Analysis of COVID-19 in Professionals Working in Geriatric Environment: Multicenter Prospective Study
Abstract
:1. Introduction
2. Methods
2.1. Design
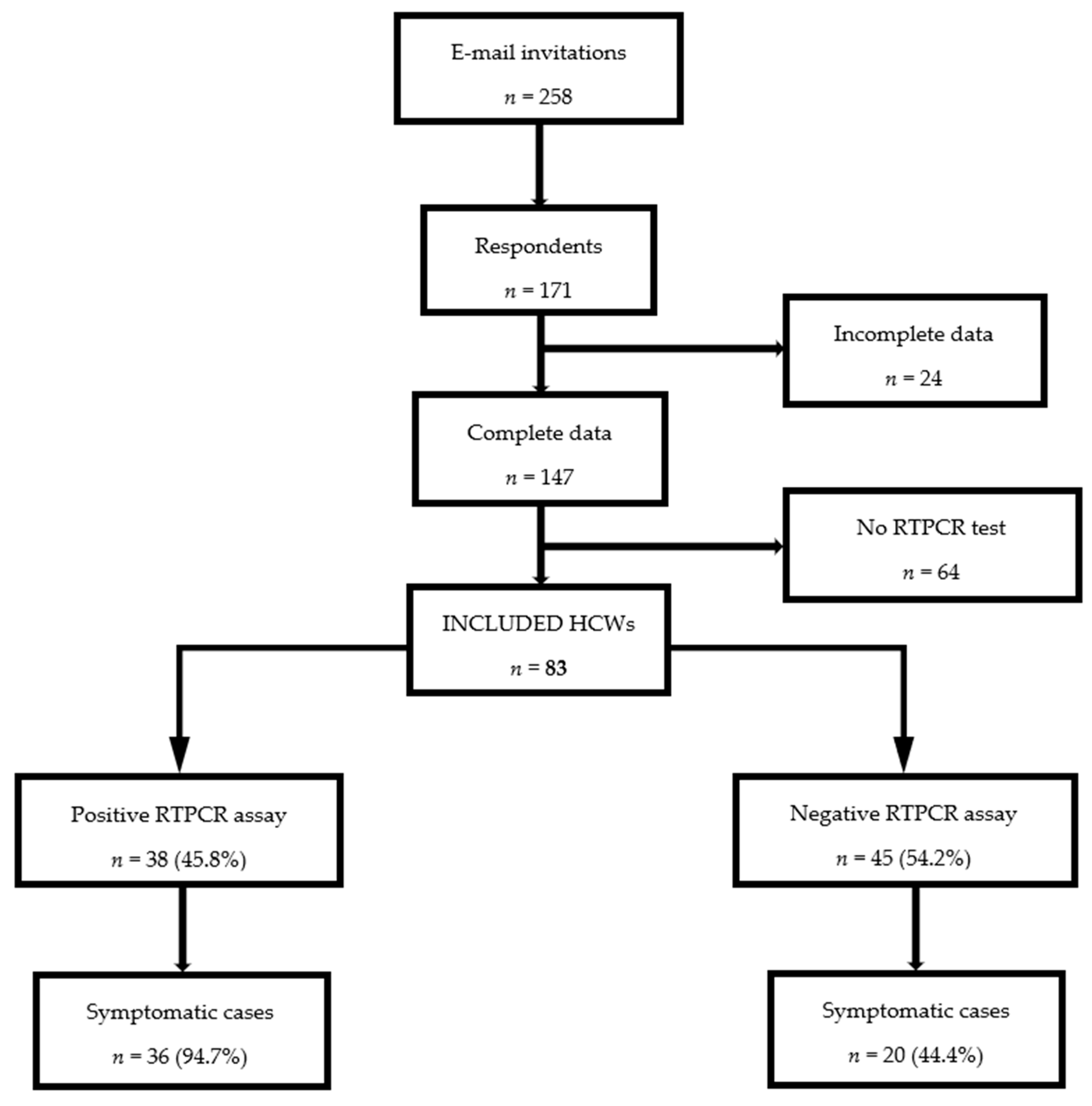
2.2. Population Selection
2.3. Diagnosis of Prevalent SARS-CoV-2 Infection
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic and Medical Data
3.2. Work-Related Characteristics
3.3. Clinical Presentation
3.4. Use of Personal Protective Equipment against COVID-19
3.5. Comparison between RTPCR+ and RTPCR− Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html (accessed on 3 September 2021).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in patients younger than 60 years is a risk factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, M.M.; Groenewold, M.R.; Lessem, S.E.; Xu, K.; Ussery, E.N.; Wiegand, R.E.; Qin, X.; Do, T.; Thomas, D.; Tsai, S.; et al. Update: Characteristics of health care personnel with COVID-19: United States, February 12–July 16 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Drew, D.A.; Joshi, A.D.; Guo, C.G.; Ma, W.; Mehta, R.S.; Sikavi, D.R.; Lo, C.H.; Kwon, S.; Song, M.; et al. Risk of COVID-19 among frontline healthcare workers and the general community: A prospective cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar]
- Sante Publique France. Recensement National des cas de CVOID-19 chez les Professionnels en Etablissements de Santé. Mise à jour le 28 juin 2021. Available online: https://www.santepubliquefrance.fr/etudes-et-enquetes/recensement-national-des-cas-de-covid-19-chez-les-professionnels-en-etablissements-de-sante/ (accessed on 10 July 2021).
- Dorlass, E.G.; Monteiro, C.O.; Viana, A.O.; Soares, C.P.; Machado, R.R.G.; Thomazelli, L.M.; Araujo, D.B.; Leal, F.B.; Candido, E.D.; Telezynski, B.L.; et al. Lower cost alternatives for molecular diagnosis of COVID-19: Conventional RT-PCR and SYBR Green-based RT-qPCR. Braz. J. Microbiol. 2020, 51, 1117–1123. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; Gubbay, J.B.; Li, Y.; Needle, R.; Arneson, S.R.; Marcino, D.; Charest, H.; Desnoyers, G.; Dust, K.; Fattouh, R.; et al. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J. Clin. Virol. 2020, 128, 104433. [Google Scholar] [CrossRef]
- Kamacooko, O.; Kitonsa, J.; Bahemuka, U.M.; Kibengo, F.M.; Wajja, A.; Basajja, V.; Lumala, A.; Kakande, A.; Kafeero, P.; Ssemwanga, E.; et al. Knowledge, Attitudes, and practices regarding COVID-19 among healthcare workers in Uganda: A cross-sectional survey. Int. J. Environ. Res. Public Health 2021, 18, 7004. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, T.G.; Kiros, K.; Gesesew, H.A.; Ward, P.R. Assessment of knowledge and practices toward COVID-19 prevention among healthcare workers in Tigray, north Ethiopia. Front. Public Health. 2021, 9, 614321. [Google Scholar] [CrossRef]
- Veronese, N.; Trabucchi, M.; Vecchiato, C.; Demurtas, J.; De Leo, D. The risk of suicide in healthcare workers in nursing home: An exploratory analysis during COVID-19 epidemic. Int. J. Geriatr. Psychiatry 2021, 36, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; Kamath, A.; Parepalli, S.A.; Brown, G.; Iharchane, S.; et al. Infection and mortality of healthcare workers worldwoide from COVID-19: A systematic review. BMJ Glob. Health 2020, 5, e003097. [Google Scholar] [CrossRef] [PubMed]
- Contejean, A.; Leporrier, J.; Canoui, E.; Alby-Laurent, F.; Lafont, E.; Beaudeau, L.; Parize, P.; Lecieux, F.; Greffet, A.; Chéron, G.; et al. Comparing dynamics and determinants of severe acute respiratory syndrome coronavirus 2 transmissions among healthcare workers of adult and pediatric settings in central Paris. Clin. Infect. Dis. 2021, 72, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Mhango, M.; Dzobo, M.; Chitungo, I.; Dzinamarira, T. COVID-19 Risk factors among health workers: A rapid review. Saf. Health. Work 2020, 11, 262–265. [Google Scholar] [CrossRef]
- Sante Publique France. Coronavirus: Chiffres clés et évolution de la COVID-19 en France et dans le Monde. Mise à Jour le 09 Juillet 2021. Available online: https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde/ (accessed on 10 July 2021).
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ. Evid. Based Med. 2020, 2020, 111549. [Google Scholar]
- Farsalinos, K.; Barbouni, A.; Niaura, R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: Could nicotine be a therapeutic option? Intern. Energy Med. 2020, 15, 845–852. [Google Scholar]
- Usman, M.S.; Siddiqi, T.J.; Khan, M.S.; Patel, U.K.; Shahid, I.; Ahmed, J.; Kalra, A.; Michos, E.D. Is there a smoker’s paradox in COVID-19? BMJ Evid. Based Med. 2020, 2020, 111492. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Patanavanich, R.; Glantz, S.A. Smoking is associated with covid-19 progression: A meta-analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Kelleni, M.T. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed. Pharmacother. 2021, 133, 110982. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Conduite à Tenir en cas de Contact d’une Personne ayant des Antécédents Evocateurs de Covid-19 avec une Personne Malade du Covid-19. Available online: https://www.hcsp.fr/Explore.cgi/AvisRapportsDomaine?clefr=819/ (accessed on 10 July 2021).
- Chu, J.; Yang, N.; Wei, Y.; Yue, H.; Zhang, F.; Zhao, J.; He, L.; Sheng, G.; Chen, P.; Li, G.; et al. Clinical characteristics of 54 medical staff with COVID-19: A retrospective study in a single center in Wuhan, China. J. Med. Virol. 2020, 92, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Del Mar, C.B.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Bawazeer, G.A.; van Driel, M.L.; Jones, M.A.; Thorning, S.; Beller, E.M.; et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. 2020, 11, CD006207. [Google Scholar] [PubMed]
| No. | Question | Response |
|---|---|---|
| 1 | How old are you? | Value: |
| 2 | What is your sex? | □Woman □Man |
| 3 | What is your body mass index? | Value: |
| 4 | What is your smoking status? | □Non-smoker □Smoker |
| 5 | Did you receive a flu vaccine in 2019–2020? | □Yes □No |
| 6 | Is there more than one person living in your home? | □Yes □No |
| 7 | Do you live in a household with children? | □Yes □No |
| 8 | Do you live in a household with older person(s) (≥75 years)? | □Yes □No |
| 9 | What is your work in the unit/department? | □Physician □Nurse □Assistant nurses □Physiotherapist □Occupational therapist □Dietitian □Psychologist □Member of administrative staff □Other |
| 10 | In what unit/department do you work? | □AGU □GRU □NH □No fixed unit |
| 11 | Do you work full time? | □Yes □No |
| 12 | Are you occupying a night shift? | □Yes □No |
| 13 | How long have you been working in this hospital (years)? | Value: |
| 14 | Do you suffer from a chronic illness? | □Yes □No |
| 15 | In the last 6 months have you used (topically or orally) NSAIDs or corticosteroids? | □Yes □No |
| 16 | In the last 6 months have you had symptoms consistent with COVID-19? | □Yes □No |
| 17 | What symptoms have you had? | □Fever □Cough □Dyspnoea □Asthenia □Myalgia □Sputum production □Headache □Sore Throat □Nasal obstruction □Diarrhea □ Anorexia □Nausea □Anosmia □Ageusia □Another |
| 18 | What was the first symptom? | □Fever □Cough □Dyspnea □Asthenia □Myalgia □Sputum production □Headache □Sore Throat □Nasal obstruction □Diarrhea □Anorexia □Nausea □Anosmia □Ageusia □Another |
| 19 | How long did the symptoms last (days)? | Value: |
| 20 | Were you hospitalized because of these symptoms? | □Yes □No |
| 21 | Do you have residual symptoms? If yes, what are those symptoms? | □Yes □No Response: |
| 22 | Have you been tested for SARS-CoV-2 infection by nasopharyngeal RTPCR test? | □Yes □No |
| 23 | If you were tested for COVID-19, what was the result of the RTPCR test? | □Negative □Positive |
| 24 | If you were tested, was the test performed before or after the start of symptoms? | □Yes □No |
| 25 | Did you go on sick leave?If yes, how long was your sick leave (days)? | □Yes □No Value: |
| 26 | Did you have a chest computed tomography scan? | □Yes □No |
| 27 | If yes, were the lung lesions compatible with a SARS-CoV-2 infection? | □Yes □No |
| 28 | What type of acquired infection do you suspect? | Response: |
| 29 | Were there COVID-19 cases in your entourage? | □Yes □No |
| 30 | Did these cases occur before or after your viral infection? | □Yes □No |
| 31 | Since the beginning of the pandemic, did you have access to personal protective equipment? | □Yes □No |
| 32 | Since the beginning of the pandemic, when in close contact with a COVID-19 infected patient or resident, what PPE were you wearing? | Response: |
| 33 | Do you strictly respect the protective personal hygiene measures (regular hand washing, proper dressing and undressing before and after contact with infected patient/resident, proper mask use) during working hours? | □ Yes □No |
| 34 | During break hours, in the presence of one or more than one colleagues, what protective personal hygiene measures do you use? | Response: |
| 35 | Do you strictly respect the protective personal hygiene measures outside of working hours? | □Yes □No |
| Variable | Total (n = 83) | RTPCR+ Group (n = 38) | RTPCR− Group (n = 45) | p * | |
|---|---|---|---|---|---|
| Mean ± SD or % (n) | Mean ± SD or % (n) | Mean ± SD or % (n) | |||
| Age (years) | Mean | 42.4 ± 10.5 | 41 ± 9.4 | 43.5 ± 11.2 | 0.30 |
| ≤25 | 3.6 (3) | 5.3 (2) | 2.2 (1) | 0.52 | |
| 26–35 | 24.1 (20) | 21.1 (8) | 26.7 (12) | 0.56 | |
| 36–45 | 34.9 (29) | 42.1 (16) | 28.9 (13) | 0.22 | |
| 46–55 | 24.1 (20) | 26.3 (10) | 22.2 (10) | 0.67 | |
| 56–65 | 13.3 (11) | 5.3 (2) | 20 (9) | 0.055 | |
| Sex | Female | 88 (73) | 92.1 (35) | 84.4 (38) | 0.28 |
| Male | 12 (10) | 7.9 (3) | 15.6 (7) | ||
| Household type | Several persons | 84.3 (70) | 81.6 (31) | 86.7 (39) | 0.73 |
| With children | 48.2 (40) | 42.1 (16) | 53.3 (24) | 0.13 | |
| With senior citizens | 1.2 (1) | 2.6 (1) | 0 (0) | 0.22 | |
| BMI | Mean ± SD | 25.4 ± 5.2 | 24.6 ± 4.9 | 26.1 ± 5.3 | 0.15 |
| ≤18.5 | 2.4 (2) | 2.6 (1) | 2.2 (1) | 0.91 | |
| 18.5–24.9 | 57.8 (48) | 71.1 (27) | 46.7 (21) | 0.03 | |
| 25–29.9 | 22.9 (19) | 13.2 (5) | 31.1 (14) | 0.06 | |
| ≥30 | 16.9 (14) | 13.2 (5) | 20 (9) | 0.42 | |
| Comorbidities | Cardiovascular diseases | 9.6 (8) | 5.3 (2) | 13.3 (6) | 0.42 |
| Autoimmune disease | 4.8 (4) | 7.9 (3) | 2.2 (1) | 0.13 | |
| Lung diseases | 3.6 (3) | 5.3 (2) | 2.2 (1) | 0.33 | |
| Type 2 diabetes | 1.2 (1) | 0 (0) | 2.2 (1) | 0.63 | |
| Other | 3.6 (3) | 0 (0) | 6.7 (3) | 0.23 | |
| Drugs | NSAID | 22.9 (19) | 10.5 (4) | 33.3 (15) | 0.01 |
| Corticosteroids | 8.4 (7) | 10.5 (4) | 6.7 (3) | 0.53 | |
| Active smokers | 21.7 (18) | 10.5 (4) | 31.1 (14) | 0.03 | |
| Flu vaccine | 65.1 (54) | 50 (19) | 77.8 (35) | 0.01 | |
| Variable | Total (n = 83) | RTPCR+ Group (n = 38) | RTPCR− Group (n = 45) | p * | |
|---|---|---|---|---|---|
| Mean ± SD or % (n) | Mean ± SD or % (n) | Mean ± SD or % (n) | |||
| Duration of working in geriatric environment (years) | 10.7 ± 9.7 | 8.6 ± 6.9 | 12.4 ± 12 | 0.06 | |
| Type of work schedule | Full-time | 85.5 (71) | 84.2 (32) | 86.7 (39) | 0.71 |
| Part-time | 14.5 (12) | 15.8 (6) | 13.3 (6) | 0.46 | |
| Night shifts | 8.4 (7) | 10.5 (4) | 6.7 (3) | 0.56 | |
| Type of worker | Physicians | 31.3 (26) | 21.1 (8) | 40 (18) | 0.20 |
| Nurses | 27.7 (23) | 42.1 (16) | 15.6 (7) | 0.18 | |
| Assistant nurses | 22.9 (19) | 18.4 (7) | 26.7 (12) | 0.38 | |
| Administrative staff | 15.7 (13) | 13.2 (5) | 17.8 (8) | 0.88 | |
| Physiotherapists | 2.4 (2) | 5.3 (2) | 0 (0) | 0.40 | |
| Department of work | Acute geriatric unit | 37.3 (31) | 63.2 (24) | 15.6 (7) | <0.001 |
| Geriatric rehabilitation unit | 19.3 (16) | 15.8 (6) | 22.2 (10) | 0.47 | |
| Rolling worker on several departments | 18.1 (15) | 18.4 (7) | 17.8 (8) | 0.93 | |
| Nursing home | 16.9 (14) | 2.6 (1) | 28.9 (13) | 0.001 | |
| Long-term care unit | 2.4 (2) | 0 (0) | 4.4 (2) | 0.53 | |
| Other (mobile palliative care or geriatric team) | 6 (5) | 0 (0) | 11.1 (5) | 0.04 | |
| Variable | Total (n = 83) | RTPCR+ Group (n = 38) | RTPCR− Group (n = 45) | p * | |
|---|---|---|---|---|---|
| Mean ± SD or % (n) | Mean ± SD or % (n) | Mean ± SD or % (n) | |||
| Duration of symptoms (days) | 17.7 ± 18.3 | 21.7 ± 20.9 | 10.4 ± 9.1 | 0.007 | |
| Sick leave (number) | 59 (49) | 94.7 (36) | 28.9 (13) | <0.001 | |
| Duration of sick leave (days) | 14.9 ± 10.5 | 16.2 ± 9.6 | 11.2 ± 11.8 | 0.19 | |
| First symptoms | Headache | 23.2 (13) | 19.4 (7) | 30 (6) | 0.45 |
| Asthenia | 12.5 (7) | 13.9 (5) | 10 (2) | 0.67 | |
| Cough | 12.5 (7) | 5.6 (2) | 25 (5) | 0.06 | |
| Fever | 10.7 (6) | 8.3 (3) | 15 (3) | 0.51 | |
| Myalgia | 10.7 (6) | 13.9 (5) | 5 (1) | 0.33 | |
| Anosmia | 5.4 (3) | 8.3 (3) | 0 (0) | 0.24 | |
| Total | 67.5 (56) | 94.7 (36) | 44.4 (20) | 0.006 | |
| Residual signs | Asthenia | 48.5 (16) | 50 (13) | 42.8 (3) | 0.14 |
| Dyspnea | 39.4 (13) | 38.5 (10) | 42.8 (3) | 0.37 | |
| Anguish | 12.1 (4) | 11.5 (3) | 14.3 (1) | 0.34 | |
| Thoracic angina | 12.1 (4) | 11.5 (3) | 14.3 (1) | 0.34 | |
| Myalgia | 12.1 (4) | 11.5 (3) | 14.3 (1) | 0.34 | |
| Cough | 12.1 (4) | 11.5 (3) | 14.3 (1) | 0.34 | |
| Total | 39.8 (33) | 68.4 (26) | 15.6 (7) | 0.003 | |
| Variable | Total (n = 83) | RTPCR+ Group (n = 38) | RTPCR− Group (n = 45) | p * | |
|---|---|---|---|---|---|
| % (n) | % (n) | % (n) | |||
| Symptoms | Asthenia | 56.6 (47) | 81.6 (31) | 35.6(16) | <0.001 |
| Headache | 48.2 (40) | 65.8 (25) | 33.3 (15) | 0.004 | |
| Ageusia | 32.5 (27) | 68.4 (26) | 2.2 (1) | <0.001 | |
| Anosmia | 32.5 (27) | 65.8 (25) | 4.4 (2) | <0.001 | |
| Fever | 24.1 (20) | 31.6 (12) | 17.8 (8) | 0.16 | |
| Chills | 22.9 (19) | 28.9 (11) | 17.8 (8) | 0.24 | |
| Cough | 19.3 (16) | 21.1 (8) | 17.8 (8) | 0.71 | |
| Myalgia | 18.1 (15) | 26.3 (10) | 11.1 (5) | 0.08 | |
| Anorexia | 15.7 (13) | 28.9 (11) | 4.4 (2) | 0.003 | |
| Dyspnea | 13.3 (11) | 23.7 (9) | 4.4 (2) | 0.001 | |
| Rhinorrhea | 10.8 (9) | 7.9 (3) | 13.3 (6) | 0.46 | |
| Insomnia | 9.6 (8) | 13.2 (5) | 6.7 (3) | 0.35 | |
| Thoracic angina | 7.2 (6) | 13.2 (5) | 2.2 (1) | 0.07 | |
| Tachycardia | 6 (5) | 5.3 (2) | 6.7 (3) | 0.82 | |
| Palpitations | 4.8 (4) | 2.6 (1) | 6.7 (3) | 0.46 | |
| Urticaria | 1.2 (1) | 2.6 (1) | 0 (0) | 0.46 | |
| Variable | Total (n = 83) | RTPCR+ Group (n = 38) | RTPCR− Group (n = 45) | p * | ||
|---|---|---|---|---|---|---|
| % (n | % (n) | % (n) | ||||
| PPE | PPE available | 79.5 (66) | 73.7 (28) | 84.4 (38) | 0.23 | |
| Types of PPE | Surgical mask | 75.9 (63) | 86.8 (33) | 66.7 (30) | 0.035 | |
| FFP2 mask | 34.9 (29) | 21.1 (8) | 46.7 (21) | 0.016 | ||
| Gown/Apron | 8.4 (7) | 7.9 (3) | 8.9 (4) | 0.88 | ||
| None | 6 (5) | 7.9 (3) | 4.4 (2) | 0.55 | ||
| Protective personal hygiene measures | Distance of 1 m | 87.9 (73) | 86.8 (33) | 88.9 (40) | 0.49 | |
| Surgical mask | 54.2 (45) | 73.7 (28) | 44.4 (20) | 0.049 | ||
| FFP2 mask | 2.4 (2) | - | 4.4 (2) | 0.62 | ||
| None | 3.6 (3) | 5.3 (2) | 2.2 (1) | 0.24 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, A.-M.; Barben, J.; Dipanda, M.; Vovelle, J.; Nuss, V.; Baudin-Senegas, C.; Putot, A.; Manckoundia, P. Analysis of COVID-19 in Professionals Working in Geriatric Environment: Multicenter Prospective Study. Int. J. Environ. Res. Public Health 2021, 18, 9735. https://doi.org/10.3390/ijerph18189735
Mihai A-M, Barben J, Dipanda M, Vovelle J, Nuss V, Baudin-Senegas C, Putot A, Manckoundia P. Analysis of COVID-19 in Professionals Working in Geriatric Environment: Multicenter Prospective Study. International Journal of Environmental Research and Public Health. 2021; 18(18):9735. https://doi.org/10.3390/ijerph18189735
Chicago/Turabian StyleMihai, Anca-Maria, Jérémy Barben, Mélanie Dipanda, Jérémie Vovelle, Valentine Nuss, Camille Baudin-Senegas, Alain Putot, and Patrick Manckoundia. 2021. "Analysis of COVID-19 in Professionals Working in Geriatric Environment: Multicenter Prospective Study" International Journal of Environmental Research and Public Health 18, no. 18: 9735. https://doi.org/10.3390/ijerph18189735
APA StyleMihai, A.-M., Barben, J., Dipanda, M., Vovelle, J., Nuss, V., Baudin-Senegas, C., Putot, A., & Manckoundia, P. (2021). Analysis of COVID-19 in Professionals Working in Geriatric Environment: Multicenter Prospective Study. International Journal of Environmental Research and Public Health, 18(18), 9735. https://doi.org/10.3390/ijerph18189735







