Removal of Arsenic Oxyanions from Water by Ferric Chloride—Optimization of Process Conditions and Implications for Improving Coagulation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Stock Solutions and Synthetic Test Samples
2.3. Jar Test Procedure and Experimental Conditions
2.4. Mathematical Analysis
2.5. Natural Water Sample
3. Results and Discussion
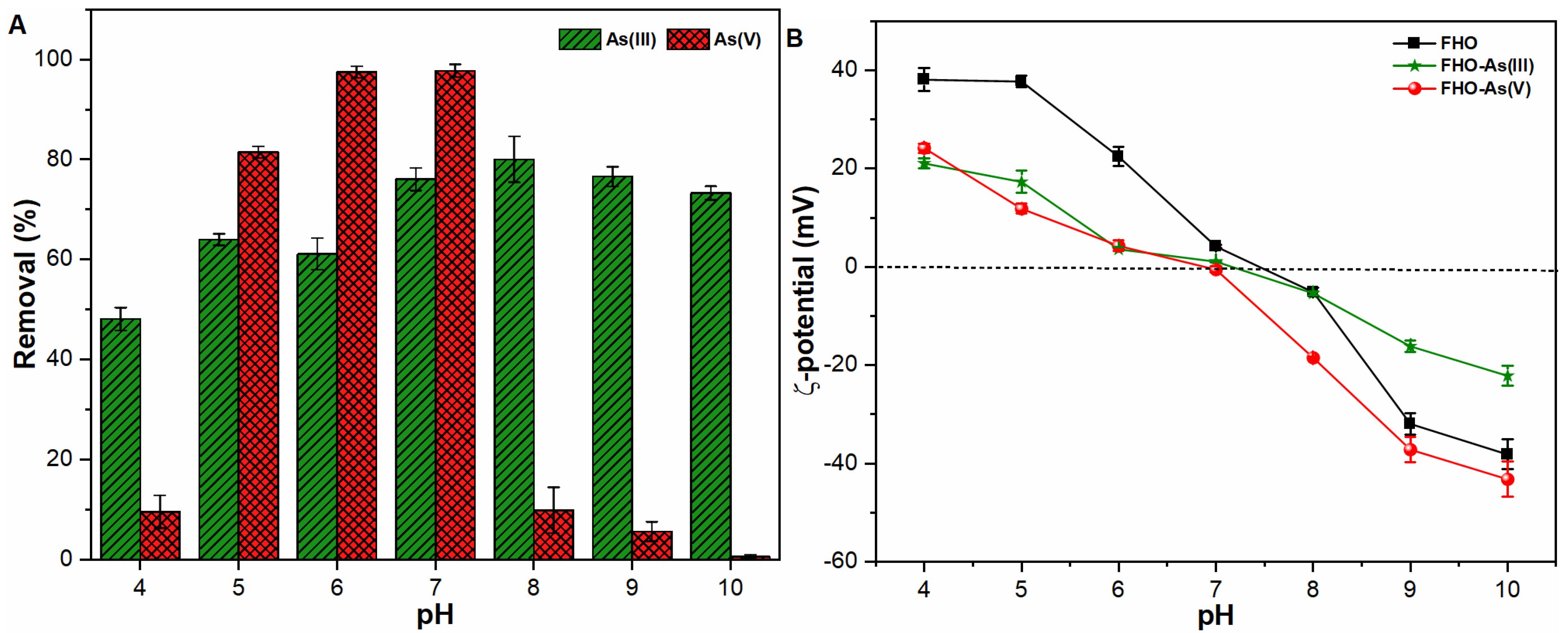
3.1. Influence of pH on ζ-Potential of Precipitates and As Removal
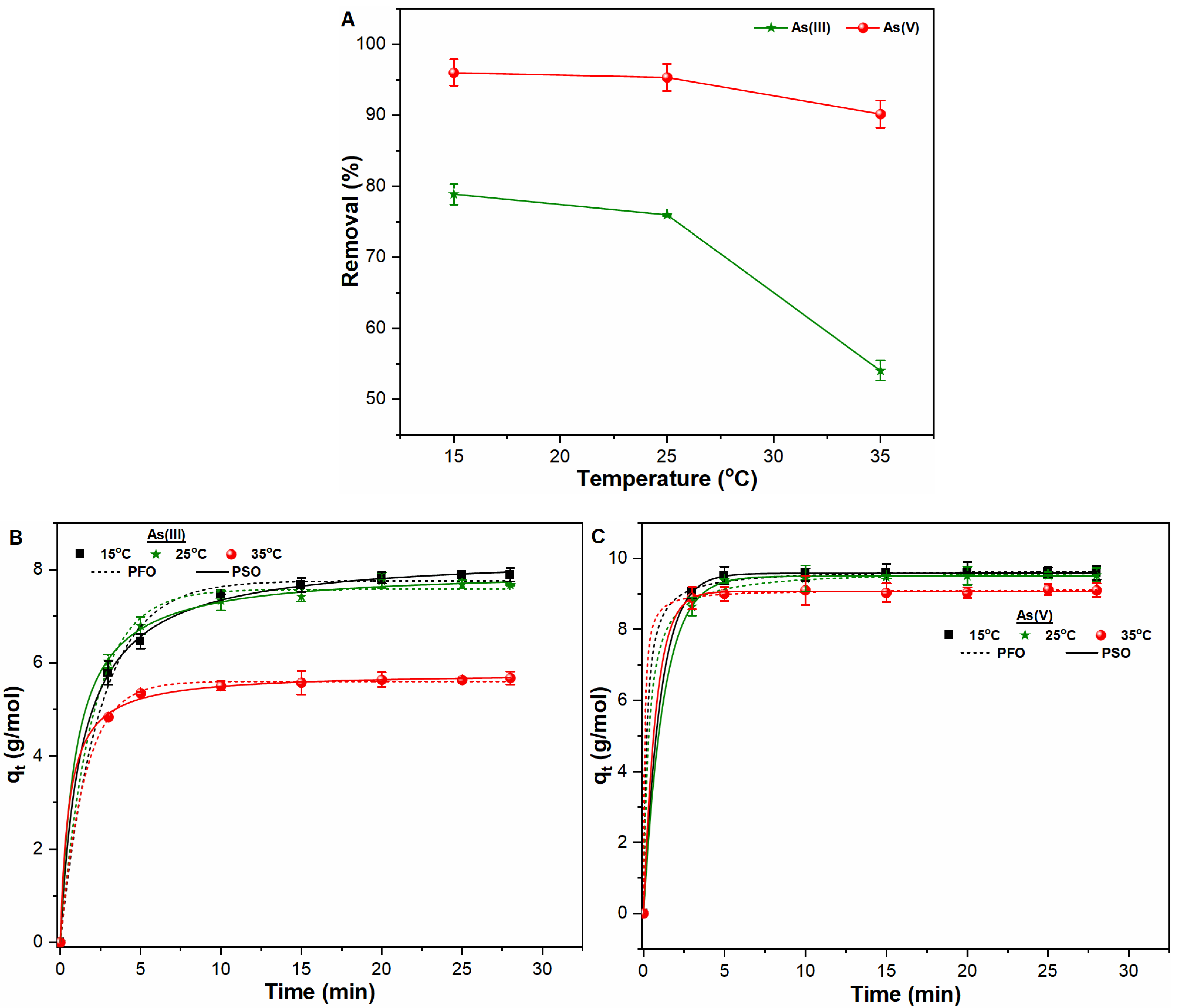
3.2. Influence of Contact Time and Temperature on As Removal
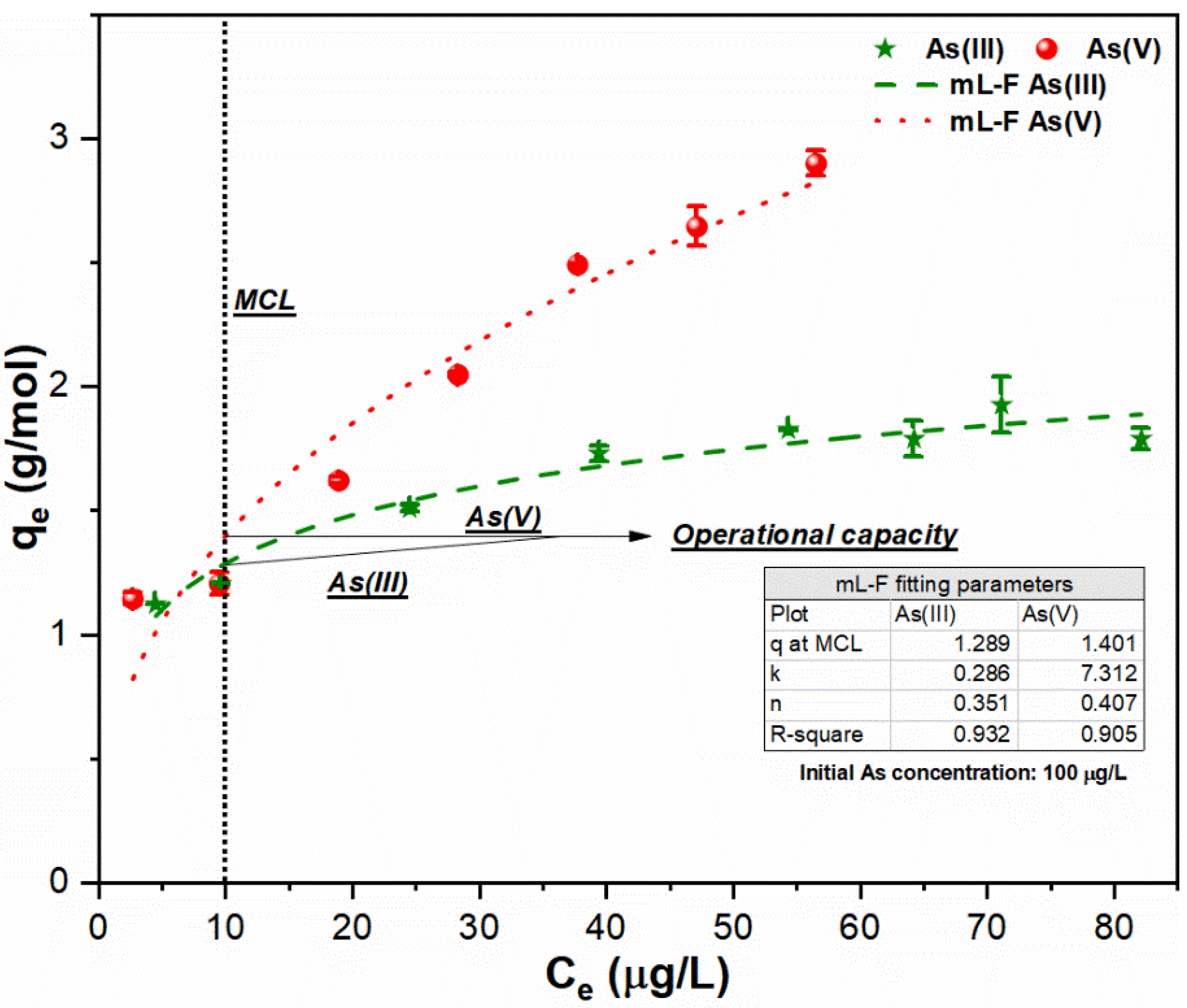
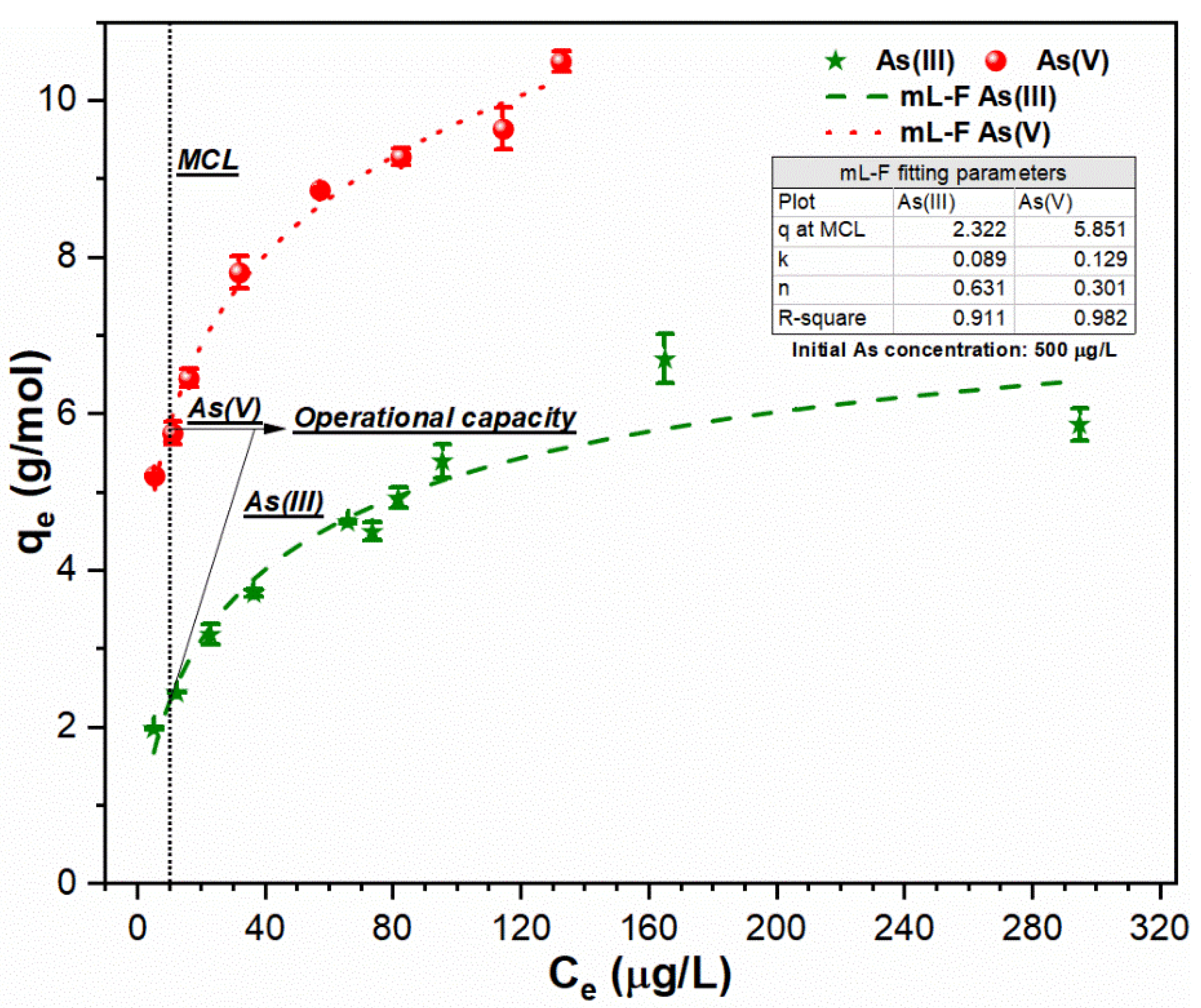
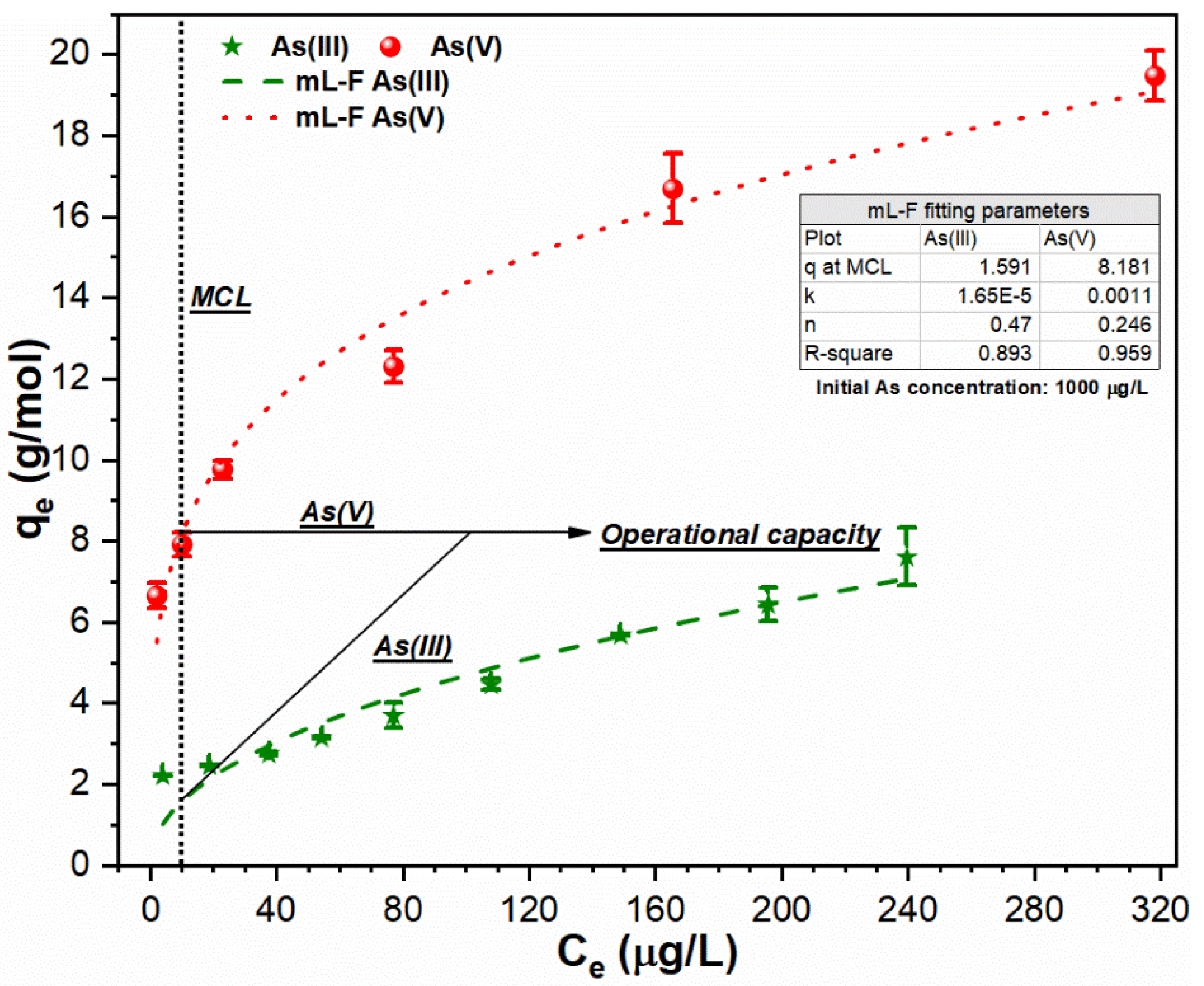
3.3. Relationship between FC Dose and As Uptake Index
3.4. Application of Experimental Strategy on Simulated Water Sample
3.5. Environmental Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.A.; Khan, R.; Park, D.R.; Ali, B.A.; Uddin, A.; Yeom, I.T. Influence of pH and Contaminant Redox Form on the Competitive Removal of Arsenic and Antimony from Aqueous Media by Coagulation. Minerals 2018, 8, 574. [Google Scholar] [CrossRef] [Green Version]
- Edition, F. Guidelines for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Ritchie, V.J.; Ilgen, A.G.; Mueller, S.H.; Trainor, T.P.; Goldfarb, R.J. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chem. Geol. 2013, 335, 172–188. [Google Scholar] [CrossRef]
- Hiller, E.; Lalinská, B.; Chovan, M.; Jurkovič, Ľ.; Klimko, T.; Jankulár, M.; Hovorič, R.; Šottník, P.; Fľaková, R.; Ženišová, Z. Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl. Geochem. 2012, 27, 598–614. [Google Scholar] [CrossRef]
- Sanjrani, M.A.; Mek, T.; Sanjrani, N.D.; Leghari, S.J.; Moryani, H.T.; Shabnam, A. Current situation of aqueous arsenic contamination in Pakistan, focused on Sindh and Punjab Province, Pakistan: A review. J. Pollut. Eff. Cont. 2017, 5, 2. [Google Scholar]
- Daud, M.K.; Nafees, M.; Ali, S.; Rizwan, M.; Bajwa, R.A.; Shakoor, M.B.; Arshad, M.U.; Chatha, S.A.S.; Deeba, F.; Murad, W.; et al. Drinking Water Quality Status and Contamination in Pakistan. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Kumar, P.R.; Chaudhari, S.; Khilar, K.C.; Mahajan, S.P. Removal of arsenic from water by electrocoagulation. Chemosphere 2004, 55, 1245–1252. [Google Scholar] [CrossRef]
- Baskan, M.B.; Pala, A. Determination of arsenic removal efficiency by ferric ions using response surface methodology. J. Hazard. Mater. 2009, 166, 796–801. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, J.; Liu, S.; Li, W.; van Leeuwen, J.; Mulcahy, D. Removal of As (III) and As (V) by ferric salts coagulation–Implications of particle size and zeta potential of precipitates. Sep. Purif. Technol. 2014, 135, 64–71. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Akram, M.; Khan, S.; Park, D.R.; Yeom, I.T. Interaction of Arsenic Species with Organic Ligands: Competitive Removal from Water by Coagulation-Flocculation-Sedimentation (C/F/S). Molecules 2019, 24, 1619. [Google Scholar] [CrossRef] [Green Version]
- Nagar, R.; Sarkar, D.; Makris, K.C.; Datta, R. Effect of solution chemistry on arsenic sorption by Fe-and Al-based drinking-water treatment residuals. Chemosphere 2010, 78, 1028–1035. [Google Scholar] [CrossRef]
- Deschamps, E.; Ciminelli, V.S.T.; Höll, W.H. Removal of As (III) and As (V) from water using a natural Fe and Mn enriched sample. Water Res. 2005, 39, 5212–5220. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Cao, Z.; Xu, J.; Zhou, X.; Zhu, J.; Liu, X.; Baig, S.A.; Zhou, J.; Xu, X. Enhanced removal of As (III)/(V) from water by simultaneously supported and stabilized Fe-Mn binary oxide nanohybrids. Chem. Eng. J. 2017, 322, 710–721. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vieira, B.R.C.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Arsenate and arsenite adsorption onto iron-coated cork granulates. Sci. Total Environ. 2018, 642, 1075–1089. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Inam, M.W.; Yeom, I.T. Kinetic and isothermal sorption of antimony oxyanions onto iron hydroxide during water treatment by coagulation process. J. Water Process. Eng. 2021, 41, 102050. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, S.; Toyohara, Y.; Saha, G.C.; Awual, M.R.; Kuba, T. Development of synthetic zeolites from bio-slag for cesium adsorption: Kinetic, isotherm and thermodynamic studies. J. Water Process. Eng. 2020, 33, 101055. [Google Scholar] [CrossRef]
- Lee, M.-G.; Kam, S.-K.; Lee, C.-H. Kinetic and isothermal adsorption properties of strontium and cesium ions by zeolitic materials synthesized from Jeju volcanic rocks. Environ. Eng. Res. 2020, 26, 194–202. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Z.; Wang, H.; Liu, S.; Wu, F.; Giesy, J.P. Removal of antimonate (Sb (V)) and antimonite (Sb (III)) from aqueous solutions by coagulation-flocculation-sedimentation (CFS): Dependence on influencing factors and insights into removal mechanisms. Sci. Total Environ. 2018, 644, 1277–1285. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef]
- Yan, D.; Li, H.-J.; Cai, H.-Q.; Wang, M.; Wang, C.-C.; Yi, H.-B.; Min, X.-B. Microscopic insight into precipitation and adsorption of As (V) species by Fe-based materials in aqueous phase. Chemosphere 2018, 194, 117–124. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Competitive adsorption of As (III), As (V), Sb (III) and Sb (V) onto ferrihydrite in multi-component systems: Implications for mobility and distribution. J. Hazard. Mater. 2017, 330, 142–148. [Google Scholar] [CrossRef]
- Leuz, A.-K.; Mönch, H.; Johnson, C.A. Sorption of Sb (III) and Sb (V) to goethite: Influence on Sb (III) oxidation and mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef]
- Taylor, K.C.; Nasr-El-Din, H.A.; Al-Alawi, M.J. Systematic study of iron control chemicals used during well stimulation. SPE J. 1999, 4, 19–24. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, J.; Liu, H.; Liu, R.; Wu, R. Preparation and evaluation of a novel Fe–Mn binary oxide adsorbent for effective arsenite removal. Water Res. 2007, 41, 1921–1928. [Google Scholar] [CrossRef]
- Zhang, G.-S.; Qu, J.-H.; Liu, H.-J.; Liu, R.-P.; Li, G.-T. Removal mechanism of As (III) by a novel Fe− Mn binary oxide adsorbent: Oxidation and sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Adsorption behavior and mechanism of arsenate at Fe–Mn binary oxide/water interface. J. Hazard. Mater. 2009, 168, 820–825. [Google Scholar] [CrossRef]
- Guan, X.; Dong, H.; Ma, J.; Jiang, L. Removal of arsenic from water: Effects of competing anions on As (III) removal in KMnO4–Fe (II) process. Water Res. 2009, 43, 3891–3899. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.; Borgfeld, N.; Kim, J.; Parsons, J.G. Removal of arsenic from aqueous solution: A study of the effects of pH and interfering ions using iron oxide nanomaterials. Microchem. J. 2012, 101, 30–36. [Google Scholar] [CrossRef]
| Cations | mg/L | Anions | mg/L |
|---|---|---|---|
| Na+ | 88.8 | HCO3− | 138 |
| Ca2+ | 40.0 | SO42− | 50 |
| Mg2+ | 12.7 | Cl− | 71 |
| NO3− | 2 | ||
| F− | 1 | ||
| PO43− | 0.04 | ||
| SiO2 | 22.4 |
| PFO | PSO | ||||||
|---|---|---|---|---|---|---|---|
| Ions | Temperature | k1 (1/min) | qe (g/mol) | R2 | k2 (mol)/g min) | qe (g/mol) | R2 |
| As (III) | 15 °C | 0.414 | 7.758 | 0.994 | 0.089 | 8.332 | 0.999 |
| 25 °C | 0.504 | 7.575 | 0.996 | 0.132 | 7.996 | 0.999 | |
| 35 °C | 0.654 | 5.603 | 0.999 | 0.322 | 5.789 | 0.999 | |
| As (V) | 15 °C | 0.954 | 9.585 | 0.999 | 0.551 | 9.701 | 0.999 |
| 25 °C | 0.808 | 9.505 | 0.999 | 0.342 | 9.685 | 0.999 | |
| 35 °C | 1.294 | 9.074 | 0.999 | 1.506 | 9.123 | 0.999 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, M.A.; Khan, R.; Lee, K.-H.; Wie, Y.-M. Removal of Arsenic Oxyanions from Water by Ferric Chloride—Optimization of Process Conditions and Implications for Improving Coagulation Performance. Int. J. Environ. Res. Public Health 2021, 18, 9812. https://doi.org/10.3390/ijerph18189812
Inam MA, Khan R, Lee K-H, Wie Y-M. Removal of Arsenic Oxyanions from Water by Ferric Chloride—Optimization of Process Conditions and Implications for Improving Coagulation Performance. International Journal of Environmental Research and Public Health. 2021; 18(18):9812. https://doi.org/10.3390/ijerph18189812
Chicago/Turabian StyleInam, Muhammad Ali, Rizwan Khan, Kang-Hoon Lee, and Young-Min Wie. 2021. "Removal of Arsenic Oxyanions from Water by Ferric Chloride—Optimization of Process Conditions and Implications for Improving Coagulation Performance" International Journal of Environmental Research and Public Health 18, no. 18: 9812. https://doi.org/10.3390/ijerph18189812
APA StyleInam, M. A., Khan, R., Lee, K.-H., & Wie, Y.-M. (2021). Removal of Arsenic Oxyanions from Water by Ferric Chloride—Optimization of Process Conditions and Implications for Improving Coagulation Performance. International Journal of Environmental Research and Public Health, 18(18), 9812. https://doi.org/10.3390/ijerph18189812







