Quantifying Coordination between Agonist and Antagonist Elbow Muscles during Backhand Crosscourt Shots in Adult Female Squash Players
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experiment Protocol
2.3. Data Recording
2.4. Co-Activation Index
2.5. Statistical Analysis
3. Results
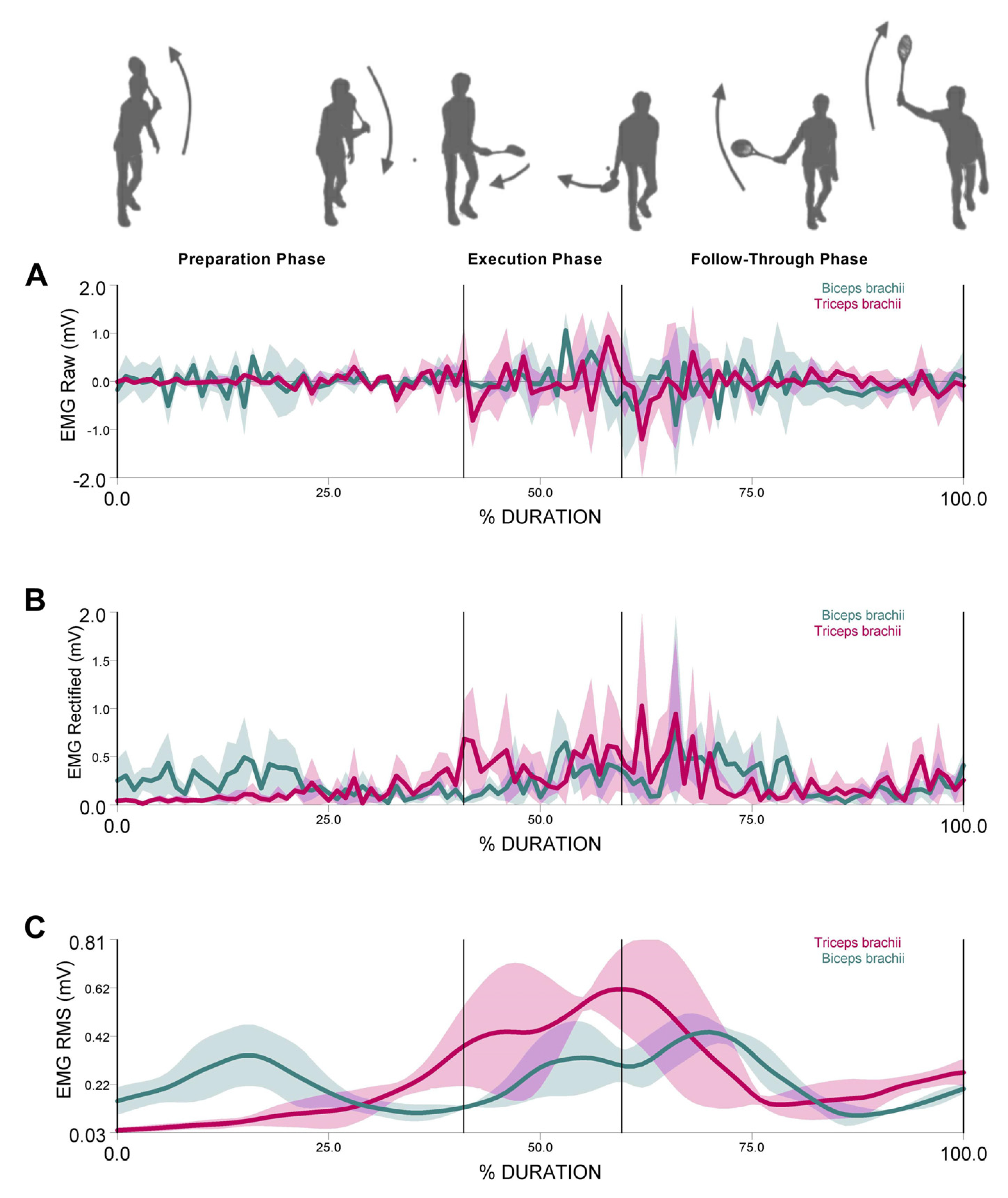
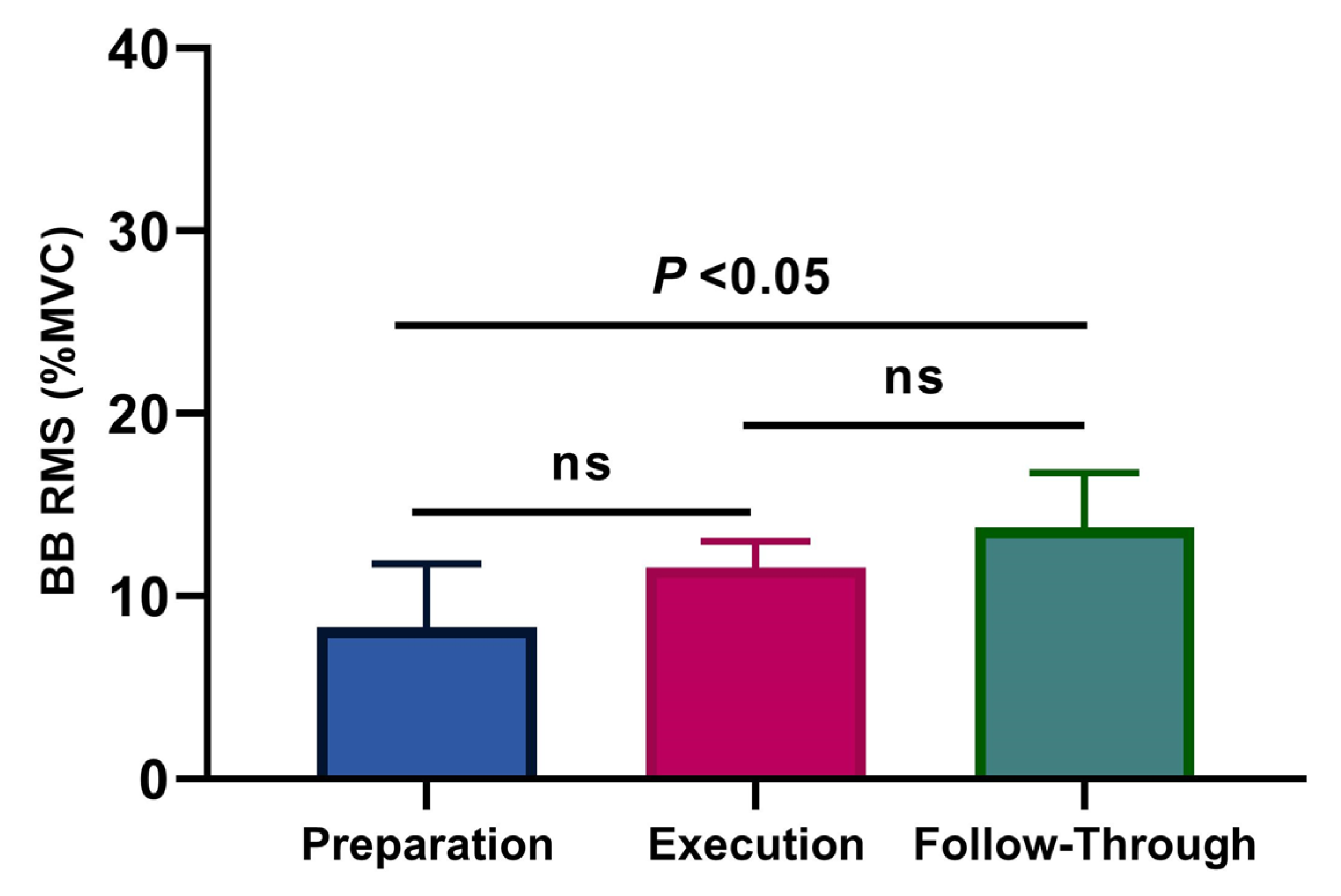
3.1. Muscular Activity
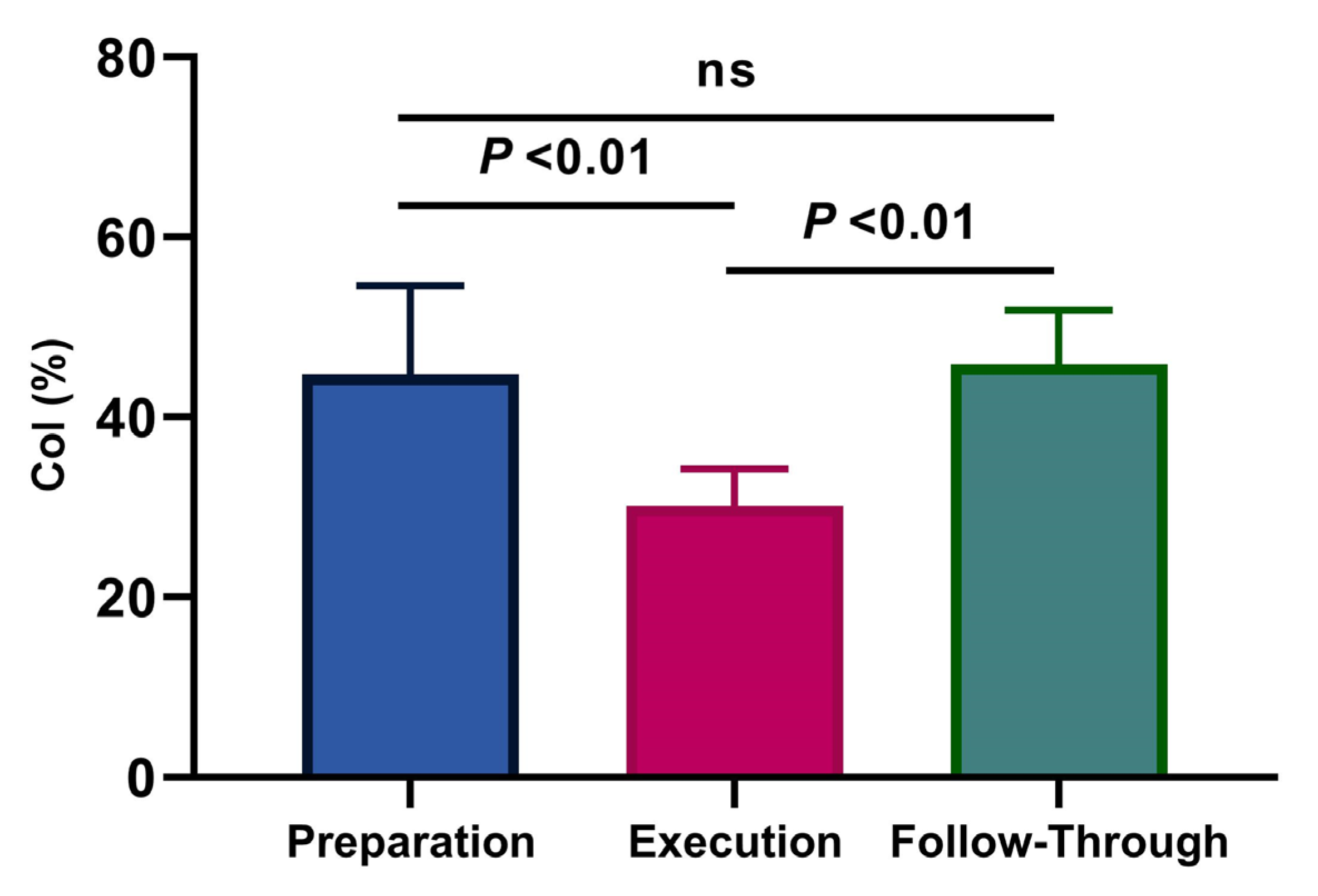
3.2. Co-Activation Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, B.K.; Sanders, R.H.; Ryu, J.H.; Graham-Smith, P.; Sinclair, P.J. The kinematic differences between skill levels in the squash forehand drive, volley and drop strokes. J. Sports Sci. 2020, 38, 1550–1559. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.; James, N.; Pers, J.; Mandeljc, R.; Vuckovic, G. Using a Situation Awareness Approach to Identify Differences in the Performance Profiles of the World’s Top Two Squash Players and Their Opponents. Front. Psychol. 2019, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Horobeanu, C.; Johnson, A.; Pullinger, S.A. The Prevalence of Musculoskeletal Injuries in Junior Elite Squash Players. Asian J. Sports Med. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Gamal, T.; Akl, A.-R. Electromyographic comparison of squash forehand shot after midcourt and frontcourt traditional movement patterns. Sport Sci. Pract. Asp. 2016, 13, 19–25. [Google Scholar]
- Okhovatian, F.; Ezatolahi, A. Sport injuries in squash. Pak. J. Med Sci. 2009, 25, 413–417. [Google Scholar]
- Lees, A. Technique analysis in sports: A critical review. J. Sports Sci. 2002, 20, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.F.; Eime, R.M. The epidemiology of squash injuries. Int. SportMed J. 2001, 2, 1–11. [Google Scholar]
- Elliott, B.; Marshall, R.; Noffal, G. The role of upper limb segment rotations in the development of racket-head speed in the squash forehand. J. Sports Sci. 1996, 14, 159–165. [Google Scholar] [CrossRef]
- Cho, K.-K.; Kim, Y.-S. The kinematic analysis and the study of muscle activities during backhand drive in squash. Korean J. Sport Biomech. 2007, 17, 11–21. [Google Scholar]
- Hong, Y.; Chang, T.C.-M.; Chan, D. A comparison of the game strategies employed by national and international squash players in competitive situation by notational analysis. J. Hum. Mov. Stud. 1996, 31, 89–104. [Google Scholar]
- An, Y.-H.; Ryu, J.-S.; Ryu, H.-Y.; Soo, J.-M.; Lim, Y.-T. The kinematic analysis of the upper extremity during backhand stroke in squash. Korean J. Sport Biomech. 2007, 17, 145–156. [Google Scholar]
- Seoung Eun, K.; Seung Nam, M.; Murali, S. Motion analysis of squash backhand drop shot—A kinematic analysis study. In Proceedings of 2nd International conference on Advances in Mechanical Engineering (ICAME 2018); IOP Publishing Ltd.: Kattankulathur, India, 2018. [Google Scholar]
- Vuckovic, G.; Dezman, B.; Erculj, F.; Kovacic, S.; Pers, J. Comparative movement analysis of winning and losing players in men’s elite squash. Kinesiol. Slov. 2003, 9, 74–84. [Google Scholar]
- Jones, T.W.; Williams, B.K.; Kilgallen, C.; Horobeanu, C.; Shillabeer, B.C.; Murray, A.; Cardinale, M. A review of the performance requirements of squash. Int. J. Sports Sci. Coach. 2018, 13, 1223–1232. [Google Scholar] [CrossRef]
- McGarry, T. Identifying patterns in squash contests using dynamical analysis and human perception. Int. J. Perform. Anal. Sport 2017, 6, 134–147. [Google Scholar] [CrossRef]
- Vanrenterghem, J.; Nedergaard, N.J.; Robinson, M.A.; Drust, B. Training Load Monitoring in Team Sports: A Novel Framework Separating Physiological and Biomechanical Load-Adaptation Pathways. Sports Med. 2017, 47, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.K.; Finch, C.F. The Relationship Between Training Load and Injury, Illness and Soreness: A Systematic and Literature Review. Sports Med. 2016, 46, 861–883. [Google Scholar] [CrossRef]
- Gibson, N.; Gibbon, K.; Bell, P.; Clyne, A.; Lobban, G.; Aitken, L. Physical preparation for elite-level squash players: Monitoring, assessment, and training practices for the strength and conditioning coach. Strength Cond. J. 2019, 41, 51–62. [Google Scholar] [CrossRef]
- Horsley, I.G.; O’Donnell, V.; Leeder, J. The epidemiology of injuries in English professional squash; A retrospective analysis between 2004 and 2015. Phys. Ther. Sport 2020, 46, 1–6. [Google Scholar] [CrossRef]
- Nutt, C.; Mirkovic, M.; Hill, R.; Ranson, C.; Cooper, S.M. Reference Values for Glenohumeral Joint Rotational Range of Motion in Elite Tennis Players. Int. J. Sports Phys. Ther. 2018, 13, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Pallis, J.M.; McNitt-Gray, J.L.; Hung, G.K. Biomechanical Principles and Applications in Sports; Springer: Heidelberg, Germany, 2019. [Google Scholar]
- Perri, T.; Norton, K.I.; Bellenger, C.R.; Murphy, A.P. Training loads in typical junior-elite tennis training and competition: Implications for transition periods in a high-performance pathway. Int. J. Perform. Anal. Sport 2018, 18, 327–338. [Google Scholar] [CrossRef]
- Gescheit, D.T.; Cormack, S.J.; Reid, M.; Duffield, R. Consecutive days of prolonged tennis match play: Performance, physical, and perceptual responses in trained players. Int. J. Sports Physiol. Perform. 2015, 10, 913–920. [Google Scholar] [CrossRef]
- Hirokawa, S.; Solomonow, M.; Luo, Z.; Lu, Y.; D’Ambrosia, R. Muscular co-contraction and control of knee stability. J. Electromyogr. Kinesiol. 1991, 1, 199–208. [Google Scholar] [CrossRef]
- Lehman, G.J. Resistance training for performance and injury prevention in golf. J. Can. Chiropr. Assoc. 2006, 50, 27–42. [Google Scholar] [PubMed]
- Salem, A.; Hassan, A.; Tilp, M.; Akl, A.-R. Antagonist Muscle Co-Activation during Kettlebell Single Arm Swing Exercise. Appl. Sci. 2021, 11, 4033. [Google Scholar] [CrossRef]
- Akl, A.R.; Goncalves, P.; Fonseca, P.; Hassan, A.; Vilas-Boas, J.P.; Conceicao, F. Muscle Co-Activation around the Knee during Different Walking Speeds in Healthy Females. Sensors 2021, 21, 677. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Kellis, E.; Arabatzi, F.; Papadopoulos, C. Muscle co-activation around the knee in drop jumping using the co-contraction index. J. Electromyogr. Kinesiol. 2003, 13, 229–238. [Google Scholar] [CrossRef]
- Lauer, J.; Figueiredo, P.; Vilas-Boas, J.P.; Fernandes, R.J.; Rouard, A.H. Phase-dependence of elbow muscle coactivation in front crawl swimming. J. Electromyogr. Kinesiol. 2013, 23, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Rouard, A.H.; Clarys, J.P. Cocontraction in the elbow and shoulder muscles during rapid cyclic movements in an aquatic environment. J. Electromyogr. Kinesiol. 1995, 5, 177–183. [Google Scholar] [CrossRef]
- Gribble, P.L.; Ostry, D.J. Independent coactivation of shoulder and elbow muscles. Exp. Brain Res. 1998, 123, 355–360. [Google Scholar] [CrossRef]
- Wang, L.; Niu, W.; Wang, K.; Zhang, S.; Li, L.; Lu, T.; SpringerLink. Badminton players show a lower coactivation and higher beta band intermuscular interactions of ankle antagonist muscles during isokinetic exercise. Med. Biol. Eng. Comput. 2019, 57, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Pizzamiglio, S.; De Lillo, M.; Naeem, U.; Abdalla, H.; Turner, D.L. High-Frequency Intermuscular Coherence between Arm Muscles during Robot-Mediated Motor Adaptation. Front. Physiol. Front. Physiol. 2017, 7, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lu, A.; Zhang, S.; Niu, W.; Zheng, F.; Gong, M. Fatigue-related electromyographic coherence and phase synchronization analysis between antagonistic elbow muscles. Exp. Brain Res. 2015, 233, 971–982. [Google Scholar] [CrossRef]
- van Asseldonk, E.H.; Campfens, S.F.; Verwer, S.J.; van Putten, M.J.; Stegeman, D.F. Reliability and agreement of intramuscular coherence in tibialis anterior muscle. PLoS ONE 2014, 9, e88428. [Google Scholar] [CrossRef] [PubMed]
- Masakado, Y.; Ushiyama, J.; Katsu, M.; Kimura, A.; Liu, M.; Ushiba, J.; Yokohama, J. Muscle fatigue-induced enhancement of corticomuscular coherence following sustained submaximal isometric contraction of the tibialis anterior muscle. NSR Neurosci. Res. 2011, 71, e347. [Google Scholar] [CrossRef]
- Mima, T.; Steger, J.; Schulman, A.E.; Gerloff, C.; Hallett, M. Electroencephalographic measurement of motor cortex control of muscle activity in humans. Clin. Neurophysiol. 2000, 111, 326–337. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Riccio, M.E.; Felici, F. Tennis players show a lower coactivation of the elbow antagonist muscles during isokinetic exercises. J. Electromyogr. Kinesiol. 2008, 18, 752–759. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Sbriccoli, P.; Marzattinocci, G.; Felici, F. Coactivation of the elbow antagonist muscles is not affected by the speed of movement in isokinetic exercise. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2006, 33, 191–199. [Google Scholar] [CrossRef]
- Darainy, M.; Ostry, D.J. Muscle cocontraction following dynamics learning. Exp. Brain Res. 2008, 190, 153–163. [Google Scholar] [CrossRef]
- Wagner, H.; Pfusterschmied, J.; Tilp, M.; Landlinger, J.; von Duvillard, S.P.; Muller, E. Upper-body kinematics in team-handball throw, tennis serve, and volleyball spike. Scand. J. Med. Sci. Sports 2014, 24, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M.; Polen, R.R.; Byrd, B.N. Contraction mode and intensity effects on elbow antagonist muscle co-activation. J. Electromyogr. Kinesiol. 2019, 44, 101–107. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akl, A.-R.; Hassan, A.; Elgizawy, H.; Tilp, M. Quantifying Coordination between Agonist and Antagonist Elbow Muscles during Backhand Crosscourt Shots in Adult Female Squash Players. Int. J. Environ. Res. Public Health 2021, 18, 9825. https://doi.org/10.3390/ijerph18189825
Akl A-R, Hassan A, Elgizawy H, Tilp M. Quantifying Coordination between Agonist and Antagonist Elbow Muscles during Backhand Crosscourt Shots in Adult Female Squash Players. International Journal of Environmental Research and Public Health. 2021; 18(18):9825. https://doi.org/10.3390/ijerph18189825
Chicago/Turabian StyleAkl, Abdel-Rahman, Amr Hassan, Helal Elgizawy, and Markus Tilp. 2021. "Quantifying Coordination between Agonist and Antagonist Elbow Muscles during Backhand Crosscourt Shots in Adult Female Squash Players" International Journal of Environmental Research and Public Health 18, no. 18: 9825. https://doi.org/10.3390/ijerph18189825
APA StyleAkl, A.-R., Hassan, A., Elgizawy, H., & Tilp, M. (2021). Quantifying Coordination between Agonist and Antagonist Elbow Muscles during Backhand Crosscourt Shots in Adult Female Squash Players. International Journal of Environmental Research and Public Health, 18(18), 9825. https://doi.org/10.3390/ijerph18189825








