Evaluation of Knowledge and Risk Perception about Antibiotic Resistance in Biology and Mathematics Young Students in Nîmes University in France
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
3. Results
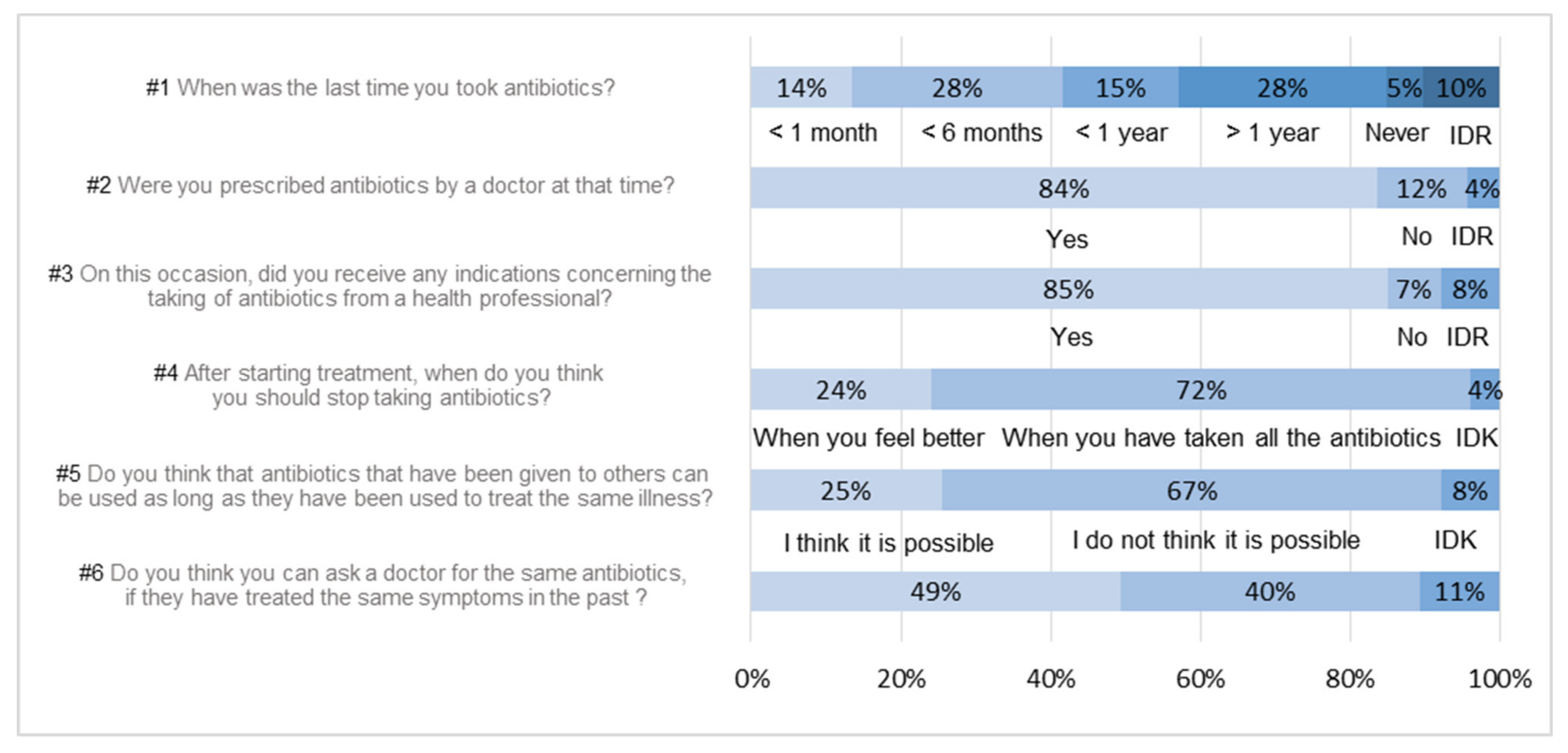
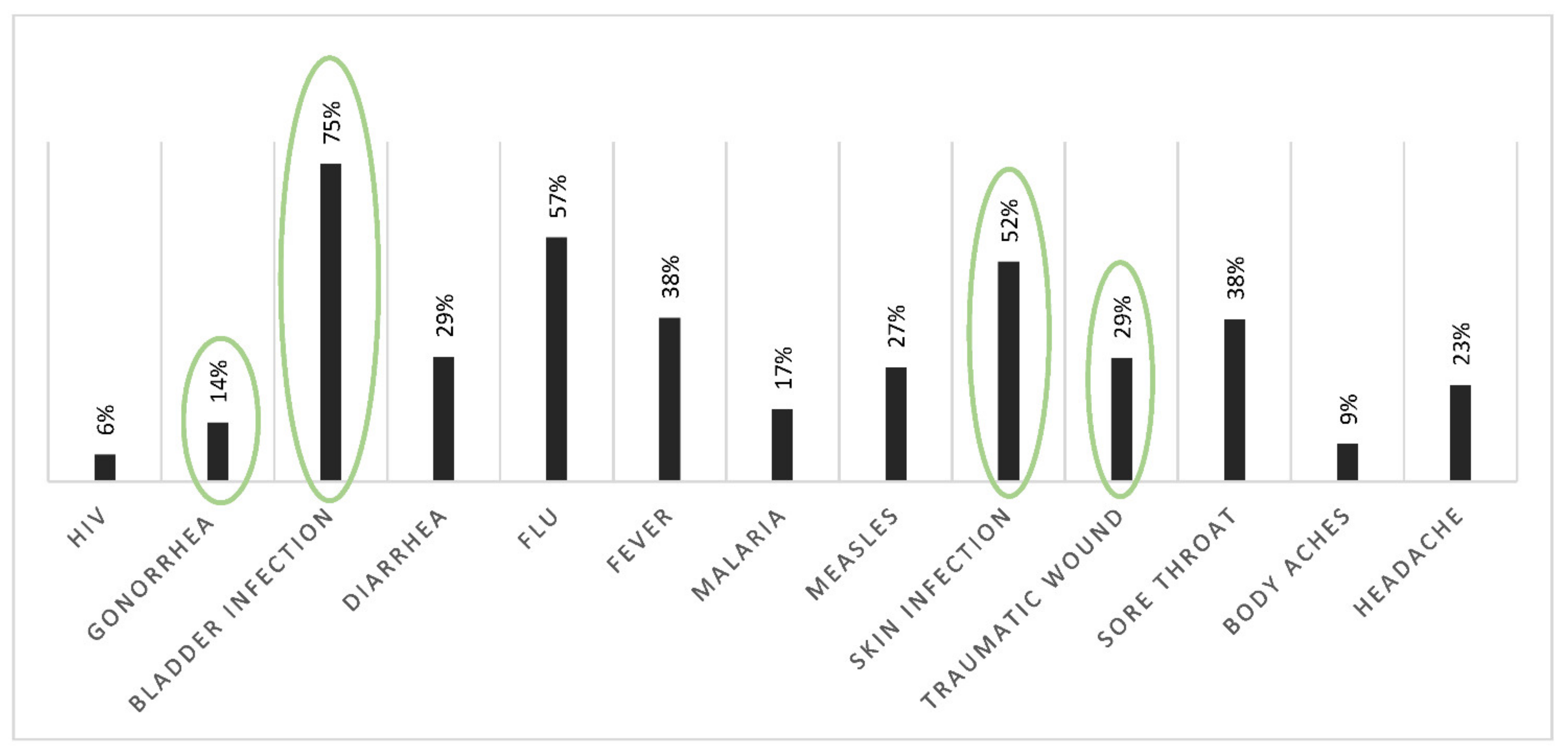
3.1. Uses and Knowledge
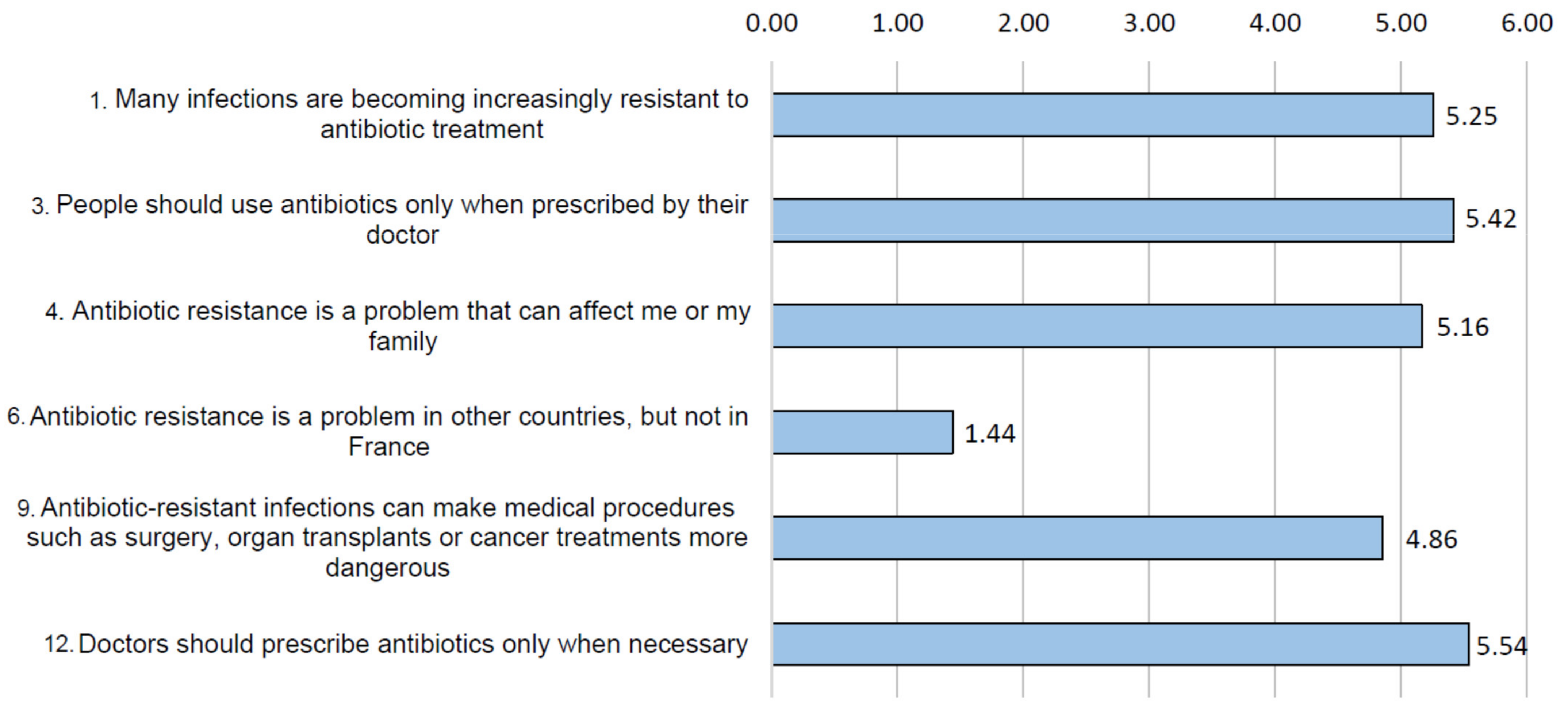
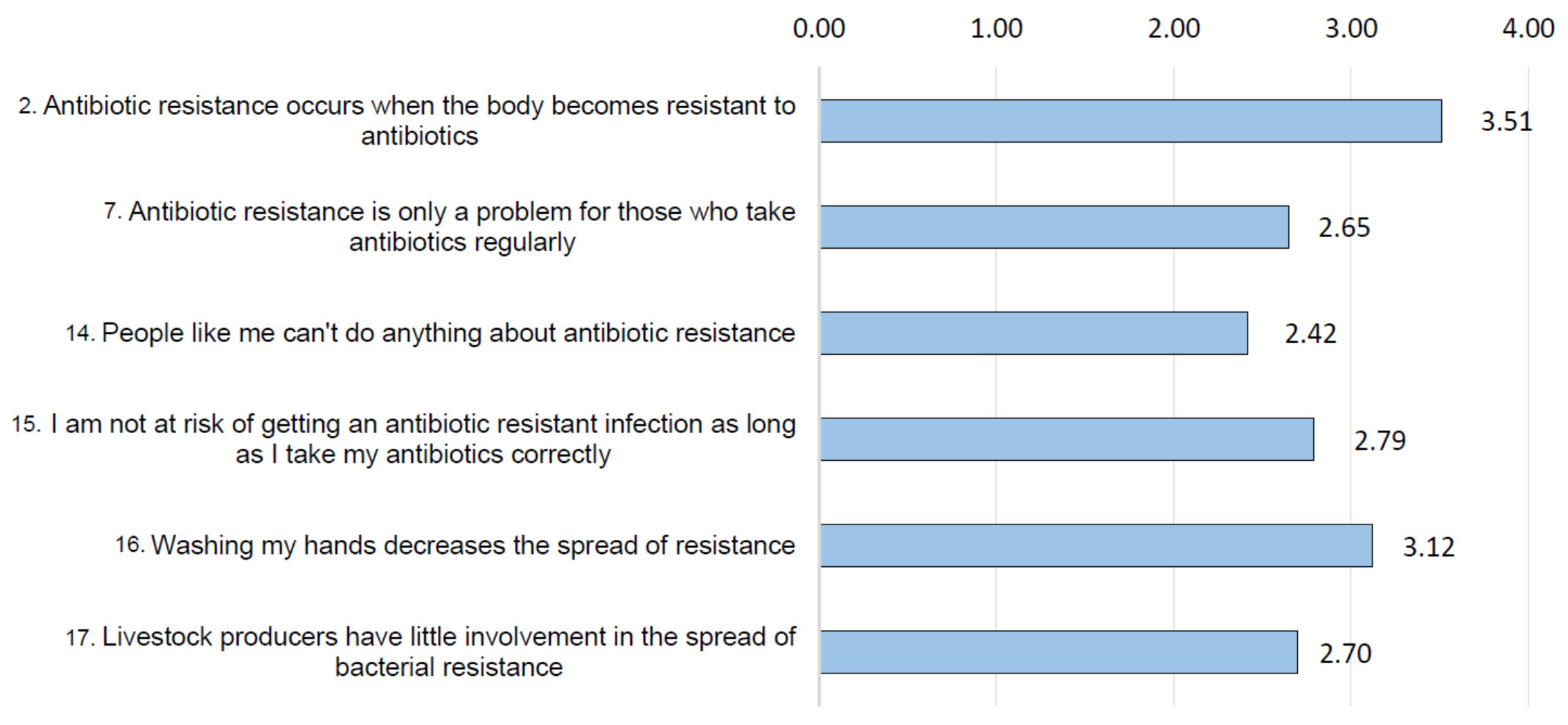
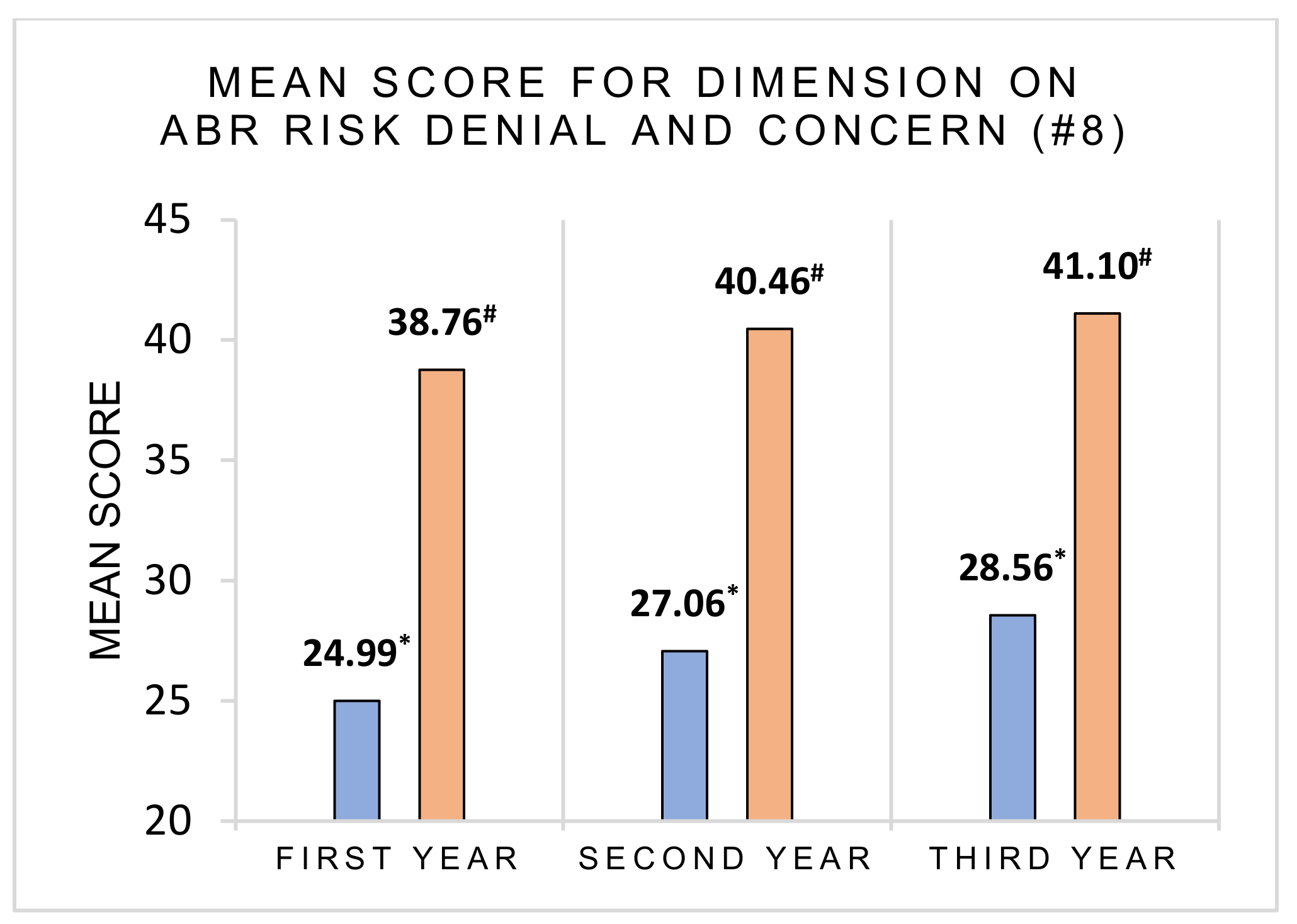
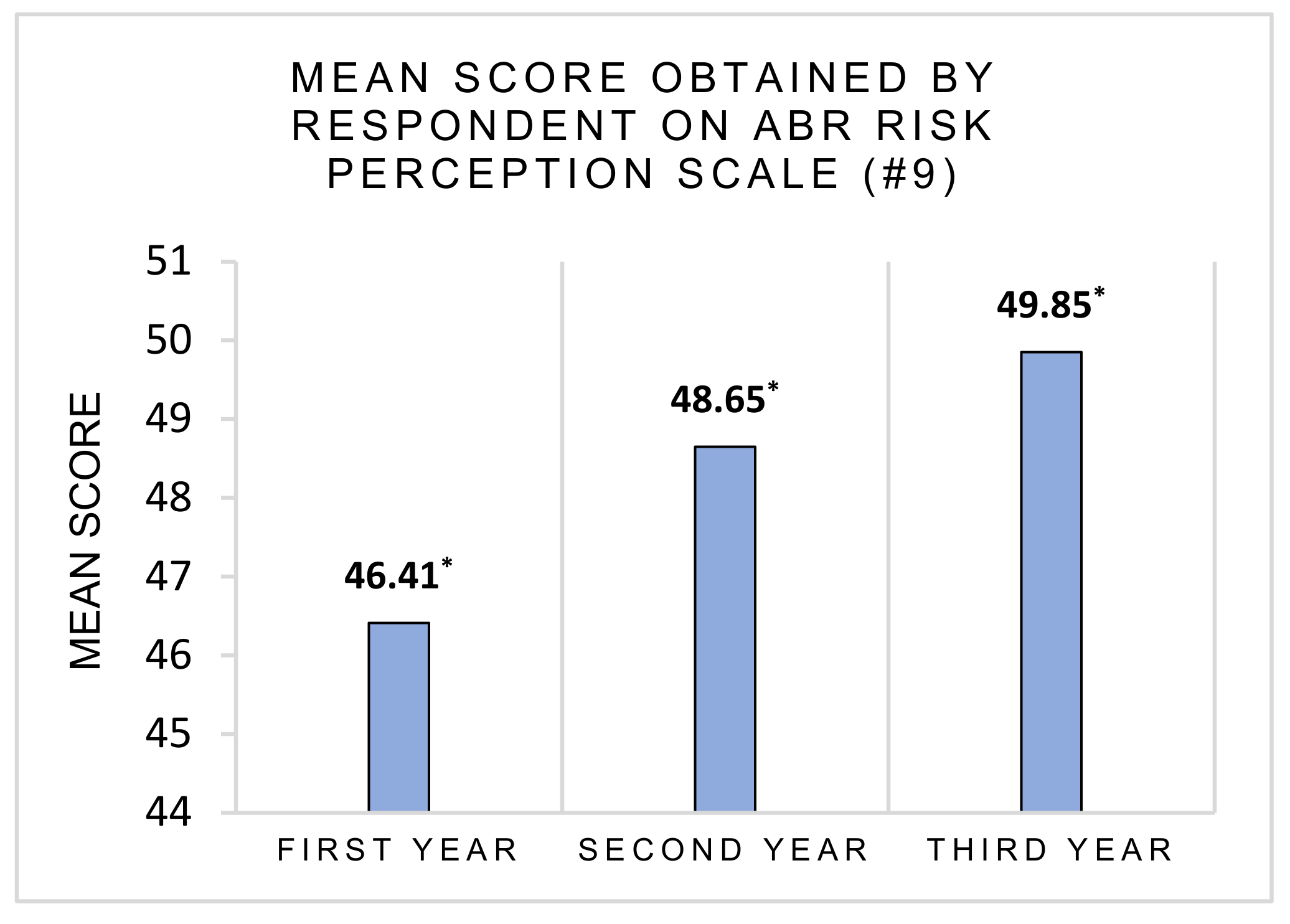
3.2. Risk Perception and Health Consequences Concern
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, D.; Guidos, R.; Boucher, H.W.; Edwards, J.; Spellberg, B.; Scheld, W.M.; Bradley, J.; Bartlett, J.G. The Epidemic of Antibiotic-Resistant Infections: A Call to Action for the Medical Community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 46, 155–164. [Google Scholar] [CrossRef]
- Noah, R.-F. The Landscape of Antibiotic Resistance. Environ. Health Perspect. 2009, 11, 245–250. [Google Scholar]
- Pidot, A.S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci. Transl. Med. 2018, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.R.; Simor, A.E. Antimicrobial resistance in hospitals: How concerned should we be? Cmaj 2009, 180, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Tello, A.; Austin, B.; Telfer, T.C. Selective pressure of antibiotic pollution. Environ. Health Perspect. 2012, 120, 1100–1106. [Google Scholar] [CrossRef]
- Pruden, A.; Larsson, D.G.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management of Options for Reducing the Release of Antibiotics. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Neill, J.O. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nation. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 13 September 2021).
- Reardon, S. Phage therapy gets revitalized. Nature 2014, 510, 15–16. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Action Plan on Antimicrobial Resistance. Microbe Mag. 2015, 10, 354–355. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 1–25. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Special Eurobarometer 445 Antimicrobial Resistance; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Wester, C.W.; Durairaj, L.; Evans, A.T.; Schwartz, D.N.; Husain, S.; Martinez, E. Antibiotic resistance. Arch. Int. Med. 2002, 162, 2210–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulcini, C.; Williams, F.; Molinari, N.; Davey, P.; Nathwani, D. Junior doctors’ knowledge and perceptions of antibiotic resistance and prescribing: A survey in France and Scotland. Clin. Microbiol. Infect. 2011, 17, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Antibiotic Resistance: Multi-Country Public Awareness Survey; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Prigitano, A.; Romanò, L.; Auxilia, F.; Castaldi, S.; Tortorano, A.M. Antibiotic resistance: Italian awareness survey 2016. J. Infect. Public Health 2018, 11, 30–34. [Google Scholar] [CrossRef]
- Chahwakilian, P.; Huttner, B.; Schlemmer, B.; Harbarth, S. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. J. Antimicrob. Chemother. 2011, 66, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Vander Stichele, R.H.; Elseviers, M.M.; Ferech, M.; Blot, S.; Goossens, H. Hospital consumption of antibiotics in 15 European countries: Results of the ESAC retrospective data collection (1997–2002). J. Antimicrob. Chemother. 2006, 58, 159–167. [Google Scholar] [CrossRef]
- Slovic, P. The Perception of Risk; Earthscan Publications: London, UK, 2000. [Google Scholar]
- Flament, C.; Rouquette, M.-L. Anatomie des Idées Ordinaires: Comment Étudier les Représentations Sociales; Armand Colin: Paris, France, 2003. [Google Scholar]
- Cheaito, L.; Azizi, S.; Saleh, N.; Salameh, P. Assessment of self-medication in population buying antibiotics in pharmacies: A pilot study from Beirut and its suburbs. Int. J. Public Health 2013, 59, 319–327. [Google Scholar] [CrossRef]
- Núñez, M.; Tresierra-Ayala, M.; Gil-Olivares, F. Antibiotic self-medication in university students from Trujillo, Peru. Med. Univ. 2016, 18, 205–209. [Google Scholar] [CrossRef]
- Bonten, M.J.M.; Prins, J.M. Antibiotics in pandemic flu. Br. Med. J. 2006, 332, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Shafto, P.; Coley, J.D. Development of Categorization and Reasoning in the Natural World: Novices to Experts, Naive Similarity to Ecological Knowledge. J. Exp. Psychol. Learn. Mem. Cogn. 2003, 29, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovchelovitch, S. Re-thinking the diversity of knowledge: Cognitive polyphasia, belief and representation. Psychol. Soc. 2007, 5, 121–138. [Google Scholar]
| Item | Factor 1 | Factor 2 |
|---|---|---|
| 1 | 0.630 | 0.046 |
| 2 | −0.070 | 0.521 |
| 4 | 0.588 | −0.070 |
| 5 | 0.465 | 0.172 |
| 6 | 0.314 | 0.275 |
| 7 | 0.053 | 0.625 |
| 8 | 0.385 | 0.575 |
| 9 | 0.511 | 0.220 |
| 11 | 0.385 | 0.277 |
| 12 | 0.559 | −0.019 |
| 13 | 0.688 | 0.025 |
| 14 | 0.154 | 0.555 |
| 18 | 0.301 | 0.399 |
| 19 | 0.219 | 0.437 |
| Item | Factor 1 |
|---|---|
| 1 | 0.749 |
| 2 | 0.785 |
| 3 | 0.570 |
| 4 | 0.336 |
| 5 | 0.386 |
| 7 | 0.481 |
| 8 | 0.600 |
| First Hypothesis: Subjects with Inappropriate Practices Would Have a Lower ABR Risk Perception, Concern and More Denial About It | |||
|---|---|---|---|
| Question #4 | Question #5 | Question #6 | |
| Good answer data | D: m = 26.90, SD = 5.17 HC: m = 40.24, SD = 5.03 | HC: m = 40.32, SD = 5.15 RP: m = 48.61, SD = 9.89 | D: m = 27.48, SD = 5.00 HC: m = 41.02, SD = 4.86 RP: m = 50.66, SD = 9.90 |
| Wrong answer data | D: m = 24.94, SD = 4.33 | HC: m = 38.88, SD = 5.27 RP: m = 46.12, SD = 8.84 | D: m = 25.43, SD = 5.00 HC: m = 39.13, SD = 5.31 RP: m = 46. 24, SD = 8.72 |
| Global data | HC: t(269) = −2.834, p = 0.005, d = 0.39, 95% CI [−3.31, −0.60] | HC: t(266) = −2.037, p = 0.043, d = 0.28, 95% CI [−2.82, −0.05] RP: t(281) = −1.939, p = 0.053, d = 0.26, 95% CI [−4.97, −0.04] | D: t(253) = −3.258, p = 0.001, d = 0.41, 95% CI [−3.29, −0.81] HC: t(259) = −2.962, p = 0.003, d = 0.37, 95% CI [−3.14, −0.63] RP: t(269) = −3.914, p < 0.001, d = 0.48, 95% CI [−6.66, −2.20] |
| Third Hypothesis: Students with a High Expertise in Life Science Would Have a Higher ABR Risk Perception, Concern and Lower ABR Risk Denial. | |||
|---|---|---|---|
| Denial about ABR Risk | Concern for Health Consequences | Perception of ABR Risk | |
| Life science students | m = 27.04, SD = 5.06 | m = 40.33, SD = 4.83 | m = 48.87, SD = 9.39 |
| Mathematics students | m = 23.81, SD = 3.86 | m = 37.44, SD = 6.23 | m = 44.11, SD = 9.09 |
| Global data | t(284) = −4.566, p < 0.001, d = 0.67, 95% CI [−4.62, −1.84] | t(288) = −3.811, p < 0.001, d = 0.56, 95% CI [−4.39, −1.40] | t(304) = −3.673, p < 0.001, d = 0.51, 95% CI [−7.31, −2.21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duvauchelle, V.; Causse, E.; Michon, J.; Rateau, P.; Weiss, K.; Meffre, P.; Benfodda, Z. Evaluation of Knowledge and Risk Perception about Antibiotic Resistance in Biology and Mathematics Young Students in Nîmes University in France. Int. J. Environ. Res. Public Health 2021, 18, 9692. https://doi.org/10.3390/ijerph18189692
Duvauchelle V, Causse E, Michon J, Rateau P, Weiss K, Meffre P, Benfodda Z. Evaluation of Knowledge and Risk Perception about Antibiotic Resistance in Biology and Mathematics Young Students in Nîmes University in France. International Journal of Environmental Research and Public Health. 2021; 18(18):9692. https://doi.org/10.3390/ijerph18189692
Chicago/Turabian StyleDuvauchelle, Valentin, Elsa Causse, Julien Michon, Patrick Rateau, Karine Weiss, Patrick Meffre, and Zohra Benfodda. 2021. "Evaluation of Knowledge and Risk Perception about Antibiotic Resistance in Biology and Mathematics Young Students in Nîmes University in France" International Journal of Environmental Research and Public Health 18, no. 18: 9692. https://doi.org/10.3390/ijerph18189692
APA StyleDuvauchelle, V., Causse, E., Michon, J., Rateau, P., Weiss, K., Meffre, P., & Benfodda, Z. (2021). Evaluation of Knowledge and Risk Perception about Antibiotic Resistance in Biology and Mathematics Young Students in Nîmes University in France. International Journal of Environmental Research and Public Health, 18(18), 9692. https://doi.org/10.3390/ijerph18189692










