Workers with Cardiac AIMD Exposed to EMF: Methods and Case Studies for Risk Analysis in the Framework of the European Regulations
Abstract
:1. Introduction
- RFID;
- Wi-Fi and Bluetooth;
- UMTS and LTE.
2. Materials and Methods
General Procedure for the Risk Assessment Required for an AIMD Employee
- All of the EMF sources are listed in the table;
- All of the EMF sources are used in accordance with the indication reported in the “exceptions and remarks” column;
- The AIMD employee has not received specific warnings from the responsible physician that the AIMD may be susceptible to electromagnetic interference (EMI) from one of the present equipment.
- Step 1: Identification of the exposure scenarios
- Step 2: EMF source characterization:
- Step 3: Literature review
- Step 4: Identification of the applicable technical standards
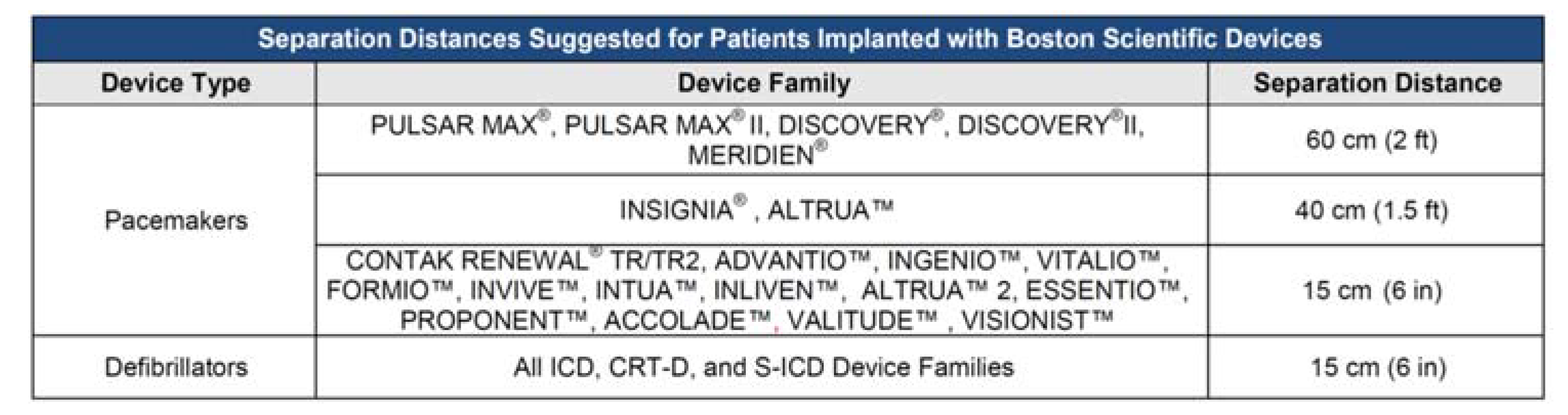
- Step 5: Specific warnings provided by PM and ICD manufacturers
- Step 6: Risk Assessment
3. Results
3.1. Case Report 1: Workers with PM or ICD Exposed to RFID Readers
3.1.1. Identification of the Exposure Scenarios
- The worker that passes through or stops close to the RFID gate;
- The worker that uses or is exposed to a hand-held RFID reader.
3.1.2. EMF Source Characterization
- 125/134 kHz (LF Low Frequencies, valid worldwide)
- 13.56 MHz (HF High Frequencies, valid worldwide)
- 860–960 MHz (UHF Ultra High Frequencies, depending on the continents they have maximum powers and different frequency bands)
3.1.3. Literature Review
3.1.4. Identification of the Applicable Technical Standards
- LF—125 kHz and 134 kHz. Several types of tests need to be performed:
- Clause 27.3: A continuous sinusoidal signal at various frequencies is applied to the PM/ICD. The amplitude of this signal is 6.25 Vpp (peak-to-peak) at 125 kHz and 6.7 Vpp at 134 kHz. Compliance is confirmed if, once the signal has been applied and then removed, the PM/ICD works as before the test;
- Clause 27.4: By applying the same signal, but with an amplitude of 1 Vpp, the PM/ICD must continue to operate without disturbances or in a safe mode defined by the manufacturer even during the application of the interference signal;
- Clause 27.5.1: A pulse modulated signal at various frequencies is applied to the PM/ICD. The amplitude of this signal is 0.750 Vpp (peak-to-peak) at 125 kHz and 0.804 Vpp at 134 kHz. Compliance is confirmed if the PM/ICD always works without malfunctions;
- Clause 27.8: The PM/ICD is exposed to a magnetic field which varies over time and after the removal of the magnetic field there must be no malfunctions. At the frequency of 125 and 134 kHz, the magnetic field amplitude is 120 A/m and 112 A/m, respectively.
- HF—13.56 MHz. Clause 27.5.3. The test signal is a modulated signal with a carrier frequency of 20 MHz. The signal must be modulated in amplitude to create pulses of 100 ms duration with a peak-to-peak amplitude of 10 V. Compliance is confirmed if the PM/ICD works without malfunctions;
- UHF—865 MHz 915 MHz. Clause 27.5.4 (clause 4.9 of ANSI/AAMI/ISO 14,117 [25]). Radiated tests should be performed using a dipole antenna fed with a pulse modulated signal with a net RF power of 120 mW (RMS). An additional 8 W (RMS) test can be performed voluntarily. The PM/ICD must not exhibit any deviation from the expected behavior during exposure to the RF field.
3.1.5. Specific Warnings Provided by PM and ICD Manufacturers
3.1.6. Risk Assessment
3.2. Case Report 2: Wi-Fi and Bluetooth
3.2.1. Identification of the Exposure Scenarios
3.2.2. EMF Source Characterization
- 2.4 GHz:100 mW (20 dBm)
- 5 GHz channel from 36 to 64:200 mW (23 dBm)
- 5 GHz channel from 100 to 140:1000 mW (30 dBm)
- 5 GHz channel from 155 to 171:4000 mW (36 dBm)
- Class 1: 100 mW ERP
- Class 2: 2.5 mW ERP
- Class 3: 1 mW ERP
3.2.3. Literature Review
3.2.4. Identification of the Applicable Technical Standards
3.2.5. Specific Warnings Provided by PM and ICD Manufacturers
3.2.6. Risk Assessment
3.3. Case Report 3: UMTS and LTE
3.3.1. Identification of the Exposure Scenarios
3.3.2. EMF Source Characterization
- 800 MHz frequency band
- 850 MHz frequency band
- 1800 MHz frequency band
- 1900 MHz frequency band
- 2100 MHz frequency band
- 2600 MHz frequency band
3.3.3. Literature Review
3.3.4. Identification of the Applicable Technical Standards
3.3.5. Specific Warnings Provided by PM and ICD Manufacturers
3.3.6. Risk Assessment
4. Discussion
- (1)
- The analysis and the data produced are sufficient to determine the risk for the worker and to implement the proper risk mitigation strategies;
- (2)
- The occurrence of malfunction in the considered scenarios cannot be excluded and there are not sufficient data to implement proper mitigation strategies. It is thus necessary to further proceed with the analysis and conduct a specific risk assessment.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Directive 89/391/EEC of the European Parliament and of the Council of 12 June 1989 on the Introduction of Measures to Encourage Improvements in the Safety and Health of Workers at Work. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A31989L0391 (accessed on 21 July 2021).
- Directive 2013/35/EU of the European Parliament and of the Council of 26 June 2013 on the Minimum Health and Safety Requirements regarding the Exposure of Workers to the Risks Arising from Physical Agents (Electromagnetic Fields) (20th Individual Directive within the Meaning of Article 16(1) of Directive 89/391/EEC) and Repealing Directive 2004/40/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32013L0035 (accessed on 21 July 2021).
- Food and Drug Administration. MAUDE—Manufacturer and User Facility Device Experience; Food and Drug Administration: Washington, DC, USA, 2021. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm (accessed on 21 July 2021).
- Napp, A.; Stunder, D.; Maytin, M.; Kraus, T.; Marx, N.; Driessen, S. Are patients with cardiac implants protected against electromagnetic interference in daily life and occupational environment? Eur. Heart J. 2015, 36, 1798–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zecchin, M.; Torre, M.; Carrani, E.; Sampaolo, L.; Ciminello, E.; Ortis, B.; Ricci, R.; Proclemer, A.; Sinagra, G.; Boriani, G. Seventeen-year trend (2001–2017) in pacemaker and implantable cardioverter-defibrillator utilization based on hospital discharge database data: An analysis by age groups. Eur. J. Intern. Med. 2021, 84, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No. 178/2002 and Regulation (EC) No. 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. Available online: https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=CELEX%3A32017R0745 (accessed on 21 July 2021).
- Calcagnini, G.; Censi, F.; Bartolini, P. Electromagnetic immunity of medical devices: The European regulatory framework. Ann. Ist. Super. Sanita 2007, 43, 268–276. [Google Scholar] [PubMed]
- European Committee for Electrotechnical Standardization. EN 45502-1:2015, Implants for Surgery. Active Implantable Medical Devices. General Requirements for Safety, Marking and for Information to be Provided by the Manufacturer; European Committee for Electrotechnical Standardization: Brussels, Belgium, 2015. [Google Scholar]
- European Committee for Electrotechnical Standardization. EN 45502-2-1:2005-Active Implantable Medical Devices—Part 2-1: Particular Requirements for Active Implantable Medical Devices Intended to Treat Bradyarrhythmia (Cardiac Pacemakers); European Committee for Electrotechnical Standardization: Brussels, Belgium, 2005. [Google Scholar]
- European Committee for Electrotechnical Standardization. EN 45502-2-2:2008-Active Implantable Medical Devices. Particular Requirements for Active Implantable Medical Devices Intended to Treat Tachyarrhythmia (Includes Implantable Defibrillators); European Committee for Electrotechnical Standardization: Brussels, Belgium, 2008. [Google Scholar]
- Council of the European Union. 1999/519/EC: Council Recommendation of 12 July 1999 on the limitation of exposure of the general public to electromagnetic fields (0 Hz to 300 GHz). Off. J. Eur. Commun. 1999, 199, 59–70.
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 99, 818–836. [Google Scholar]
- European Committee for Electrotechnical Standardization. EN 50527-1:2016, Procedure for the Assessment of the Exposure to Electromagnetic Fields of Workers Bearing Active Implantable Medical Devices—Part 1: General; European Committee for Electrotechnical Standardization: Brussels, Belgium, 2016. [Google Scholar]
- European Committee for Electrotechnical Standardization. EN 50527-2-1:2016, Procedure for the Assessment of the Exposure to Electromagnetic Fields of Workers Bearing Active Implantable Medical Devices—Part 2-1: Specific Assessment for Workers with Cardiac Pacemakers; European Committee for Electrotechnical Standardization: Bruxelles, Belgium, 2016. [Google Scholar]
- European Committee for Electrotechnical Standardization. EN 50527-2-2:2018, Procedure for the Assessment of the Exposure to Electromagnetic Fields of Workers Bearing Active Implantable Medical Devices—Part 2-2: Specific Assessment for Workers with Cardioverter Defibrillators (ICD); European Committee for Electrotechnical Standardization: Brussels, Belgium, 2018. [Google Scholar]
- Directive 2014/53/EU of the European Parliament and of the Council of 16 April 2014 on the Harmonisation of the Laws of the Member States Relating to the Making Available on the Market of RADIO Equipment and Repealing Directive 1999/5/EC Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014L0053 (accessed on 21 July 2021).
- European Telecommunications Standards Institute. ETSI EN 300 330-2: Electromagnetic Compatibility and Radio Spectrum Matters (ERM); Short Range Devices (SRD); Radio Equipment in the Frequency Range 9 kHz to 25 MHz and Inductive Loop Systems in the Frequency Range 9 kHz to 30 MHz; Part 2: Harmonized EN under Article 3.2 of the R&TTE Directive; European Telecommunications Standards Institute: Sophia Antipolis, France, 2015. [Google Scholar]
- European Telecommunications Standards Institute. ETSI EN 300 220-1: Electromagnetic Compatibility and Radio Spectrum Matters (ERM); Short Range Devices (SRD); Radio Equipment to be Used in the 25 MHz to 1000 MHz Frequency Range with Power Levels Ranging up to 500 mW; Part 1: Technical Characteristics and Test Methods; European Telecommunications Standards Institute: Sophia Antipolis, France, 2017. [Google Scholar]
- European Telecommunications Standards Institute. ETSI EN 302 208: Radio Frequency Identification Equipment Operating in the Band 865 MHz to 868 MHz with Power Levels up to 2 W and in the Band 915 MHz to 921 MHz with Power Levels up to 4 W; Harmonised Standard for Access to Radio Spectrum; European Telecommunications Standards Institute: Sophia Antipolis, France, 2020. [Google Scholar]
- Seidman, S.J.; Brockman, R.; Lewis, B.M.; Guag, J.; Shein, M.J.; Clement, W.J.; Kippola, J.; Digby, D.; Barber, C.; Huntwork, D. In vitro tests reveal sample radiofrequency identification readers inducing clinically significant electromagnetic interference to implantable pacemakers and implantable cardioverter-defibrillators. Heart Rhythm 2010, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mattei, E.; Censi, F.; Delogu, A.; Ferrara, A.; Calcagnini, G. Setups for in vitro assessment of RFID interference on pacemakers. Phys. Med. Biol. 2013, 58, 5301–5316. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Atkinson, D.B.; Bezzerides, V.J.; Yuki, K.; Franklin, K.; Casta, A.; Alexander, M.E. Pausing with the Gauze: Inhibition of Temporary Pacemakers by Radiofrequency Scan During Cardiac Surgery. Anesth. Analg. 2016, 123, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Seidman, S.J.; Guag, J.W. Adhoc electromagnetic compatibility testing of non-implantable medical devices and radio frequency identification. Biomed. Eng. Online 2013, 12, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, E.; Lucano, E.; Censi, F.; Triventi, M.; Calcagnini, G. Provocative Testing for the Assessment of the Electromagnetic Interference of RFID and NFC Readers on Implantable Pacemaker. IEEE Trans. Electromagn. Compat. 2016, 58, 314–322. [Google Scholar] [CrossRef]
- Mattei, E.; Censi, F.; Triventi, M.; Bartolini, P.; Calcagnini, G. Radiofrequency identification and medical devices: The regulatory framework on electromagnetic compatibility. Part II: Active implantable medical devices. Expert Rev. Med. Devices 2012, 9, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Padgette, J.; Bahr, J.; Batra, M.; Holtmann, M.; Smithbey, R.; Chen, L.; Scarfone, K. Guide to Bluetooth Security, Special Publication (NIST SP); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017; Available online: https://doi.org/10.6028/NIST.SP.800-121r2 (accessed on 21 July 2021).
- ISO. ANSI/AAMI/ISO 14117:2012: Active Implantable Medical Devices—Electromagnetic Compatibility—EMC Test Protocols for Implantable Cardiac Pacemakers, Implantable Cardioverter Defibrillators, and Cardiac Resynchronization Devices; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Tri, J.L.; Trusty, J.M.; Hayes, D.L. Potential for Personal Digital Assistant interference with implantable cardiac devices. Mayo Clin. Proc. 2004, 79, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Mattei, E.; Censi, F.; Triventi, M.; Calcagnini, G. Electromagnetic immunity of implantable pacemakers exposed to wi-fi devices. Health Phys. 2014, 107, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Badreldin, A.M.; Heldwein, M.; Hekmat, K. Third-generation mobile phones (UMTS) do not interfere with permanent implanted pacemakers. Pacing Clin. Electrophysiol. 2010, 33, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Mondouagne Engkolo, L.P.; Dayal, N.; Etemadi, A.; Makhlouf, A.M.; Stettler, C.; Trentaz, F. Low risk of electromagnetic interference between smartphones and contemporary implantable cardioverter defibrillators. Europace 2016, 18, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Mattei, E.; Calcagnini, G.; Censi, F.; Pinto, I.; Bogi, A.; Falsaperla, R. Workers with Active Implantable Medical Devices Exposed to EMF: In Vitro Test for the Risk Assessment. Environments 2019, 6, 119. [Google Scholar] [CrossRef] [Green Version]
| Operating Frequency | LF 125–134 kHz | HF 13.56 MHz | UHF 868–915 MHz |
| Maximum Reading Distance | 0.5 m | 1–1.5 m | 3 m |
| Data Transfer Rate | low | Good | high |
| Reading Capability in Presence of Metal Surface or Liquids | good | Fair | low |
| Tag Dimension | medium/small | medium/small | small |
| Specific Standard Defining Transmission Protocol | no | ISO/IEC 15693 ISO/IEC 14443 | no |
| RFID Systems | |
|---|---|
| Frequency Range | Maximum Field Strength |
| Low Frequency (LF) 125 and 134 kHz | ~64 dBμA/m, at 10 m |
| High Frequency (HF) 13.56 MHz | 42–60 dBµA/m, at 10 m |
| Ultra High Frequency (UHF) 865–915 MHz | 2 W (4 W for 915 MHz in US and Canada only) |
| Designation of Workplace | Examples of Equipment | Exceptions and Remarks |
|---|---|---|
| All places | Lighting equipment | Excluding specialized lighting for industrial purposes where the energy is deployed by microwave or radio frequency fields. |
| All places | Computer and IT equipment not containing wireless communication | No restrictions Hard disks (other than solid state harddiscs) of portable computers and external hard disks should be treated as equipment producing static magnetic fields and be used only with minimum distance of 15 cm between the hard disk and the device. |
| All places | Computer and IT equipment wireless transmitters communication using Bluetooth Class 1 or WiFi (both typically 100 mW) | lf such equipment contains RFincluding operating at frequencies greater than 385 MHz with peak power radiation greater than 120 mW either follow manufacturer’s recommendations associated with the device restricting their use or perform a special assessment using one of the methods specified in 4.1.2. |
| Generation | Voice | Data | Carrier Band (MHz) | Pulsing (Hz) | Max. Power (W) |
|---|---|---|---|---|---|
| 2G | GSM | EDGE | 900 1800 | 2 8 217 1733 | 2 (900 MHz) 1 (1800 MHz) |
| 3G | UMTS | 2100 (900) | 100 1500 | 0.25 | |
| 4G | VoLTE | LTE | 800 1800 2600 | 1000 | 0.25 |
| Designation of Workplace | Examples of Equipment | Exceptions and Remarks |
|---|---|---|
| From Table A.1 of the EV50527-2-1 | ||
| All places | Mobile phones, smart phones and cordless phones | For pacemakers the interference distance between a GSM phone and pacemaker is 15 cm for radiated peak powers up to 2 W. For DECT phones (250 mW), it is lower. |
| From Table A.1 of the EV50527-2-2 | ||
| All places | Mobile phones, smart phones and cordless phones | For devices the interference distance between a mobile phone and device is 15 cm for radiated peak powers up to 2 W. For DECT phones (250 mW), it is lower. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattei, E.; Censi, F.; Calcagnini, G.; Falsaperla, R. Workers with Cardiac AIMD Exposed to EMF: Methods and Case Studies for Risk Analysis in the Framework of the European Regulations. Int. J. Environ. Res. Public Health 2021, 18, 9709. https://doi.org/10.3390/ijerph18189709
Mattei E, Censi F, Calcagnini G, Falsaperla R. Workers with Cardiac AIMD Exposed to EMF: Methods and Case Studies for Risk Analysis in the Framework of the European Regulations. International Journal of Environmental Research and Public Health. 2021; 18(18):9709. https://doi.org/10.3390/ijerph18189709
Chicago/Turabian StyleMattei, Eugenio, Federica Censi, Giovanni Calcagnini, and Rosaria Falsaperla. 2021. "Workers with Cardiac AIMD Exposed to EMF: Methods and Case Studies for Risk Analysis in the Framework of the European Regulations" International Journal of Environmental Research and Public Health 18, no. 18: 9709. https://doi.org/10.3390/ijerph18189709
APA StyleMattei, E., Censi, F., Calcagnini, G., & Falsaperla, R. (2021). Workers with Cardiac AIMD Exposed to EMF: Methods and Case Studies for Risk Analysis in the Framework of the European Regulations. International Journal of Environmental Research and Public Health, 18(18), 9709. https://doi.org/10.3390/ijerph18189709







