analysis of a case-control studies. Epidemiology 2017, 28, 288–299. [Google Scholar] [CrossRef]
- Ferrante, D.; Chellini, E.; Merler, E.; Pavone, V.; Silvestri, S.; Miligi, L.; Gorini, G.; Bressan, V.; Girardi, P.; Ancona, L.; et al. Italian pool asbestos workers cohorts: Mortality trends of asbestos-related neoplasms after long time since first exposure. Occup. Environ. Med. 2017, 74, 887–898. [Google Scholar] [CrossRef]
- Il Registro Nazionale dei Mesoteliomi (ReNaM). VI Rapporto. Roma: INAIL; 2015. Available online: https://www.inail.it/cs/internet/comunicazione/pubblicazioni/catalogo-generale/pubbl-registro-nazionale-mesoteliomi-6-rapporto.html (accessed on 15 June 2021).
- Virta, R.L. Worldwide Asbestos Supply and Consumption Trends from 1900 Through 2003: U.S. Geological Survey Circular 1298, 80p. 2006. Available online: https://pubs.usgs.gov/circ/2006/1298/c1298.pdf (accessed on 15 June 2021).
- Marinaccio, A.; Binazzi, A.; Marzio, D.D.; Scarselli, A.; Verardo, M.; Mirabelli, D.; Gennaro, V.; Mensi, C.; Riboldi, L.; Merler, E.; et al. Pleural malignant mesothelioma epidemic: Incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int. J. Cancer 2012, 130, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Comba, P.; Zona, A. Adverse health effects of fluoro-edenitic fibers: Epidemiological evidence and public health priorities. Ann. N. Y. Acad. Sci. 2006, 1076, 778–783. [Google Scholar] [CrossRef]
- Caputo, A.; De Santis, M.; Manno, V.; Cauzillo, G.; Bruni, B.M.; Palumbo, L.; Conti, S.; Comba, P. Health impact of asbestos fibres naturally occurring in Mount Pollino area (Basilicata Region, Southern Italy). Epidemiol. Prev. 2018, 42, 142–150. (In Italian) [Google Scholar] [CrossRef]
- Maule, M.M.; Magnani, C.; Dalmasso, P.; Mirabelli, D.; Merletti, F.; Biggeri, A. Modeling mesothelioma risk associated with environmental asbestos exposure. Environ. Health Perspect. 2007, 115, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Musti, M.; Pollice, A.; Cavone, D.; Dragonieri, S.; Bilancia, M. The relationship between malignant mesothelioma and an asbestos cement plant environmental risk: A spatial case-control study in the city of Bari (Italy). Int. Arch. Occup. Environ. Health 2009, 82, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Consonni, D.; De Matteis, S.; Dallari, B.; Pesatori, A.C.; Riboldi, L.; Mensi, C. Impact of an asbestos cement factory on mesothelioma incidence in a community in Italy. Environ. Res. 2020, 183, 108968. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S. Managing asbestos in Italy: Twenty years after the ban. New Solut. J. Environ. Occup. Health Policy 2012, 22, 489–496. [Google Scholar] [CrossRef]
- Ji, J.; Sundquist, J.; Sundquist, K. Incidence and familial risk of pleural mesothelioma in Sweden: A national cohort study. Eur. Respir. J. 2016, 48, 873–879. [Google Scholar] [CrossRef]
- Price, B.; Ware, A. Time trend of mesothelioma incidence in the United States and projection of future cases: An update based on SEER data for 1973 through 2005. Crit. Rev. Toxicol. 2009, 39, 576–588. [Google Scholar] [CrossRef]
- Oddone, E.; Bollon, J.; Nava, C.R.; Consonni, D.; Marinaccio, A.; Magnani, C.; Gasparrini, A.; Barone-Adesi, F. Effect of asbestos consumption on malignant pleural mesothelioma in Italy: Forecasts of mortality up to 2040. Cancers 2021, 13, 3338. [Google Scholar] [CrossRef]
- Coviello, V.; Buzzoni, C.; Fusco, M.; Barchielli, A.; Cuccaro, F.; De Angelis, R.; Giacomin, A.; Luminari, S.; Randi, G.; Mangone, L. AIRTUM Working Group. Survival of cancer patients in Italy. Epidemiol. Prev. 2017, 41 (Suppl. 1), 1–244. [Google Scholar] [CrossRef]
- Marinaccio, A.; Corfiati, M.; Binazzi, A.; Di Marzio, D.; Scarselli, A.; Ferrante, P.; Bonafede, M.; Verardo, M.; Mirabelli, D.; Gennaro, V.; et al. The epidemiology of malignant mesothelioma in women: Gender differences and modalities of asbestos exposure. Occup. Environ. Med. 2018, 75, 254–262. [Google Scholar] [CrossRef]
- Moon, E.K.; Son, M.; Jin, Y.-W.; Park, S.; Lee, W.J. Variations of lung cancer risk from asbestos exposure: Impact on estimation of population attributable fraction. Ind. Health 2013, 51, 128–133. [Google Scholar] [CrossRef]
- Harris, E.J.A.; Musk, A.; De Klerk, N.; Reid, A.; Franklin, P.; Brims, F.J.H. Diagnosis of asbestos-related lung diseases. Expert. Rev. Respir. Med. 2019, 13, 241–249. [Google Scholar] [CrossRef]
- Frost, G.; Darnton, A.; Harding, A. The effect of smoking on the risk of lung cancer mortality for asbestos workers in Great Britain (1971–2005). Ann. Occup. Hyg. 2011, 55, 239–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngamwong, Y.; Tangamornsuksan, W.; Lohitnavy, O.; Chaiyakunapruk, N.; Scholfield, C.N.; Reisfeld, B.; Lohitnavy, M. Additive synergism between asbestos and smoking in lung cancer risk: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0135798. [Google Scholar] [CrossRef]
- Marinaccio, A.; Scarselli, A.; Binazzi, A.; Mastrantonio, M.; Ferrante, P.; Iavicoli, S. Magnitude of asbestos-related lung cancer mortality in Italy. Br. J. Cancer 2008, 99, 173–175. [Google Scholar] [CrossRef]
- Albin, M.; Magnani, C.; Krstev, S.; Rapiti, E.; Shefer, I. Asbestos and cancer: An overview of current trends in Europe. Environ. Health Perspect. 1999, 107, 289–298. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.; Peto, J.; Byrnes, G.; Straif, K.; Boffetta, P. Estimating the asbestos-related lung cancer burden from mesothelioma mortality. Br. J. Cancer 2012, 106, 575–584. [Google Scholar] [CrossRef]
- Magnani, C.; Silvestri, S.; Angelini, A.; Ranucci, A.; Azzolina, D.; Cena, T.; Chellini, E.; Merler, E.; Pavone, V.; Miligi, L.; et al. Italian pool of asbestos workers cohorts: Asbestos related mortality by industrial sector and cumulative exposure. Ann. Ist. Super. Sanita 2020, 56, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Mensi, C.; Macchione, M.; Termine, L.; Canti, Z.; Rivolta, G.; Riboldi, L.; Chiappino, G. Asbestos exposure in the non-asbestos textile industry: The experience of the Lombardy Mesothelioma Registry. Epidemiol. Prev. 2007, 31 (Suppl. 1), 27–30. (In Italian) [Google Scholar]
- Odgerel, C.O.; Takahashi, K.; Sorahan, T.; Driscoll, T.; Fitzmaurice, C.; Yoko-o, M.; Sawanyawisuth, K.; Furuya, S.; Tanaka, F.; Horie, S.; et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup. Environ. Med. 2017, 74, 851–858. [Google Scholar] [CrossRef]
- Ducic, S. Accuracy in reporting the causes of death. A comparison with diagnosis at autopsy in a series of mesotheliomas and other malignant tumours of the lung. Can. J. Public Health 1971, 62, 395–402. [Google Scholar]
- Newhouse, M.L. Mesothelioma and death certificate. Lancet 1982, 2, 991. [Google Scholar] [CrossRef]
- Connelly, R.R.; Spirtas, R.; Myers, M.H.; Percy, C.L.; Fraumeni, J.F., Jr. Demographic patterns for mesothelioma in the United States. J. Nat. Cancer Inst. 1987, 78, 1053–1060. [Google Scholar]
- Ribak, J.; Lillis, R.; Suzuki, Y.; Penner, L.; Selikoff, J. Death certificate categorization of malignant pleural and peritoneal mesothelioma in a cohort of asbestos insulation workers. J. Soc. Occup. Med. 1991, 41, 137–139. [Google Scholar] [CrossRef]
- Delendi, M.; Riboli, E.; Peruzzo, P.; Stanta, G.; Cocchi, A.; Gardiman, D.; Sasco, A.J.; Giarelli, L. Comparison of diagnosis of cancer of the respiratory system on death certificates and autopsy. IARC Sci. Publ. 1991, 112, 55–62. [Google Scholar]
- Bruno, C.; Comba, P.; Maiozzi, P.; Vetrugno, T. Accuracy of death certification of pleural mesothelioma in Italy. Eur. J. Epidemiol. 1996, 12, 421–423. [Google Scholar] [CrossRef]
- Magnani, C.; Ferrante, D.; Barone-Adesi, F.; Bertolotti, M.; Todesco, A.; Mirabelli, D.; Terracini, B. Cancer risk after cessation of asbestos exposure: A cohort study of Italian asbestos cement workers. Occup. Environ. Med. 2008, 6, 164–170. [Google Scholar] [CrossRef]
- Loomis, D.; Richardson, D.B.; Elliott, L. Quantitative relationships of exposure to chrysotile asbestos and mesothelioma mortality. Am. J. Ind. Med. 2019, 62, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Kopylev, L.; Sullivan, P.A.; Vinikoor, L.C.; Bateson, F.T. Monte Carlo analysis of impact of underascertainment of mesothelioma cases on underestimation of risk. Open Epidemiol. J. 2011, 4, 45–53. [Google Scholar] [CrossRef]
- Barbieri, P.G.; Finotto, L.; Belli, S.; Festa, R.; Comba, P. Death certification of pleural malignant mesothelioma from the Italian National Institute of Statistics: A comparison on 269 clinical diagnosis confirmed at autopsy (1997–2016). Epidemiol. Prev. 2021, 45, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Fazzo, L.; Minelli, G.; De Santis, M.; Bruno, C.; Zona, A.; Marinaccio, A.; Conti, S.; Pirastu, R.; Comba, P. Mesothelioma mortality surveillance and asbestos exposure tracking in Italy. Ann. Ist. Super. Sanita 2012, 48, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Nesti, M.; Adamoli, S.; Ammirabile, F.; Ascoli, V.; Barbieri, P.G.; Cacciarini, V.; Candela, S.; Cavone, D.; Cuzillo, G.; Chellini, E.; et al. Linee Guida per la Rilevazione e la Definizione dei Casi di Mesotelioma Maligno e la Trasmissione delle Informazioni all’ISPESL da Parte dei Centri Operativi Regionali. Roma, Ispesl 2003. Available online: https://www.inail.it/cs/internet/docs/all-linee-guida-renam.pdf?section=attivita (accessed on 5 August 2021).
- Conti, S.; Minelli, G.; Ascoli, V.; Marinaccio, A.; Bonafede, M.; Manno, V.; Crialesi, R.; Straif, K. Peritoneal mesothelioma in Italy: Trends and geography of mortality and incidence. Am. J. Ind. Med. 2015, 58, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Germani, D.; Belli, S.; Bruno, C.; Grignoli, M.; Nesti, M.; Pirastu, R.; Comba, P. Cohort mortality study of women compensated for asbestosis in Italy. Am. J. Ind. Med. 1999, 36, 129–134. [Google Scholar] [CrossRef]
- Fazzo, L.; Minelli, G.; Bruno, C.; Comba, P.; Conti, S.; De Santis, M.; Zona, A.; Binazzi, A.; Magnani, C.; Marinaccio, A.; et al. Early mortality from malignant mesothelioma in Italy as a proxy of environmental exposure to asbestos in children. Ann. Ist. Super. Sanita 2020, 56, 478–486. [Google Scholar] [CrossRef] [PubMed]
| Gender | Case-Control Study | Proportion of Exposed Cases | OR (95% CI) | AFp | ARLC/Year * |
|---|---|---|---|---|---|
| Males | EAGLE (Lombardy) | 0.40 | 1.45 (1.21–1.74) | 0.12 | 3041 |
| ROMA | 0.50 | 1.16 (0.78–1.72) | 0.07 | 1677 | |
| TORINO-VENETO | 0.57 | 1.21 (1.00–1.48) | 0.10 | 2430 | |
| POOL | 0.47 | 1.31 (1.16–1.49) | 0.11 | 2718 | |
| Females | EAGLE (Lombardy) | 0.11 | 1.11 (0.67–1.86) | 0.01 | 95 |
| ROMA | 0.03 | Not reported | |||
| TORINO-VENETO | 0.16 | 1.14 (0.55–2.33) | 0.02 | 177 | |
| POOL | 0.12 | 1.12 (0.74–1.70) | 0.01 | 112 |
| Occupational Cohorts | Gender | R (95% CI) | MM Cases in the ReNaM Occupational Categories (Corresponding to Those of Italian Cohorts) | MM Deaths | ARLC Deaths (95% CI) | |
|---|---|---|---|---|---|---|
| Incident Cases | % of Exposed * | |||||
| Shipyards | Males | 0.94 (0.18–1.57) | 319 | 7.30 | 497 | 467 (90–780) |
| Females | - | 6 | 0.92 | 24 | - | |
| Asbestos-cement | Males | 1.13 (1.01–1.24) | 130 | 2.98 | 203 | 229 (205–252) |
| Females | 0.17 (0.07–0.25) | 23 | 3.54 | 94 | 16 (6–23) | |
| Crocidolite miners | Males | 0.08 (0.00–0.46) | 4 | 0.09 | 6 | 0.5 (0.0–2.9) |
| Females | - | 1 | 0.15 | 4 | - | |
| Ship furniture | Males | 1.21 (0.00–2.44) | 5 | 0.11 | 8 | 9 (0–19) |
| Females | - | 0 | 0.00 | 0 | - | |
| Dockyards and harbours | Males | 2.71 (1.86–3.35) | 184 | 4.21 | 287 | 778 (532–961) |
| Females | - | 1 | 0.15 | 4 | - | |
| Asphalt rolls production | Males | 0.00 | 0 | 0.00 | 0 | - |
| Females | - | 0 | 0.00 | 0 | - | |
| Rolling stock construction | Males | 0.26 (0.00–0.60) | 196 | 4.49 | 305 | 79 (0–182) |
| Females | 1.3 (0–4.21) | 3 | 0.46 | 12 | 16 (0–51) | |
| Glassworks | Males | 1.91 (0.00–4.11) | 74 | 1.69 | 115 | 220 (0–474) |
| Females | - | 17 | 2.62 | 69 | - | |
| Occupational Cohorts | R (95% CI) | MM Cases in the ReNaM Occupational Categories (Corresponding to Those of Italian Cohorts) | MM Deaths | AROC Deaths (95% CI) | |
|---|---|---|---|---|---|
| Incident Cases | % of Exposed | ||||
| Asbestos-cement | 0.07 (0–0.12) | 23 | 3.54 | 94 | 7 (0–12) |
| Glassworks | 1.28 (0–2.18) | 17 | 2.62 | 69 | 89 (0–151) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazzo, L.; Binazzi, A.; Ferrante, D.; Minelli, G.; Consonni, D.; Bauleo, L.; Bruno, C.; Bugani, M.; De Santis, M.; Iavarone, I.; et al. Burden of Mortality from Asbestos-Related Diseases in Italy. Int. J. Environ. Res. Public Health 2021, 18, 10012. https://doi.org/10.3390/ijerph181910012
Fazzo L, Binazzi A, Ferrante D, Minelli G, Consonni D, Bauleo L, Bruno C, Bugani M, De Santis M, Iavarone I, et al. Burden of Mortality from Asbestos-Related Diseases in Italy. International Journal of Environmental Research and Public Health. 2021; 18(19):10012. https://doi.org/10.3390/ijerph181910012
Chicago/Turabian StyleFazzo, Lucia, Alessandra Binazzi, Daniela Ferrante, Giada Minelli, Dario Consonni, Lisa Bauleo, Caterina Bruno, Marcella Bugani, Marco De Santis, Ivano Iavarone, and et al. 2021. "Burden of Mortality from Asbestos-Related Diseases in Italy" International Journal of Environmental Research and Public Health 18, no. 19: 10012. https://doi.org/10.3390/ijerph181910012
APA StyleFazzo, L., Binazzi, A., Ferrante, D., Minelli, G., Consonni, D., Bauleo, L., Bruno, C., Bugani, M., De Santis, M., Iavarone, I., Magnani, C., Romeo, E., Zona, A., Alessi, M., Comba, P., & Marinaccio, A. (2021). Burden of Mortality from Asbestos-Related Diseases in Italy. International Journal of Environmental Research and Public Health, 18(19), 10012. https://doi.org/10.3390/ijerph181910012











