Association of Vital Exhaustion with Risk Factors for Cardiovascular Diseases, Quality of Life and Lifestyle in 41–44-Year-Old Muscovite Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurement Methods
2.3. Statistical Analyses
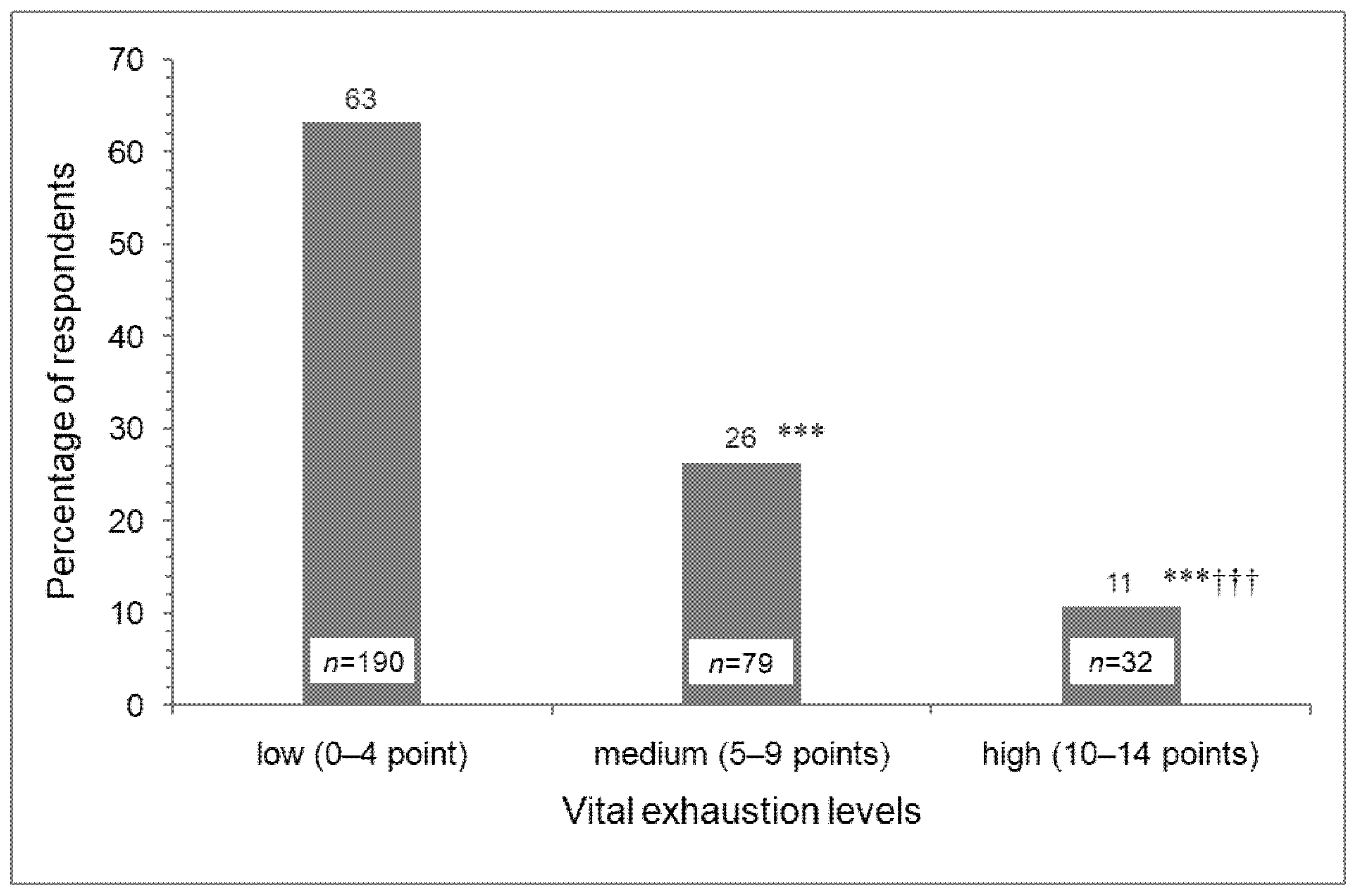
3. Results
4. Discussion
4.1. Vital Exhaustion versus Cardiovascular Risk Factors
4.2. Vital Exhaustion versus Sociopsychological Environment, Lifestyle and Quality of Life
5. Conclusions
Study Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European guidelines on cardiovascular disease prevention in clinical practice (2016 revision). Russ. J. Cardiol. 2017, 22, 7–85. [CrossRef]
- Cohen, R.; Bavishi, C.; Haider, S.; Thankachen, J.; Rozanski, A. Meta-analysis of relation of vital exhaustion to cardiovascular disease events. Am. J. Cardiol. 2017, 119, 1211–1216. [Google Scholar] [CrossRef]
- Appels, A.; Hoppener, P.; Mulder, P. A questionnaire to assess premonitory symptoms of myocardial infarction. Int. J. Cardiol. 1987, 17, 15–24. [Google Scholar] [CrossRef]
- Balog, P.; Falger, P.R.J.; Szabó, G.; Rafael, B.; Székely, A.; Thege, B.K. Are vital exhaustion and depression independent risk factors for cardiovascular disease morbidity? Health Psychol. 2017, 36, 740–748. [Google Scholar] [CrossRef]
- Gafarov, V.; Voevoda, M.; Gromova, E.; Maksimov, V.; Gagulin, I.; Yudin, N.; Gafarova, A.; Mishakova, T. Cardiovascular diseases and vital exhaustion: Longitudinal study in Russia/Siberia (WHO MONICA—psychosocial program). Russ. J. Cardiol. 2016, 4, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Frestad, D.; Prescott, E. Vital exhaustion and coronary heart disease risk: A systematic review and meta-analysis. Psychosom. Med. 2017, 79, 260–272. [Google Scholar] [CrossRef]
- Batelaan, N.M.; Seldenrijk, A.; Bot, M.; Van Balkom, A.J.L.M.; Penninx, B.W.J.H. Anxiety and new onset of cardiovascular disease: Critical review and meta-analysis. Br. J. Psychiatry 2016, 208, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noser, E.; Fischer, S.; Ruppen, J.; Ehlert, U. Psychobiological stress in vital exhaustion. Findings from the Men Stress 40+ study. J. Psychosom. Res. 2018, 105, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jousilahti, P.; Vartiainen, E.; Tuomilehto, J.; Puska, P. Sex, age, cardiovascular risk factors, and coronary heart disease: A prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999, 99, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Schnohr, P.; Marott, J.L.; Kristensen, T.S.; Gyntelberg, F.; Gronbaek, M.; Lange, P.; Jensen, M.T.; Jensen, G.B.; Prescott, E. Ranking of psychosocial and traditional risk factors by importance for coronary heart disease: The Copenhagen City Heart Study. Eur. Heart J. 2015, 36, 1385. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Hussein, S.; Lange, H.W.; Herrmann-Lingen, C. Anxiety is associated with a reduction in both mortality and major adverse cardiovascular events five years after coronary stenting. Eur. J. Prev. Cardiol. 2015, 22, 75–82. [Google Scholar] [CrossRef]
- Liu, B.; Floud, S.; Pirie, K.; Green, J.; Peto, R.; Beral, V. Does happiness itself directly affect mortality? The prospective UK Million Women Study. Lancet 2016, 387, 874–881. [Google Scholar] [CrossRef] [Green Version]
- Diest, R.V.; Appels, A. Vital exhaustion: Behavioural and biological correlates. Curr. Opin. Psychiatry 2002, 15, 639–641. [Google Scholar] [CrossRef]
- Cohen, B.E.; Edmondson, D.; Kronish, I.M. State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. Am. J. Hypertens. 2015, 28, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Schoch, J.; Noser, E.; Ehlert, U. Do implicit motives influence perceived chronic stress and vital exhaustion? Front. Psychol. 2018, 9, 1149. [Google Scholar] [CrossRef] [Green Version]
- National Cholesterol Education Program (US). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Kopina, O.S.; Suslova, E.A.; Zaikin, E.V. Population studies of psychosocial stress as a risk factor for cardiovascular diseases. Kardiologiia 1996, 36, 53–56. [Google Scholar]
- Gundarov, I.A. Quality of life. Sib. Health 1995, 1, 15–16. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms. 2005. Available online: http://www.ipaq.ki.se (accessed on 1 July 2021).
- Zohoori, N.; Mroz, T.A.; Popkin, B.; Glinskaya, E.; Lokshin, M.; Mancini, D.; Kozyreva, P.; Kosolapov, M.; Swafford, M. Monitoring the economic transition in the Russian Federation and its implications for the demographic crisis: The Russian Longitudinal Monitoring Survey. World Dev. 1998, 26, 1977–1993. [Google Scholar] [CrossRef]
- Shkolnikova, M.; Shalnova, S.; Shkolnikov, V.M.; Metelskaya, V.; Deev, A.; Andreev, E.; Jdanov, D.; Vaupel, J.W. Biological mechanisms of disease and death in Moscow: Rationale and design of the Survey on Stress, Aging and Health in Russia (SAHR). BMC Public Health 2009, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Akimova, E.V.; Akimov, M.J.; Gakova, E.I.; Kayumova, M.M.; Gafarov, V.V.; Kuznetsov, V.A. Levels of depression and life exhaustion in the open population of the middle urbanized Siberian city: Gender differences. Ther. Arch. 2019, 91, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Hoekstra, T.; Barbosa-Leiker, C.; Twisk, J.W. Vital exhaustion and markers of low-grade inflammation in healthy adults: The Amsterdam Growth and Health Longitudinal Study. Stress Health 2013, 29, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Taratukhin, E.O. The biopsychosocial approach as a novel requirement for interdisciplinarity. Russ. J. Cardiol. 2015, 20, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Balog, P.; KonkolÿThege, B. The role of vital exhaustion in predicting the recurrence of vascular events: A longitudinal study. Int. J. Clin. Health Psychol. 2019, 19, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Van Diest, R.; Appels, A. Vital exhaustion and depression: A conceptual study. J. Psychosom. Res. 1991, 35, 535–544. [Google Scholar] [CrossRef]
- Igna, C.V.; Julkunen, J.; Vanhanen, H. Vital exhaustion, depressive symptoms and serum triglyceride levels in high-risk middle-aged men. Psychiatry Res. 2011, 187, 363–369. [Google Scholar] [CrossRef]
- Just-Østergaard, E.; Mortensen, E.L.; Tolstrup, J.S.; Flensborg-Madsen, T. Vital exhaustion and risk of alcohol use disorders: A prospective cohort study. J. Psychosom. Res. 2018, 114, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Bryant, M.J.; Stevens, J.; Truesdale, K.P.; Mosley, T.; Chambless, L. Obesity and vital exhaustion: Analysis of the atherosclerosis risk in the communities study. Obesity 2008, 16, 1545–1551. [Google Scholar] [CrossRef]
- Iversen, L.B.; Strandberg-Larsen, K.; Prescott, E.; Schnohr, P.; Rod, N.H. Psychosocial risk factors, weight changes and risk of obesity: The Copenhagen City Heart Study. Eur. J. Epidemiol. 2012, 27, 119–130. [Google Scholar] [CrossRef]
- Koertge, J.C.; Ahnve, S.; Schenck-Gustafsson, K.; Orth-Gomer, K.; Wamala, S.P. Vital exhaustion in relation to lifestyle and lipid profile in healthy women. Int. J. Behav. Med. 2003, 10, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Bellingrath, S.; Weigl, T.; Kudielka, B.M. Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort-reward-imbalance. Biol. Psychol. 2008, 78, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Noguchi, H.; Tachikawa, H.; Aiba, M.; Nakamine, S.; Kawamura, A.; Takahashi, H.; Tamiya, N. Relation between social network and psychological distress among middle-aged adults in Japan: Evidence from a national longitudinal survey. Soc. Sci. Med. 2017, 175, 58–65. [Google Scholar] [CrossRef]
- Kotova, M.B.; Rozanov, V.B.; Aleksandrov, A.A.; Drapkina, O.M. Association of psychosocial stress with the social environment, lifestyle and risk factors for cardiovascular diseases in middle-aged male Muscovites. Russ. J. Cardiol. 2021, 26, 4335. [Google Scholar] [CrossRef]
- OSHwiki Contributors. Psychosocial Risks and Workers Health. 2020. Available online: http://oshwiki.eu/index.php?title=Psychosocial_risks_and_workers_health&oldid=252881 (accessed on 1 July 2021).
- Burr, H.; Formazin, M.; Pohrt, A. Methodological and conceptual issues regarding occupational psychosocial coronary heart disease epidemiology. Scand. J. Work Environ. Health 2016, 42, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliot, S.J. Psychosocial stress, women and heart health: A critical review. Soc. Sci. Med. 1995, 40, 105–115. [Google Scholar] [CrossRef]
- Van Dyck, D.; Teychenne, M.; McNaughton, S.; De Bourdeaudhuij, I.; Salmon, J. Relationship of the perceived social and physical environment with mental health-related quality of life in middle-aged and older adults: Mediating effects of physical activity. PLoS ONE 2015, 10, e0120475. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors | Vital Exhaustion Levels | Chi-Squared Tests | ||||
|---|---|---|---|---|---|---|
| 1 (n = 97) | 2 (n = 109) | 3 (n = 93) | Linear-by-Linear Association | Pearson’s Chi-Square | ||
| Overweight status + obesity | yes | 71 (73) | 73 (67) | 57 (61) | x2 = 3.05 p = 0.081 | x2 = 3.06 p = 0.26 |
| no | 26 (27) | 36 (33) | 36 (39) | |||
| Abdominal obesity | yes | 50 (52) | 54 (50) | 40 (43) | x2 = 1.37 p = 0.242 | x2 = 1.52 p = 0.468 |
| no | 47 (48) | 55 (50) | 53 (57) | |||
| Arterial hypertension | yes | 32 (33) | 37 (34) | 45 (48) *† | x2 = 4.69 p = 0.030 | x2 = 6.04 p = 0.049 |
| no | 65 (67) | 72 (66) | 48 (52) | |||
| Dyslipidemia | yes | 73 (75) | 83 (76) | 65 (70) | x2 = 0.69 p = 0.405 | x2 = 1.15 p = 0.562 |
| no | 24 (25) | 26 (24) | 28 (30) | |||
| Current smokers | yes | 40 (41) | 50 (46) | 44 (47) | x2 = 0.71 p = 0.399 | x2 = 0.79 p = 0.675 |
| no | 57 (59) | 59 (54) | 49 (53) | |||
| Low physical activity | yes | 27 (28) | 24 (22) | 27 (29) | x2 = 0.03 p = 0.863 | x2 = 1.51 p = 0.471 |
| no | 70 (72) | 85 (78) | 66 (71) | |||
| Alcohol consumption | yes | 76 (78) | 86 (79) | 80 (86) | x2 = 1.78 p = 0.182 | x2 = 2.27 p = 0.321 |
| no | 21 (22) | 23 (21) | 13 (14) | |||
| Excessive alcohol consumption | yes | 11 (11) | 26 (24) * | 30 (32) *** | x2 = 11.95 p = 0.001 | x2 = 12.16 p = 0.002 |
| no | 86 (89) | 83 (76) | 63 (68) | |||
| Studied Indicators | Vital Exhaustion Levels | F-Test for Trend | ||
|---|---|---|---|---|
| 1 (n = 97) | 2 (n = 109) | 3 (n = 93) | ||
| Age, years | 42.9 (42.8–43.0) | 43.0 (42.9–43.1) | 42.9 (42.8–43.0) | F = 0.60, p = 0.440 |
| SBP, mmHg | 121 (118–123) | 122 (119–124) | 125 (121–128) | F = 3.17, p = 0.076 |
| DBP, mmHg | 82 (80–84) | 82 (80–84) | 84 (81–86) | F = 1.67, p = 0.198 |
| Pulse, beats per minute | 73 (71–75) | 75 (73–77) | 75 (72–77) | F = 0.96, p = 0.328 |
| BMI, kg/m2 | 28.7 (27.6–29.7) | 27.5 (26.6–28.4) | 26.9 (25.9–27.8) * | F = 6.85, p = 0.009 |
| WC, cm | 96.3 (93.5–99.1) | 93.8 (91.4–96.3) | 93.3 (90.8–95.9) | F = 2.38, p = 0.124 |
| WC/HC | 0.93 (0.92–0.95) | 0.93 (0.91–0.94) | 0.93 (0.92–0.95) | F = 0.03, p = 0.871 |
| Hand-grip dynamometry, kg | 45.5 (44.2–46.8) | 43.6 (42.0–45.2) | 41.1 (39.5–42.6) *** | F = 16.41, p < 0.001 |
| TCH, mmol/L | 5.7 (5.5–6.0) | 5.7 (5.4–5.9) | 5.8 (5.5–6.0) | F = 0.01, p = 0.907 |
| HDL CH, mmol/L | 0.95 (0.90–1.01) | 0.99 (0.93–1.04) | 1.07 (0.98–1.15) * | F = 5.72, p = 0.017 |
| LDL CH, mmol/L | 4.1 (3.9–4.4) | 4.0 (3.8–4.3) | 4.0 (3.8–4.3) | F = 0.57, p = 0.450 |
| TG, mmol/L | 1.4 (1.2–1.6) | 1.5 (1.3–1.6) | 1.5 (1.2–1.7) | F = 0.34, p = 0.559 |
| Number of smoked cigarettes per day | 15 (12–17) | 18 (15–21) | 18 (15–21) | F = 2.22, p = 0.138 |
| The amount of consumed ethanol, g per week | 77.3 (55.1–99.5) | 119.1 (80.2–158.1) | 201.0 (136.7–265.2) ***† | F = 14.59, p < 0.001 |
| Physical training and sports, hours per week | 2.3 (1.7–2.9) | 2.5 (1.9–3.1) | 1.5 (1.0–2.1) | F = 3.19, p = 0.075 |
| Sedentary behavior, hours per day | 6.3 (5.7–6.8) | 7.8 (7.1–8.5) ** | 7.4 (6.7–8.1) * | F = 5.89, p = 0.016 |
| Psychosocial stress score | 3.2 (3.1–3.3) | 2.9 (2.8–3.0) *** | 2.5 (2.4–2.7) ***††† | F = 79.02, p < 0.001 |
| Studied Indicators | Vital Exhaustion Levels | F-Test | ||
|---|---|---|---|---|
| 1 (n = 97) | 2 (n = 109) | 3 (n = 93) | ||
| SBP, mmHg | 121 (118–124) | 122 (119–124) | 125 (121–128) | F = 1.58, p = 0.208 |
| DBP, mmHg | 81 (79–84) | 82 (80–84) | 84 (81–86) | F = 0.93, p = 0.397 |
| Pulse, beats per minute | 73 (71–75) | 75 (73–77) | 75 (73–77) | F = 0.86, p = 0.426 |
| BMI, kg/m2 | 28.6 (27.6–29.6) | 27.5 (26.6–28.4) | 26.9 (25.9–28.0) | F = 2.46, p = 0.087 |
| WC, cm | 95.9 (93.2–98.7) | 93.8 (91.3–96.3) | 93.7 (90.8–96.6) | F = 0.77, p = 0.463 |
| WC/HC | 0.93 (0.91–0.95) | 0.93 (0.91–0.94) | 0.94 (0.92–0.95) | F = 0.30, p = 0.783 |
| Hand-grip dynamometry, kg | 45.3 (43.7–46.9) | 43.6 (42.2–45.1) | 41.3 (39.6–42.9) ** | F = 5.36, p < 0.005 |
| TCH, mmol/L | 5.7 (5.5–6.0) | 5.7 (5.5–5.9) | 5.8 (5.5–6.0) | F = 0.10, p = 0.909 |
| HDL CH, mmol/L | 0.96 (0.89–1.03) | 0.99 (0.93–1.05) | 1.06 (0.99–1.13) | F = 1.89, p = 0.153 |
| LDL CH, mmol/L | 4.1 (3.9–4.4) | 4.0 (3.8–4.3) | 4.0 (3.8–4.3) | F = 0.22, p = 0.801 |
| TG, mmol/L | 1.4 (1.2–1.6) | 1.5 (1.3–1.6) | 1.5 (1.3–1.7) | F = 0.39, p = 0.680 |
| Number of smoked cigarettes per day | 15 (12–18) | 18 (15–20) | 18 (15–21) | F = 1.33, p = 0.268 |
| The amount of consumed ethanol, g per week | 70.3 (23.3–117.3) | 119.5 (77.3–161.7) | 209.1 (160.3–257.9) ***† | F = 7.47, p < 0.001 |
| Physical training and sports, hours per week | 2.5 (1.9–3.1) | 2.5 (2.0–3.1) | 1.0 (0.7–1.9) *†† | F = 4.86, p = 0.008 |
| Sedentary behavior, hours per day | 6.4 (5.7–7.2) | 7.8 (7.2–8.5) ** | 7.2 (6.5–7.9) | F = 4.23, p = 0.015 |
| Studied Indicators | Vital Exhaustion Levels | F-Test | ||
|---|---|---|---|---|
| 1 (n = 97) | 2 (n = 109) | 3 (n = 93) | ||
| Education | 3.4 (3.2–3.5) | 3.2 (3.0–3.4) | 3.2 (3.0–3.4) | F = 1.74, p = 0.188 |
| Social satisfaction | 71.5 (68.3–74.7) | 64.5 (61.2–67.9) * | 51.8 (46.7–56.8) **†† | F = 48.51, p < 0.001 |
| The type of work | 3.6 (3.5–3.8) | 3.5 (3.3–3.7) | 3.3 (3.1–3.5) * | F = 7.26, p = 0.007 |
| Working conditions | 69.8 (66.0–73.5) | 61.8 (56.8–66.8) * | 57.5 (52.5–62.5) *** | F = 13.40, p < 0.001 |
| Working hours | 8.5 (7.9–9.1) | 8.7 (8.0–9.5) | 8.8 (8.0–9.5) | F = 0.29, p = 0.588 |
| Working relationship with managers | 80.5 (77.3–83.8) | 76.5 (73.0–80.0) | 66.2 (61.1–71.2) **†† | F = 25.95, p < 0.001 |
| Working relationships with colleagues | 83.9 (81.4–86.4) | 80.9 (78.1–83.8) | 72.7 (68.7–76.7) **†† | F = 24.12, p < 0.001 |
| Job satisfaction | 76.8 (73.5–80.1) | 68.5 (64.8–72.1) ** | 54.7 (49.8–59.6) **†† | F = 59.08, p < 0.001 |
| Lack of stress in the workplace | 59.4 (55.0–63.7) | 49.0 (44.5–53.4) ** | 46.5 (41.6–51.4) ** | F = 15.45, p < 0.001 |
| Spiritual needs | 62.3 (58.8–65.8) | 52.4 (48.3–56.5) ** | 46.7 (42.2–51.3) **† | F = 27.82, p < 0.001 |
| Hobby | 61.1 (55.7–66.4) | 58.9 (54.0–63.7) | 48.0 (42.5–53.6) **†† | F = 11.57, p = 0.001 |
| Personal happiness level | 77.1 (74.2–80.1) | 71.1 (68.1–74.1) ** | 56.9 (53.1–60.7) **†† | F = 72.82, p < 0.001 |
| Faith | 56.8 (49.9–63.7) | 57.7 (52.0–63.5) | 59.2 (53.0–65.5) | F = 0.28, p = 0.595 |
| Enough friends | 74.9 (70.5–79.3) | 70.7 (66.2–75.3) | 56.7 (50.8–62.6) **†† | F = 25.02, p < 0.001 |
| Satisfaction with friends | 76.1 (71.6–80.5) | 72.2 (67.7–76.6) | 61.3 (55.7–67.0) **†† | F = 17.24, p < 0.001 |
| Family and relatives support | 90.7 (87.9–93.5) | 87.7 (84.4–91.0) | 81.1 (75.6–86.6) **† | F = 10.92, p = 0.001 |
| Friends’ support | 78.9 (74.4–83.3) | 70.7 (65.9–75.5) | 56.5 (50.2–62.8) ***††† | F = 34.62, p < 0.001 |
| Satisfaction with the authorities | 51.4 (46.4–56.4) | 46.6 (42.2–51.0) | 40.8 (35.4–46.2) ** | F = 8.75, p = 0.003 |
| Availability of essential food | 82.4 (79.1–85.7) | 77.2 (73.8–80.6) * | 68.8 (64.3–73.4) **†† | F = 24.78, p < 0.001 |
| Personal safety | 67.9 (64.5–71.3) | 64.3 (60.8–67.8) | 56.5 (52.2–60.8) **†† | F = 17.49, p < 0.001 |
| Living space, sq. m | 75.2 (64.4–86.0) | 76.9 (63.1–90.7) | 56.9 (51.6–62.3) *† | F = 5.03, p = 0.026 |
| Material well-being of the household | 67.2 (63.9–70.6) | 60.1 (56.6–63.6) ** | 53.5 (49.9–57.0) **†† | F = 29.62, p < 0.001 |
| Satisfaction with earnings | 61.6 (57.1–66.1) | 52.5 (47.6–57.4) * | 47.2 (42.0–52.3) ** | F = 16.89, p < 0.001 |
| Satisfaction with housing conditions | 68.7 (64.2–73.2) | 65.0 (60.1–69.8) | 59.0 (53.3–64.6) * | F = 7.10, p = 0.008 |
| Environmental satisfaction | 64.0 (59.6–68.4) | 63.9 (59.9–67.8) | 47.7 (42.9–52.5) **†† | F = 25.65, p < 0.001 |
| Satisfaction with living conditions | 76.7 (73.3–80.2) | 72.9 (69.3–76.5) | 65.9 (61.6–70.1) ***† | F = 15.80, p < 0.001 |
| Dietary diversity | 74.1 (70.8–77.5) | 69.2 (65.8–72.5) | 59.5 (55.4–63.6) **†† | F = 30.62, p < 0.001 |
| Satisfaction with food intake | 90.2 (87.6–92.8) | 88.7 (85.9–91.5) | 79.6 (75.5–83.6) **†† | F = 21.07, p < 0.001 |
| Intimacy issues | 83.0 (79.7–86.2) | 75.6 (71.4–79.9) * | 64.5 (59.1–69.8) **†† | F = 34.18, p < 0.001 |
| Satisfaction with sleep | 76.5 (72.7–80.2) | 65.9 (61.2–70.7) ** | 53.2 (47.6–58.7) **†† | F = 45.47, p < 0.001 |
| Family happiness | 82.3 (78.5–86.2) | 76.8 (72.8–80.9) | 68.6 (63.3–73.8) **† | F = 19.22, p < 0.001 |
| Studied Indicators | Vital Exhaustion Levels | F-Test | ||
|---|---|---|---|---|
| 1 (n = 97) | 2 (n = 109) | 3 (n = 93) | ||
| Education | 3.4 (3.2–3.6) | 3.2 (3.0–3.4) | 3.1 (2.9–3.4) | F = 1.29, p = 0.276 |
| Social satisfaction | 70.9 (66.7–75.0) | 64.4 (60.7–68.2) * | 52.5 (48.2–56.7) ***††† | F = 17.12, p < 0.001 |
| The type of work | 3.6 (3.5–3.8) | 3.5 (3.3–3.7) | 3.2 (3.1–3.4) * | F = 3.89, p = 0.021 |
| Working conditions | 68.5 (63.7–73.2) | 61.8 (57.4–66.2) | 59.2 (53.9–64.5) * | F = 3.38, p = 0.035 |
| Working hours | 8.7 (7.9–9.4) | 8.8 (8.1–9.5) | 8.5 (7.7–9.3) | F = 0.18, p = 0.837 |
| Working relationship with managers | 80.3 (76.4–84.3) | 76.5 (72.7–80.3) | 66.4 (62.1–70.7) ***†† | F = 10.41, p < 0.001 |
| Working relationships with colleagues | 82.4 (79.2–85.5) | 80.8 (77.9–83.7) | 74.6 (71.2–78.1) **† | F = 5.39, p = 0.005 |
| Job satisfaction | 75.7 (71.6–79.7) | 68.5 (64.7–72.2) * | 56.1 (51.7–60.5) ***††† | F = 18.90, p < 0.001 |
| Lack of stress in the workplace | 55.5 (51.1–60.0) | 48.7 (44.6–52.9) | 51.3 (46.4–56.2) | F = 2.41, p = 0.091 |
| Spiritual needs | 62.7 (58.4–67.0) | 52.6 (48.7–56.4) ** | 46.3 (41.8–50.7) ***† | F = 12.61, p < 0.001 |
| Hobby | 60.3 (54.8–65.9) | 58.6 (53.6–63.6) | 48.8 (43.1–54.6) *† | F = 4.27, p = 0.015 |
| Personal happiness level | 76.2 (72.8–79.6) | 71.0 (67.9–74.1) * | 58.0 (54.4–61.6) ***††† | F = 25.04, p < 0.001 |
| Faith | 56.1 (49.4–62.7) | 57.6 (51.6–63.7) | 60.1 (53.2–67.0) | F = 0.31, p = 0.734 |
| Enough friends | 73.7 (68.5–78.8) | 71.3 (66.6–76.0) | 58.2 (52.8–63.5) ***††† | F = 8.92, p < 0.001 |
| Satisfaction with friends | 74.7 (69.6–79.8) | 72.3 (67.7–76.9) | 62.9 (57.6–68.2) **† | F = 5.03, p = 0.007 |
| Family and relatives support | 90.1 (85.9–94.3) | 87.5 (83.8–91.3) | 81.8 (77.5–86.2) * | F = 3.40, p = 0.035 |
| Friends’ support | 77.9 (72.3–83.4) | 70.8 (65.8–75.8) | 57.6 (51.9–63.3) ***†† | F = 11.64, p < 0.001 |
| Satisfaction with the authorities | 47.3 (42.3–52.3) | 46.2 (41.7–50.7) | 45.5 (40.3–50.6) | F = 0.12, p = 0.887 |
| Availability of essential food | 82.8 (78.9–86.8) | 77.5 (73.9–81.1) * | 68.3 (64.3–72.4) ***†† | F = 11.45, p < 0.001 |
| Personal safety | 65.6 (61.7–69.5) | 64.1 (60.6–67.6) | 59.1 (55.1–63.1) | F = 2.61, p = 0.076 |
| Living space, sq. m | 77.3 (65.9–88.7) | 77.5 (66.8–88.1) | 54.4 (42.2–66.7) *† | F = 4.48, p = 0.012 |
| Material well-being of the household | 66.7 (63.1–70.4) | 60.3 (56.9–63.6) * | 54.0 (50.2–57.9) **† | F = 9.94, p < 0.001 |
| Satisfaction with earnings | 61.9 (56.8–67.1) | 53.0 (48.4–57.6) * | 46.8 (41.5–52.1) *** | F = 7.50, p < 0.001 |
| Satisfaction with housing conditions | 68.2 (62.9–73.5) | 64.6 (59.8–69.4) | 59.5 (54.1–65.0) | F = 2.25, p = 0.107 |
| Environmental satisfaction | 62.0 (57.4–66.6) | 63.5 (59.4–67.7) | 50.0 (45.2–54.8) **†† | F = 9.42, p < 0.001 |
| Satisfaction with living conditions | 76.9 (73.0–80.8) | 73.5 (70.0–77.0) | 65.7 (61.6–69.7) ***†† | F = 7.28, p < 0.001 |
| Dietary diversity | 75.0 (71.2–78.9) | 69.2 (65.7–72.6) * | 58.5 (54.5–62.4) ***††† | F = 16.04, p < 0.001 |
| Satisfaction with food intake | 90.0 (86.6–93.3) | 88.7 (85.7–91.8) | 79.9 (76.4–83.3) ***††† | F = 9.26, p < 0.001 |
| Intimacy issues | 82.7 (78.1–87.2) | 76.3 (72.2–80.4) * | 64.9 (60.2–69.5) ***††† | F = 13.30, p < 0.001 |
| Satisfaction with sleep | 74.1 (69.1–79.1) | 65.6 (61.1–70.1) * | 55.9 (50.8–61.1) ***† | F = 11.19, p < 0.001 |
| Family happiness | 80.7 (76.3–85.0) | 77.0 (72.9–81.1) | 70.6 (65.7–75.5) * | F = 4.04, p = 0.019 |
| Independent Variables | B (95%CI) | p | b | R2 (Partial) | R2(Adjusted) | VIF |
|---|---|---|---|---|---|---|
| Constant term | 19.140 (16.546; 21.733) | <0.001 | – | – | 0.466 | – |
| Personal happiness level | −0.036 (−0.056; −0.015) | 0.001 | −0.193 | 0.255 | 1.523 | |
| Psychosocial stress | −1.494 (−2.097; −0.891) | <0.001 | −0.247 | 0.093 | 1.175 | |
| Job satisfaction | −0.027 (−0.045; −0.010) | 0.002 | −0.171 | 0.049 | 1.423 | |
| Satisfaction with sleep | −0.023 (−0.036; −0.009) | 0.001 | −0.177 | 0.028 | 1.258 | |
| Total volume of consumed ethanol, g per week | 0.002 (0.001; 0.003) | 0.001 | 0.154 | 0.023 | 1.045 | |
| Hand-grip dynamometry | −0.060 (−0.101; −0.019) | 0.001 | −0.138 | 0.016 | 1.045 | |
| Satisfaction with living conditions | −0.020 (−0.037; −0.003) | 0.023 | −0.113 | 0.011 | 1.118 | |
| Spiritual needs | −0.016 (−0.031; −0.0001) | 0.049 | −0.097 | 0.008 | 1.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotova, M.B.; Rozanov, V.B.; Kiselev, A.R.; Maksimov, S.A.; Drapkina, O.M. Association of Vital Exhaustion with Risk Factors for Cardiovascular Diseases, Quality of Life and Lifestyle in 41–44-Year-Old Muscovite Men. Int. J. Environ. Res. Public Health 2021, 18, 9691. https://doi.org/10.3390/ijerph18189691
Kotova MB, Rozanov VB, Kiselev AR, Maksimov SA, Drapkina OM. Association of Vital Exhaustion with Risk Factors for Cardiovascular Diseases, Quality of Life and Lifestyle in 41–44-Year-Old Muscovite Men. International Journal of Environmental Research and Public Health. 2021; 18(18):9691. https://doi.org/10.3390/ijerph18189691
Chicago/Turabian StyleKotova, Marina B., Vyacheslav B. Rozanov, Anton R. Kiselev, Sergey A. Maksimov, and Oxana M. Drapkina. 2021. "Association of Vital Exhaustion with Risk Factors for Cardiovascular Diseases, Quality of Life and Lifestyle in 41–44-Year-Old Muscovite Men" International Journal of Environmental Research and Public Health 18, no. 18: 9691. https://doi.org/10.3390/ijerph18189691
APA StyleKotova, M. B., Rozanov, V. B., Kiselev, A. R., Maksimov, S. A., & Drapkina, O. M. (2021). Association of Vital Exhaustion with Risk Factors for Cardiovascular Diseases, Quality of Life and Lifestyle in 41–44-Year-Old Muscovite Men. International Journal of Environmental Research and Public Health, 18(18), 9691. https://doi.org/10.3390/ijerph18189691








