Effective Removal of Cr(VI) from Wastewater Using Biochar Derived from Walnut Shell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Biomaterial Collection and Biochar Preparation
2.3. Surface Characterization
2.4. Batch Sorption
2.5. pH
2.6. Isotherm Study
2.7. Biosorbent Dose
2.8. Contact Time (Kinetic Study)
2.9. Equilibrium Modeling
3. Results and Discussion
3.1. pH
3.2. Contact Time
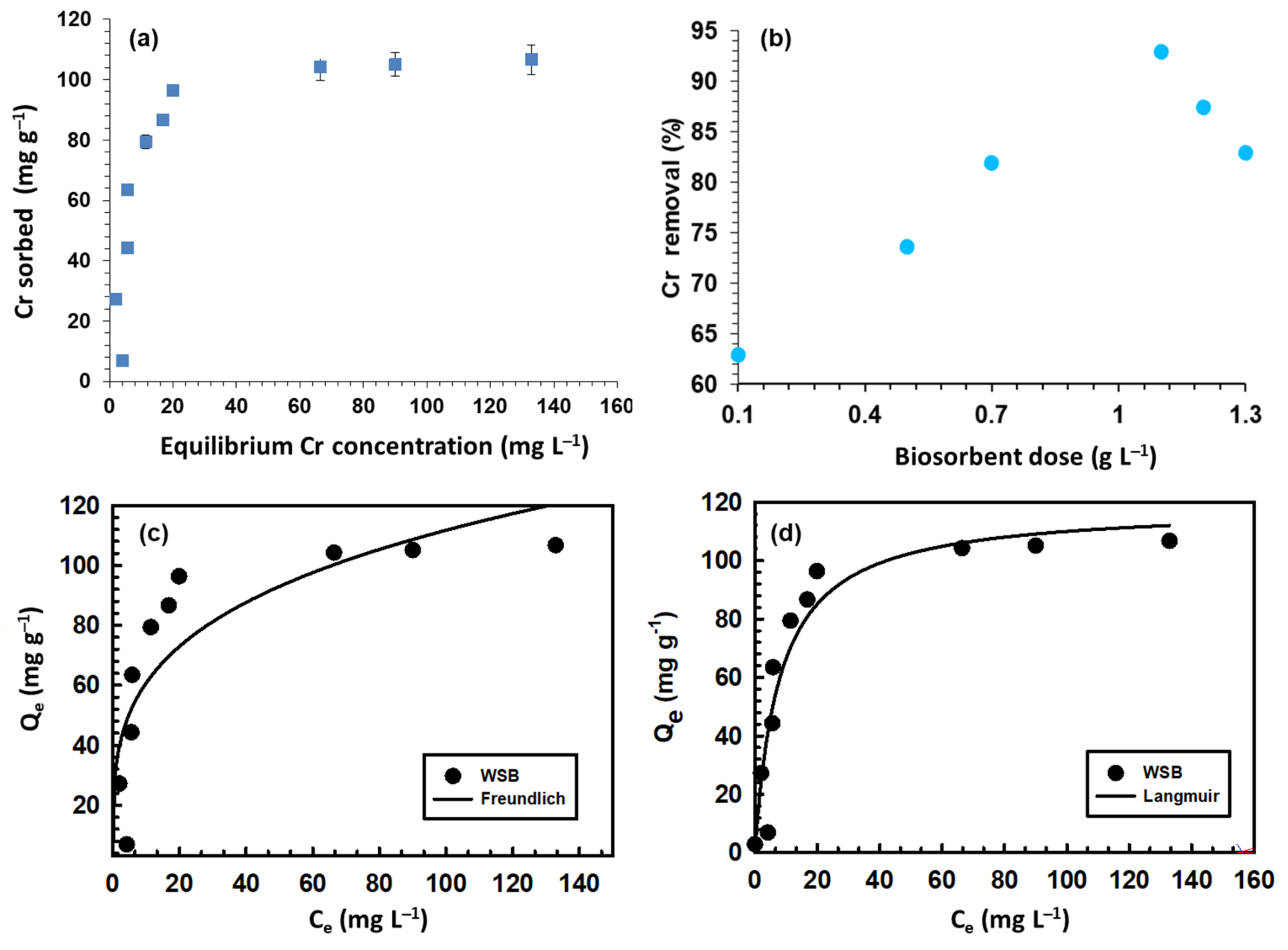
3.3. Iinitial Chromium Concentration
3.4. Effect of the Biochar Dose
3.5. Isotherm Modeling
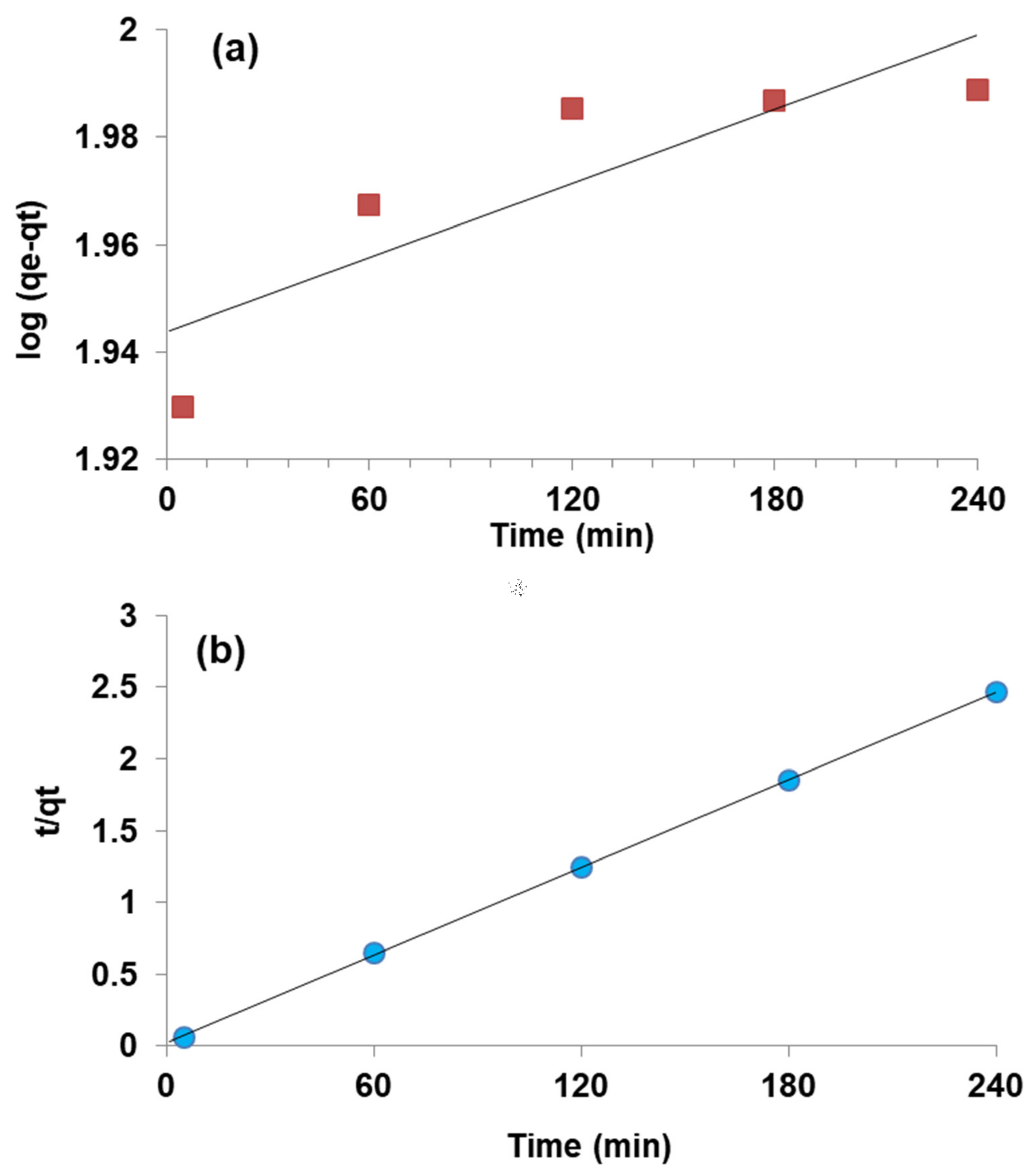
3.6. Kinetic Modeling
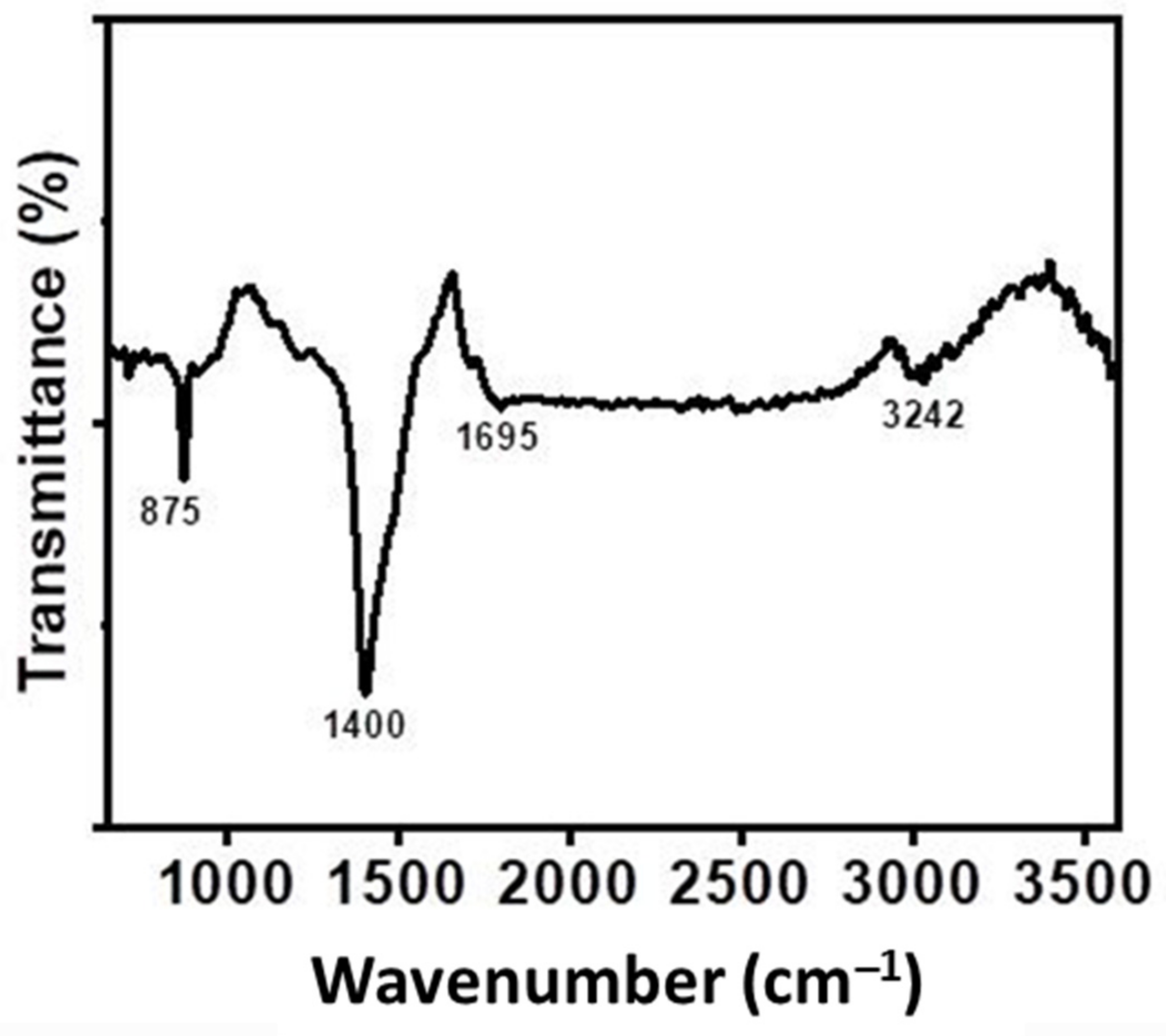
3.7. FTIR
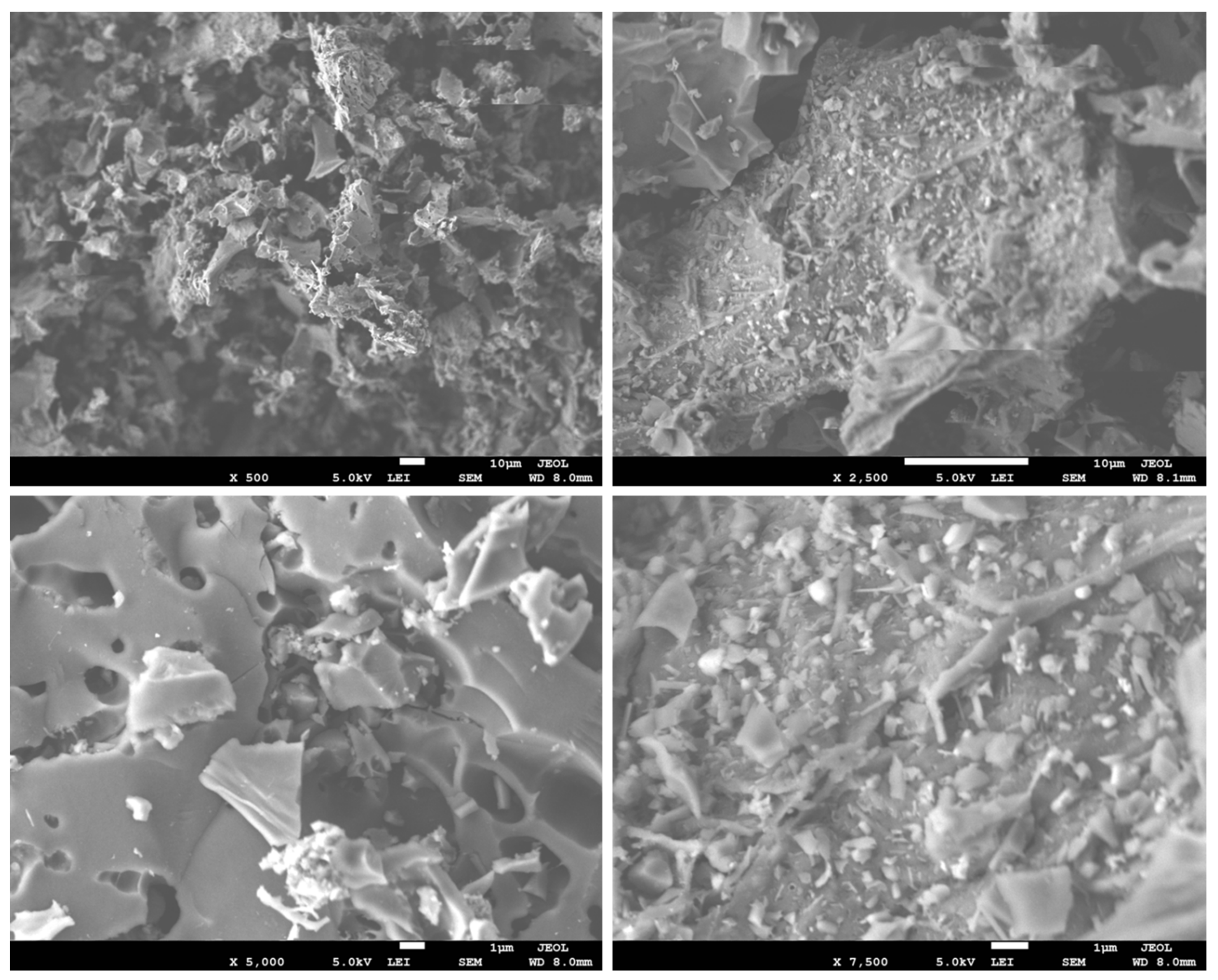
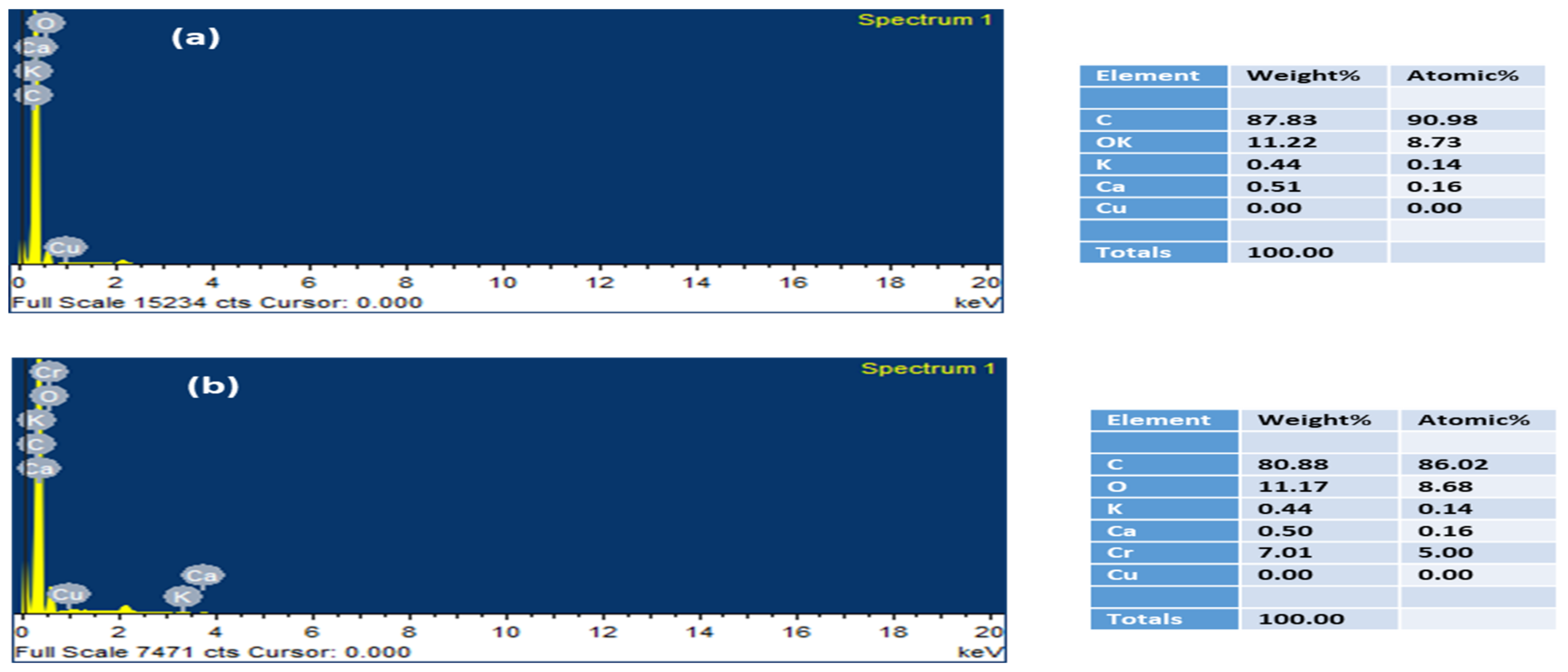
3.8. SEM-EDX
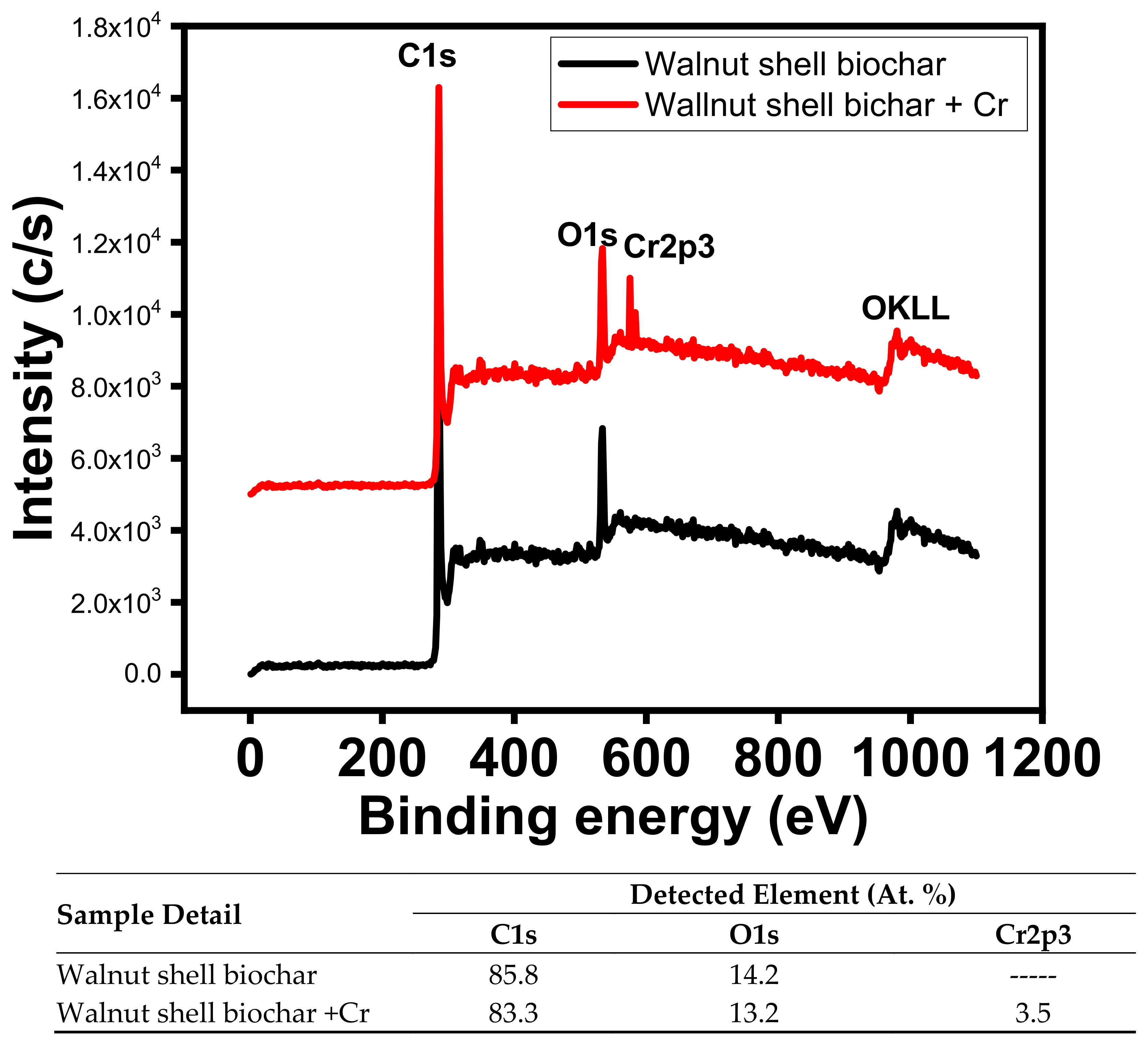
3.9. XPS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, X.T.; Wang, J.; Dong, H.; Chen, J.M.; Ge, Y. Relative importance of soil properties and heavy metals/metalloids to modulate microbial community and activity at a smelting site. J. Soils Sediments 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Cole, P.; Rodu, B. Epidemiologic studies of chrome and cancer mortality: A series of meta-analyses. Regulat. Toxicol. Pharmacol. 2005, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Mu, Y.; Chang, H.; Zhao, X.; Giesy, J.P.; Wu, K.B. Predicting water quality criteria for protecting aquatic life from physicochemical properties of metals or metalloids. Environ. Sci. Technol. 2013, 47, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Guertin, J. Toxicity and Health Effects of Chromium (All Oxidation States) Chromium (VI) Handbook; CRC Press: Boca Raton, FL, USA, 2004; pp. 215–234. [Google Scholar]
- Jiang, N.; Schwarz, W.E.; Li, J. Theoretical studies on hexanuclear oxometalates [M6L19]q−(M = Cr, Mo, W, Sg, Nd, U). electronic structures, oxidation states, aromaticity, and stability. Inorg. Chem. 2015, 54, 7171–7180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, J.; Li, A.; Mei, Y.; Ge, X.; Liu, X.; Xu, Q. Multiple exposure pathways and urinary chromium in residents exposed to chromium. Environ. Int. 2020, 141, 105753. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Iron (Fe+3) oxide/hydroxide nanoparticles-based agglomerates suspension as adsorbent for chromium (Cr+ 6) removal from water and recovery. Sep. Purif. Technol. 2011, 80, 330–337. [Google Scholar] [CrossRef]
- Babangida, S.K.; Sallau, A.B.; Muhammad, A.; Garba, A. Antioxidants in Bioremediation of Chromium (VI) by Conventional and Nanotechnological Approaches: A Review. Toxicol. Environ. Chem. 2021, 103, 162–183. [Google Scholar] [CrossRef]
- Alluri, H.K.; Ronda, S.R.; Settalluri, V.S.; Bondili, J.S.; Suryanarayana, V.; Venkateshwar, P. Biosorption: An eco-friendly alternative for heavy metal removal. Afr. J. Biotechnol. 2007, 6, 2924–2931. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015. [Google Scholar]
- Maremeni, L.C.; Modise, S.J.; Mtunzi, F.M.; Klink, M.J.; Pakade, V.E. Adsorptive removal of hexavalent chromium by diphenylcarbazide-grafted Macadamia nutshell powder. Bioinorg. Chem. Appl. 2018, 2018, 6171906. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; Van der Velde, M.; Diafas, I. Biochar Application to Soils. A Critical Scientific Review of Effects on Soil Properties, Processes, and Functions; EUR, 24099, 162; Office for the Official Publications of the European Communities: Luxembourg, 2010. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, D.M.; Vithanage, M.; Lee, S.S.; Ok, S.Y. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Planning Commission (PC); Planning Commission of Pakistan; Ministry of Planning; Development Reforms. Walnut Cluster Feasibility and Transformation Study. Available online: https://www.pc.gov.pk/uploads/report/Walnut_Cluster_Report.pdf (accessed on 19 August 2021).
- Qayyum, M.F.; Abid, M.; Danish, S.; Saeed, M.K.; Ali, M.A. Effects of various biochars on seed germination and carbon mineralization in an alkaline soil. Pak. J. Agric. Sci. 2015, 51, 977–982. [Google Scholar]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Alyemeni, M.N.; Wijaya, L. Adsorption-reduction performance of tea waste and rice husk biochars for Cr (VI) elimination from wastewater. J. Saudi Chem. Soc. 2020, 24, 799–810. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Razmovski, R.; Šćiban, M. Biosorption of Cr (VI) and Cu (II) by waste tea fungal biomass. Ecol. Eng. 2008, 34, 179–186. [Google Scholar] [CrossRef]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M. Tea waste as a potential biowaste for removal of hexavalent chromium from wastewater: Equilibrium and kinetic studies. Arab. J. Geosci. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A. Advanced biosorbents materials for removal of chromium from water and wastewaters. Environ. Prog. Sustain. Energy 2011, 30, 284–293. [Google Scholar] [CrossRef]
- Ghorbani-Khosrowshahi, S.; Behnajady, M.A. Chromium (VI) adsorption from aqueous solution by prepared biochar from Onopordom Heteracanthom. Int. J. Environ. Sci. Technol. 2016, 13, 1803–1814. [Google Scholar] [CrossRef] [Green Version]
- Kamsonlian, S.; Suresh, S.; Ramanaiah, V.; Majumder, C.; Chand, S.; Kumar, A. Biosorptive behaviour of mango leaf powder and rice husk for arsenic (III) from aqueous solutions. Int. J. Environ. Sci. Technol. 2012, 9, 565–578. [Google Scholar] [CrossRef] [Green Version]
- Sattar, M.S.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Niazi, N.K.; Jilani, A. Comparative efficiency of peanut shell and peanut shell biochar for removal of arsenic from water. Environ. Sci. Pollut. Res. 2019, 26, 18624–18635. [Google Scholar] [CrossRef]
- Kahraman, H.T.; Pehlivan, E. Cr6+ removal using oleaster (Elaeagnus) seed and cherry (Prunus avium) stone biochar. Powder Technol. 2017, 306, 61–67. [Google Scholar] [CrossRef]
- Pouya, M.R.; Behnam, S. Adsorption behavior of copper ions on alga Jania adhaerens through SEM and FTIR analyses. Sep. Sci. Technol. 2017, 52, 2062–2068. [Google Scholar] [CrossRef]
- Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and removal of chromium VI in water by powdered activated carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, M.; Rozada, F.; Moran, A.; Calvo, L.F.; Garcıa, A.I. Removal of heavy metals from aqueous solution by sewage sludge based sorbents: Competitive effects. Desalination 2009, 239, 46–57. [Google Scholar] [CrossRef]
- El-Shafey, E.I.; Cox, M.; Pichugin, A.A.; Appleton, Q. Application of a carbon sorbent for the removal of cadmium and other heavy metal ions from aqueous solution. J. Chem. Technol. Biotechnol. 2002, 77, 429–436. [Google Scholar] [CrossRef]
- Wang, C.; Gu, L.; Liu, X.; Zhang, X.; Cao, L.; Hu, X. Sorption behavior of Cr (VI) on pineapple-peel-derived biochar and the influence of coexisting pyrene. Int. Biodeterior. Biodegrad. 2016, 111, 78–84. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; Ismail, I.M.; Abdelmonem, N.M.; El Nemr, A.; Ragab, S. Amination of biochar surface from watermelon peel for toxic chromium removal enhancement. Chin. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]

| Isotherm Models | Parameters | |
|---|---|---|
| Langmuir | QL | 15.02 (mg g−1) |
| R2 | 0.89 | |
| KL | 0.12 (L g−1) | |
| Freundlich | QF | 32.98 (mg1−n g−1 Ln) |
| R2 | 0.78 | |
| n | 0.26 |
| PFO | PSO | ||||
|---|---|---|---|---|---|
| qe (mg g−1) | k1 (min−1) | R2 | qe (mg g−1) | k2 (g mg−1 min−1) | R2 |
| 87 | 0.00002 | 0.75 | 100 | 0.01 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokab, T.; Ashraf, H.S.; Shakoor, M.B.; Jilani, A.; Ahmad, S.R.; Majid, M.; Ali, S.; Farid, N.; Alghamdi, R.A.; Al-Quwaie, D.A.H.; et al. Effective Removal of Cr(VI) from Wastewater Using Biochar Derived from Walnut Shell. Int. J. Environ. Res. Public Health 2021, 18, 9670. https://doi.org/10.3390/ijerph18189670
Kokab T, Ashraf HS, Shakoor MB, Jilani A, Ahmad SR, Majid M, Ali S, Farid N, Alghamdi RA, Al-Quwaie DAH, et al. Effective Removal of Cr(VI) from Wastewater Using Biochar Derived from Walnut Shell. International Journal of Environmental Research and Public Health. 2021; 18(18):9670. https://doi.org/10.3390/ijerph18189670
Chicago/Turabian StyleKokab, Tanzeela, Hafiza Sumbal Ashraf, Muhammad Bilal Shakoor, Asim Jilani, Sajid Rashid Ahmad, Muzaffar Majid, Shafaqat Ali, Nazar Farid, Rana A. Alghamdi, Diana A. H. Al-Quwaie, and et al. 2021. "Effective Removal of Cr(VI) from Wastewater Using Biochar Derived from Walnut Shell" International Journal of Environmental Research and Public Health 18, no. 18: 9670. https://doi.org/10.3390/ijerph18189670
APA StyleKokab, T., Ashraf, H. S., Shakoor, M. B., Jilani, A., Ahmad, S. R., Majid, M., Ali, S., Farid, N., Alghamdi, R. A., Al-Quwaie, D. A. H., & Hakeem, K. R. (2021). Effective Removal of Cr(VI) from Wastewater Using Biochar Derived from Walnut Shell. International Journal of Environmental Research and Public Health, 18(18), 9670. https://doi.org/10.3390/ijerph18189670








