Modelling Spatiotemporal Patterns of Lyme Disease Emergence in Québec
Abstract
1. Introduction
2. Materials and Methods
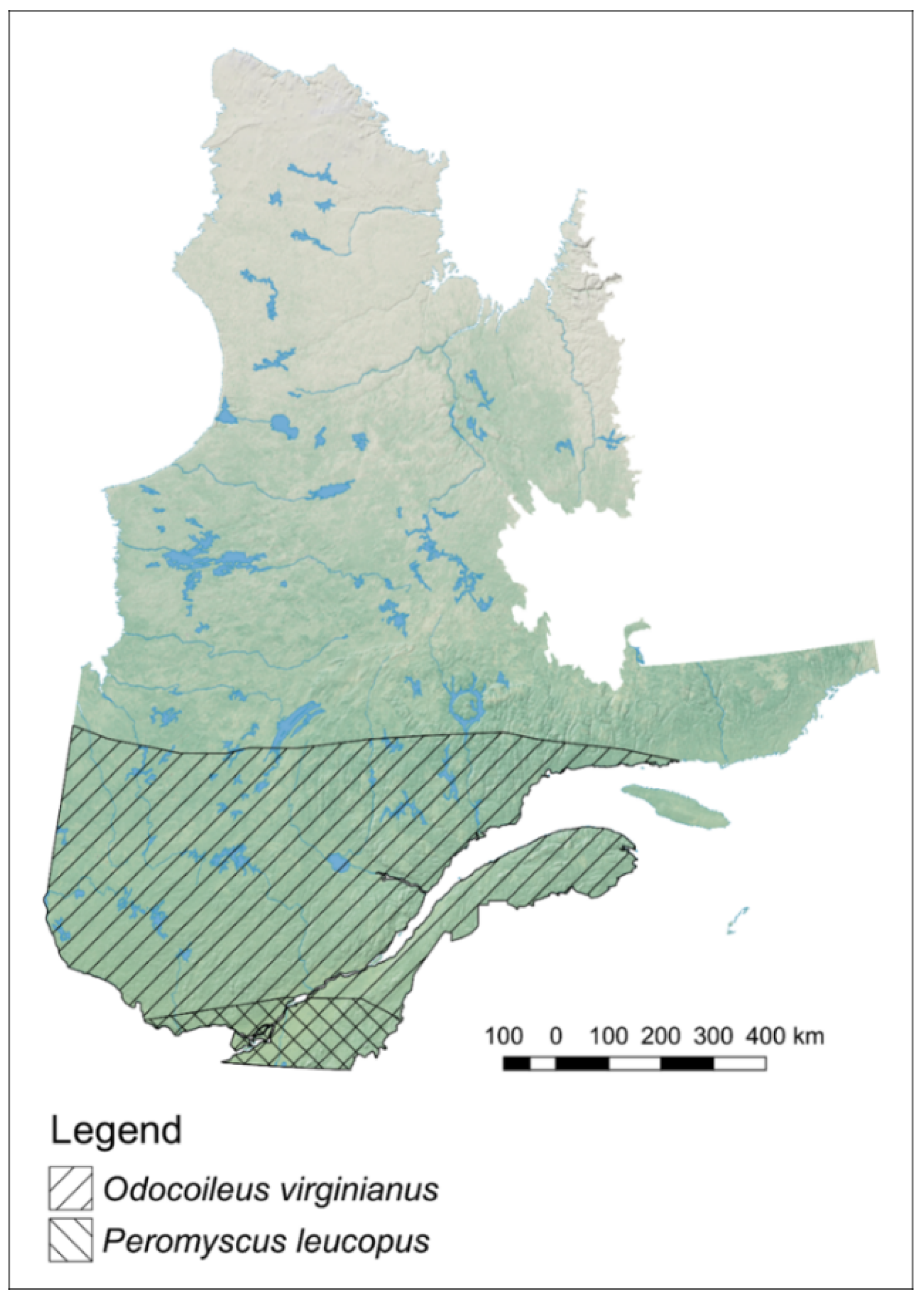
2.1. Data
2.2. Surface Trend Analysis
2.3. Cluster Analysis
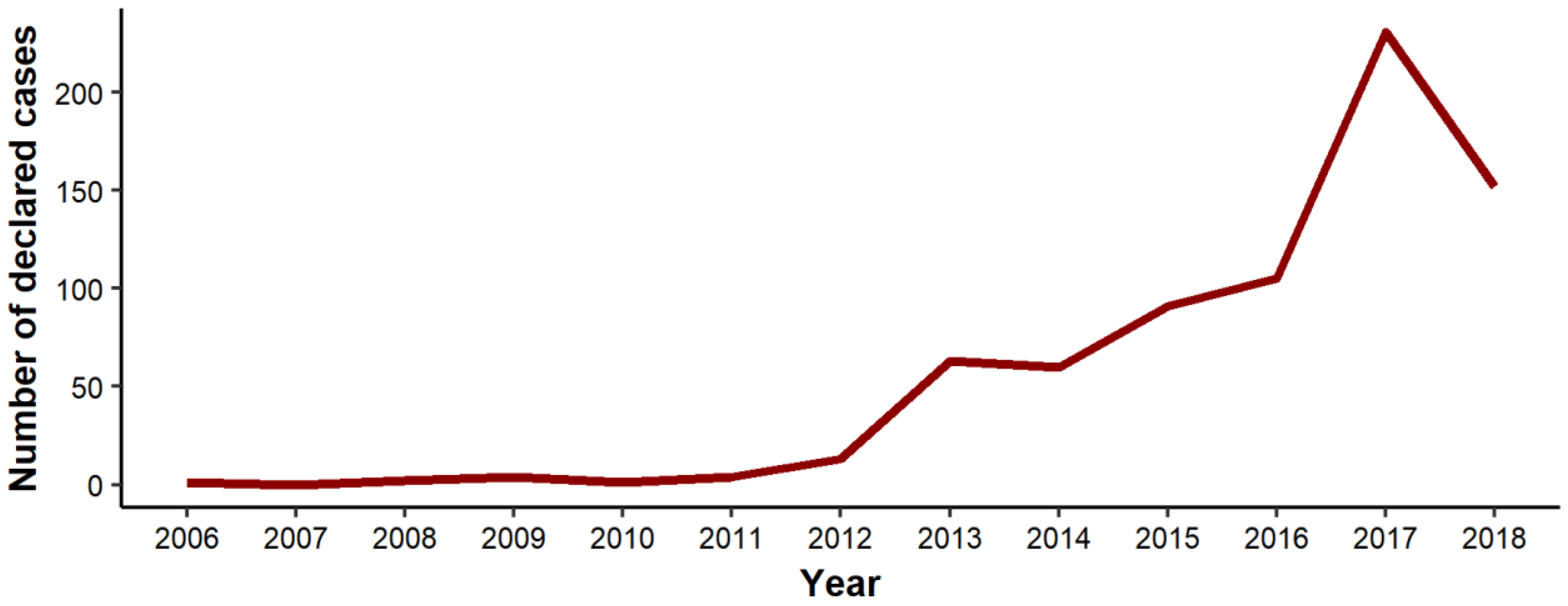
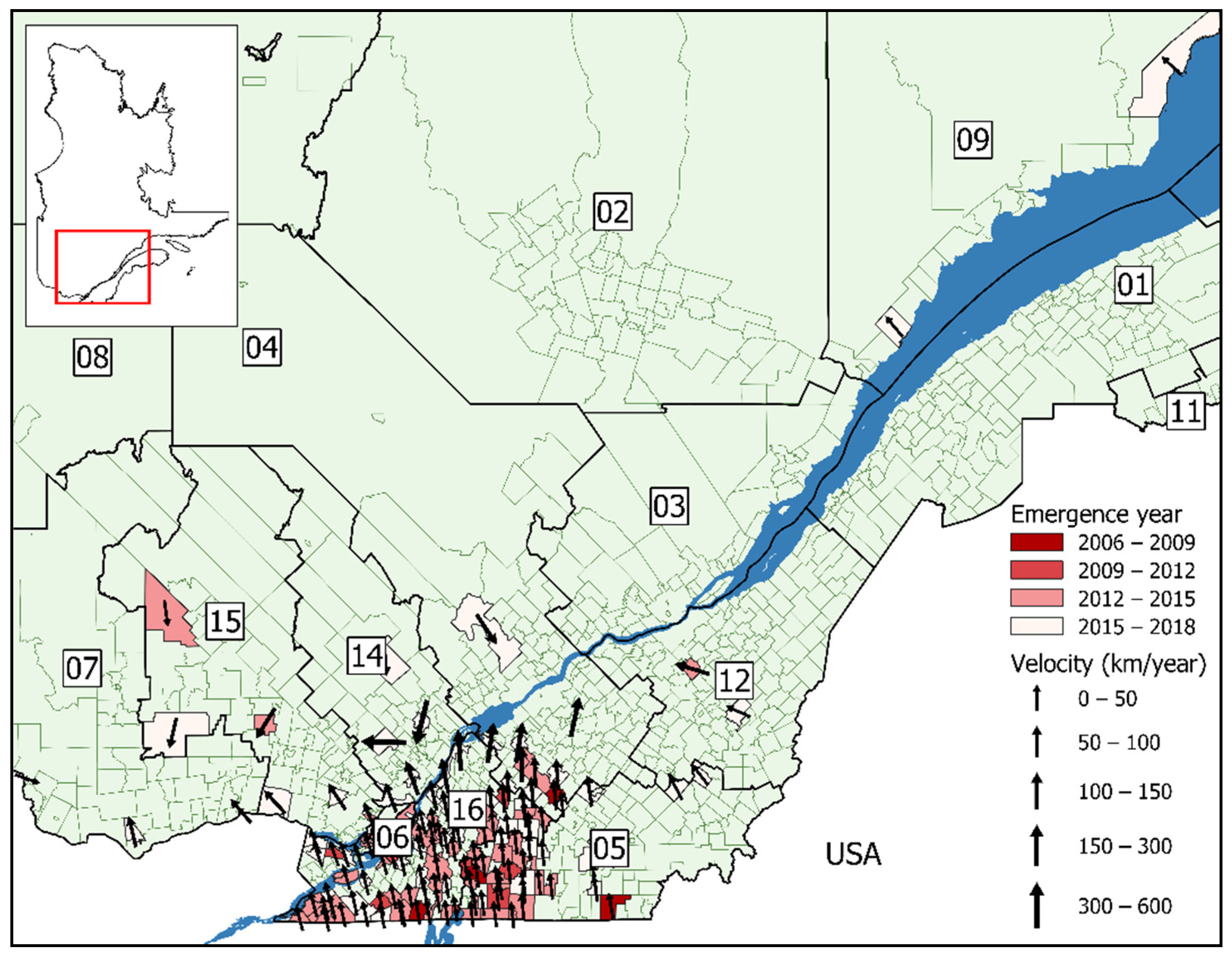
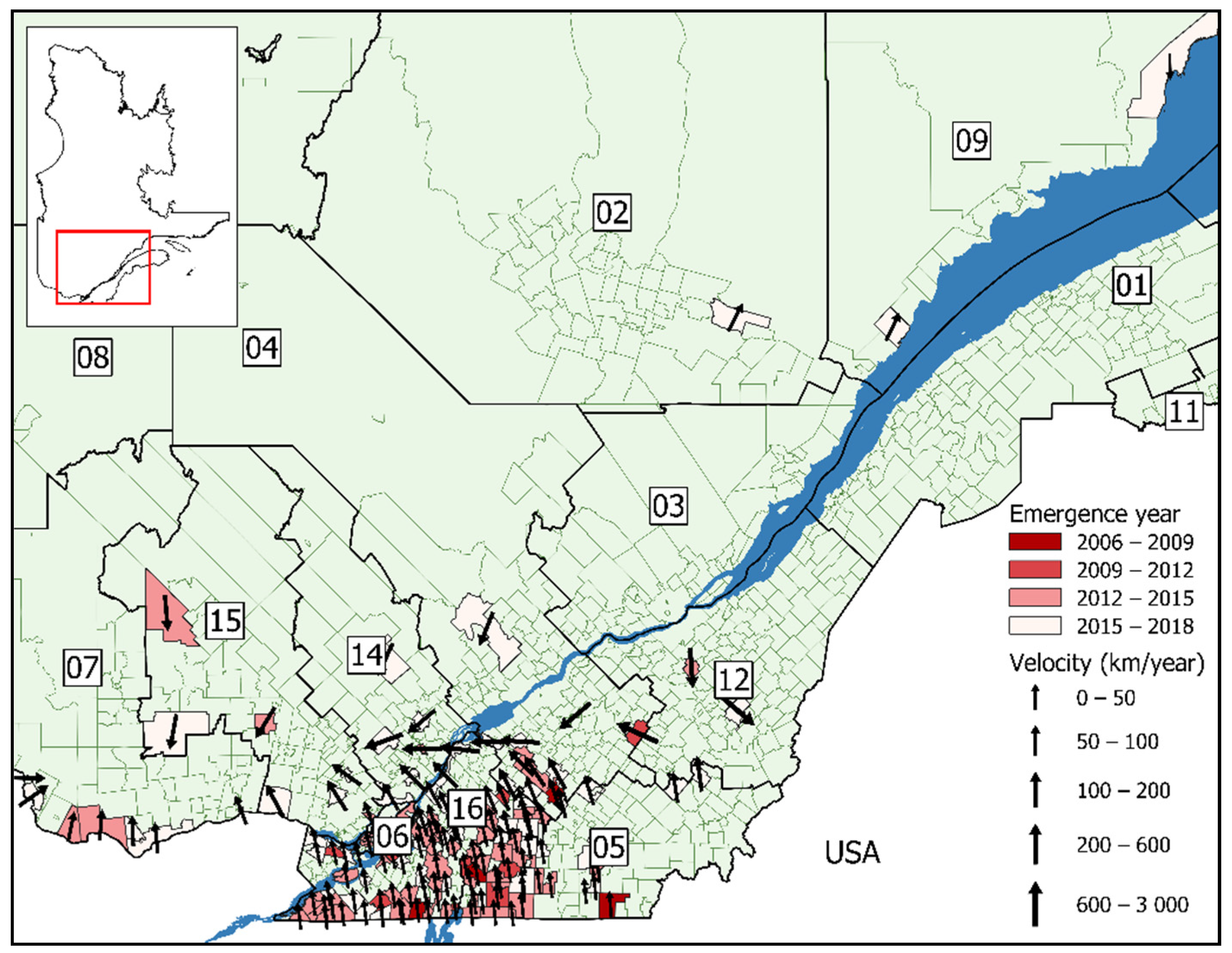
3. Results
3.1. Surface Trend Analysis
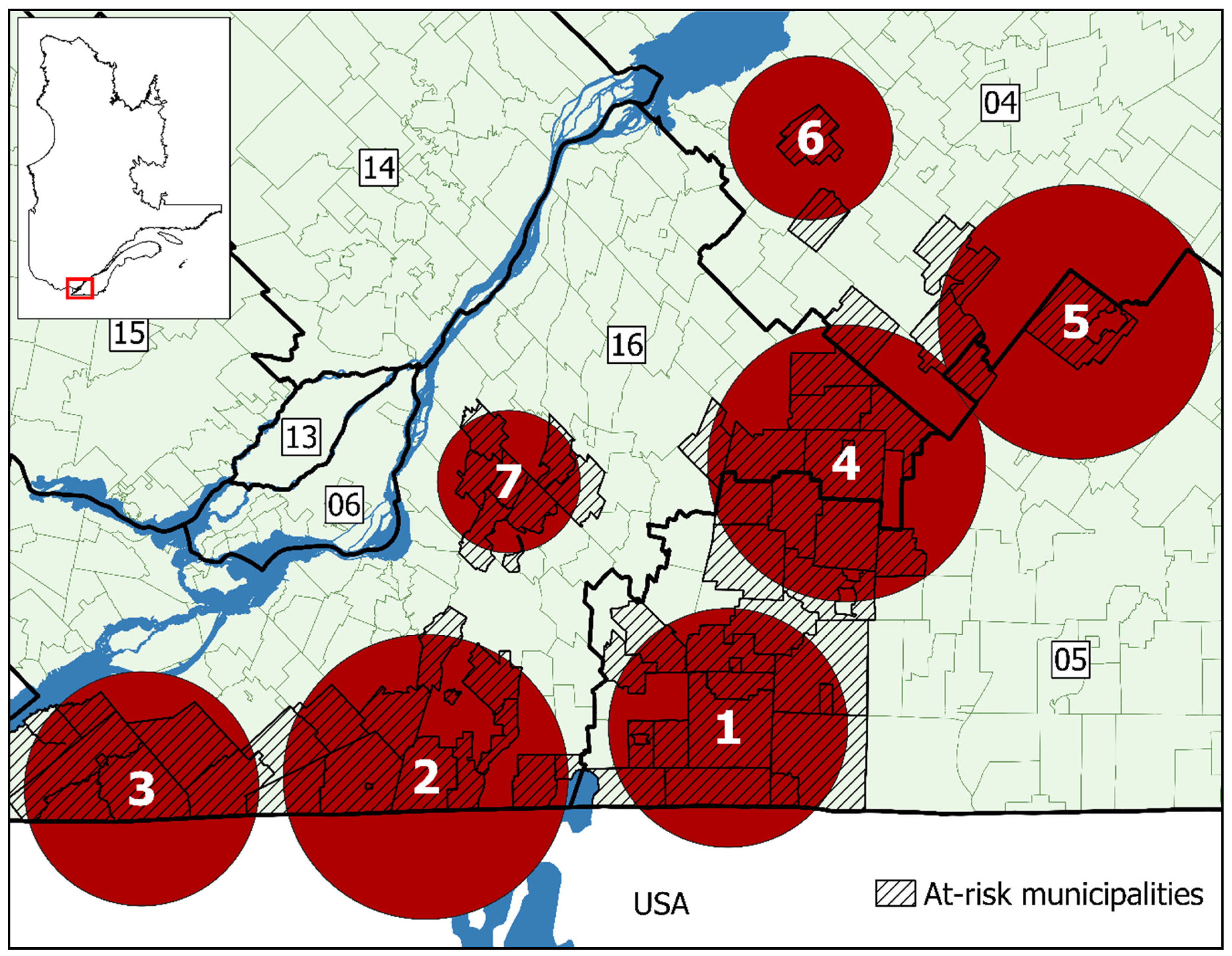
3.2. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- DPH. A Brief History of Lyme Disease in Connecticut. Available online: https://portal.ct.gov/DPH/Epidemiology-and-Emerging-Infections/A-Brief-History-of-Lyme-Disease-in-Connecticut (accessed on 22 January 2018).
- Kilpatrick, A.M.; Dobson, A.D.; Levi, T.; Salkeld, D.J.; Swei, A.; Ginsberg, H.S.; Kjemtrup, A.; Padgett, K.A.; Jensen, P.M.; Fish, D. Lyme disease ecology in a changing world: Consensus, uncertainty and critical gaps for improving control. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160117. [Google Scholar] [CrossRef]
- Stone, B.L.; Tourand, Y.; Brissette, C.A. Brave new worlds: The expanding universe of Lyme disease. Vector-Borne Zoonotic Dis. 2017, 17, 619–629. [Google Scholar] [CrossRef]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef]
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the frequency of lyme disease diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 616. [Google Scholar] [CrossRef]
- CDC. Recent Surveillance Data. Available online: https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html (accessed on 2 February 2019).
- Gouvernement du Canada. Surveillance de la Maladie de Lyme. Available online: https://www.canada.ca/fr/sante-publique/services/maladies/maladie-lyme/surveillance-maladie-lyme.html (accessed on 11 October 2017).
- Gouvernement du Canada. Pour les Professionnels de la Santé: Maladie de Lyme. Available online: https://www.canada.ca/fr/sante-publique/services/maladies/maladie-lyme/pour-professionnels-sante-maladie-lyme.html (accessed on 23 November 2018).
- Ogden, N.; Koffi, J.; Lindsay, L.; Fleming, S.; Mombourquette, D.; Sanford, C.; Badcock, J.; Gad, R.; Jain-Sheehan, N.; Moore, S. Vector-borne diseases in Canada: Surveillance for Lyme disease in Canada, 2009 to 2012. Can. Commun. Dis. Rep. 2015, 41, 132. [Google Scholar] [CrossRef]
- Gouvernement du Canada. Maladie de Lyme. Available online: https://www.canada.ca/fr/sante-publique/services/maladies/maladie-lyme.html (accessed on 22 January 2018).
- Ścieszka, J.; Dąbek, J.; Cieślik, P. Post-Lyme disease syndrome. Reumatologia 2015, 53, 46. [Google Scholar] [CrossRef] [PubMed]
- Feder, H.M., Jr.; Johnson, B.J.; O’Connell, S.; Shapiro, E.D.; Steere, A.C.; Wormser, G.P.; Ad Hoc International Lyme Disease Group. A critical appraisal of “chronic Lyme disease”. N. Engl. J. Med. 2007, 357, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S. Epidemiology of Lyme disease. Infect. Dis. Clin. 2015, 29, 187–210. [Google Scholar] [CrossRef]
- Steere, A.C.; Coburn, J.; Glickstein, L. The emergence of Lyme disease. J. Clin. Investig. 2004, 113, 1093–1101. [Google Scholar] [CrossRef]
- Wood, C.L.; Lafferty, K.D. Biodiversity and disease: A synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol. Evol. 2013, 28, 239–247. [Google Scholar] [CrossRef]
- Leighton, P.A.; Koffi, J.K.; Pelcat, Y.; Lindsay, L.R.; Ogden, N.H. Predicting the speed of tick invasion: An empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 2012, 49, 457–464. [Google Scholar] [CrossRef]
- IUCN. Peromyscus leucopus. Available online: https://www.iucnredlist.org/species/16669/115136270 (accessed on 14 July 2019).
- IUCN. Odocoileus virginianus. Available online: https://www.iucnredlist.org/species/42394/22162580 (accessed on 14 July 2019).
- Eisen, R.J.; Eisen, L.; Ogden, N.H.; Beard, C.B. Linkages of Weather and Climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), Enzootic Transmission of Borrelia burgdorferi, and Lyme Disease in North America. J. Med. Entomol. 2016, 53, 250–261. [Google Scholar] [CrossRef]
- MDDELCC. Tendances des Températures 1961–2010. Available online: http://www.mddelcc.gouv.qc.ca/climat/tendances/ (accessed on 21 March 2018).
- Gouvernement du Canada. Normales Climatiques Canadiennes. Available online: http://climat.meteo.gc.ca/climate_normals/index_f.html (accessed on 21 March 2018).
- MDDELCC. Faits Saillants. Available online: http://www.environnement.gouv.qc.ca/climat/Faits-saillants/index.htm (accessed on 14 July 2019).
- Soucy-Gonthier, N.; Marceau, D.; Delage, M.; Cogliastro, A.; Domon, G.; Bouchard, A. Détection de L’Évolution des Superficies Forestières en Montérégie Entre Juin 1999 et Août 2002 à Partir D’Images Satellitaires Landsat-TM. Available online: https://www.irbv.umontreal.ca/wp-content/uploads/cogliastro-deboisement2003.pdf (accessed on 21 March 2018).
- Cormier, C.; Côté, S.; Mercure, M.; Cerruti, A.; Minelli, F. Cadre méthodologique pour restaurer la connectivité écologique, de la planification à la conservation: Étude de cas en Montérégie. Le Nat. Can. 2012, 136, 95–100. [Google Scholar]
- Cogliastro, A.; D’Orangeville, L. Organiser la Cohabitation Agriculture-Forêt en Montérégie. Available online: https://www.irbv.umontreal.ca/wp-content/uploads/cogliastro-caaaq.pdf (accessed on 21 March 2018).
- Simon, J.A.; Marrotte, R.R.; Desrosiers, N.; Fiset, J.; Gaitan, J.; Gonzalez, A.; Koffi, J.K.; Lapointe, F.J.; Leighton, P.A.; Lindsay, L.R.; et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol. Appl. 2014, 7, 750–764. [Google Scholar] [CrossRef]
- Allan, B.F.; Keesing, F.; Ostfeld, R.S. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 2003, 17, 267–272. [Google Scholar] [CrossRef]
- Rogic, A.; Tessier, N.; Legendre, P.; Lapointe, F.J.; Millien, V. Genetic structure of the white-footed mouse in the context of the emergence of Lyme disease in southern Quebec. Ecol. Evol. 2013, 3, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Clow, K.M.; Ogden, N.H.; Lindsay, L.R.; Michel, P.; Pearl, D.L.; Jardine, C.M. The influence of abiotic and biotic factors on the invasion of Ixodes scapularis in Ontario, Canada. Ticks Tick-Borne Dis. 2017, 8, 554–563. [Google Scholar] [CrossRef]
- Fiset, J.; Tessier, N.; Millien, V.; Lapointe, F.J. Phylogeographic Structure of the White-Footed Mouse and the Deer Mouse, Two Lyme Disease Reservoir Hosts in Quebec. PLoS ONE 2015, 10, e0144112. [Google Scholar] [CrossRef] [PubMed]
- Marrotte, R.R.; Gonzalez, A.; Millien, V. Functional connectivity of the white-footed mouse in Southern Quebec, Canada. Landsc. Ecol. 2017, 32, 1987–1998. [Google Scholar] [CrossRef]
- Richard, S.; Oppliger, A. Zoonotic occupational diseases in forestry workers—Lyme borreliosis, tularemia and leptospirosis in Europe. Ann. Agric. Environ. Med. 2015, 22, 43–50. [Google Scholar] [CrossRef]
- Smith, G.; Wileyto, E.P.; Hopkins, R.B.; Cherry, B.R.; Maher, J.P. Risk factors for lyme disease in Chester County, Pennsylvania. Public Health Rep. 2001, 116 (Suppl. 1), 146–156. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Morshed, M.; Sockett, P.N.; Artsob, H. The emergence of Lyme disease in Canada. Can. Med. Assoc. J. 2009, 180, 1221–1224. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, D.M.; Woodstock, K.; Ogden, N.H.; Wu, X.T.; Wu, J.H. Analyzing the Potential Risk of Climate Change on Lyme Disease in Eastern Ontario, Canada Using Time Series Remotely Sensed Temperature Data and Tick Population Modelling. Remote Sens. 2017, 9, 609. [Google Scholar] [CrossRef]
- Koffi, J.K.; Leighton, P.A.; Pelcat, Y.; Trudel, L.; Lindsay, L.R.; Milord, F.; Ogden, N.H. Passive Surveillance for I. scapularis Ticks: Enhanced Analysis for Early Detection of Emerging Lyme Disease Risk. J. Med Entomol. 2012, 49, 400–409. [Google Scholar] [CrossRef]
- Ogden, N.H.; Mechai, S.; Margos, G. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Front. Cell. Infect. Microbiol. 2013, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bouchard, C.; Kurtenbach, K.; Margos, G.; Lindsay, L.R.; Trudel, L.; Nguon, S.; Milord, F. Active and Passive Surveillance and Phylogenetic Analysis of Borrelia burgdorferi Elucidate the Process of Lyme Disease Risk Emergence in Canada. Environ. Health Perspect. 2010, 118, 909–914. [Google Scholar] [CrossRef]
- MSSS. Maladie de Lyme. Available online: http://www.msss.gouv.qc.ca/professionnels/zoonoses/maladie-lyme/surveillance-de-la-maladie/ (accessed on 12 June 2019).
- Statistique Québec. Population et Structure Par Âge et Sexe. Available online: http://www.stat.gouv.qc.ca/statistiques/population-demographie/structure/index.html (accessed on 25 March 2019).
- Statistique Canada. 2011 Census—Boundary Files. Available online: https://www12.statcan.gc.ca/census-recensement/2011/geo/bound-limit/bound-limit-2011-eng.cfm (accessed on 29 March 2019).
- Morrison, K. R Package: Outbreakvelocity. Available online: https://github.com/kathryntmorrison/outbreakvelocity (accessed on 18 December 2018).
- Zinszer, K.; Morrison, K.; Anema, A.; Majumder, M.S.; Brownstein, J.S. The velocity of Ebola spread in parts of West Africa. Lancet Infect. Dis. 2015, 15, 1005–1007. [Google Scholar] [CrossRef]
- Zinszer, K.; Morrison, K.; Brownstein, J.S.; Marinho, F.; Santos, A.F.; Nsoesie, E.O. Reconstruction of Zika virus introduction in Brazil. Emerg. Infect. Dis. 2017, 23, 91. [Google Scholar] [CrossRef]
- INSPQ. La Maladie de Lyme et Les Maladies Transmises Par Les Tiques. Available online: https://www.inspq.qc.ca/zoonoses/maladie-de-lyme (accessed on 26 June 2019).
- Kulldorff, M. SaTScan User Guide for Version 9.6. Available online: https://www.satscan.org/ (accessed on 14 December 2018).
- Lantos, P.M.; Nigrovic, L.E.; Auwaerter, P.G.; Fowler, V.G., Jr.; Ruffin, F.; Brinkerhoff, R.J.; Reber, J.; Williams, C.; Broyhill, J.; Pan, W.K. Geographic expansion of Lyme disease in the southeastern United States, 2000–2014. Open Forum Infect. Dis. 2015, 3, ofv143. [Google Scholar] [CrossRef]
- Ogden, N.H.; Barker, I.K.; Beauchamp, G.; Brazeau, S.; Charron, D.F.; Maarouf, A.; Morshed, M.G.; O’Callaghan, C.J.; Thompson, R.A.; Waltner-Toews, D.; et al. Investigation of ground level and remote-sensed data for habitat classification and prediction of survival of Ixodes scapularis in habitats of southeastern Canada. J. Med. Entomol. 2006, 43, 403–414. [Google Scholar] [CrossRef][Green Version]
- Roy-Dufresne, E.; Logan, T.; Simon, J.A.; Chmura, G.L.; Millien, V. Poleward Expansion of the White-Footed Mouse (Peromyscus leucopus) under Climate Change: Implications for the Spread of Lyme Disease. PLoS ONE 2013, 8, e80724. [Google Scholar] [CrossRef]
- Kulkarni, M.A.; Narula, I.; Slatculescu, A.M.; Russell, C. Lyme Disease Emergence after Invasion of the Blacklegged Tick, Ixodes scapularis, Ontario, Canada, 2010–2016. Emerg. Infect. Dis. 2019, 25, 328. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.; Koffi, J.; Pelcat, Y.; Lindsay, L. Lyme disease: Environmental risk from Lyme disease in central and eastern Canada: A summary of recent surveillance information. Can. Commun. Dis. Rep. 2014, 40, 74. [Google Scholar] [CrossRef]
- Bouchard, C.; Leonard, E.; Koffi, J.K.; Pelcat, Y.; Peregrine, A.; Chilton, N.; Rochon, K.; Lysyk, T.; Lindsay, L.R.; Ogden, N.H. The increasing risk of Lyme disease in Canada. Can. Vet. J. 2015, 56, 693. [Google Scholar]
- Ogden, N.; Bouchard, C.; Badcock, J.; Drebot, M.; Elias, S.; Hatchette, T.; Koffi, J.; Leighton, P.; Lindsay, L.; Lubelczyk, C. What is the real number of Lyme disease cases in Canada? BMC Public Health 2019, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Leo, S.S.T.; Gonzalez, A.; Millien, V. The Genetic Signature of Range Expansion in a Disease Vector—The Black-Legged Tick. J. Hered. 2016, 108, 176–183. [Google Scholar] [CrossRef]
- Ogden, N.; Barker, I.; Francis, C.; Heagy, A.; Lindsay, L.; Hobson, K. How far north are migrant birds transporting the tick Ixodes scapularis in Canada? Insights from stable hydrogen isotope analyses of feathers. Ticks Tick-Borne Dis. 2015, 6, 715–720. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of climate change on Lyme disease risk in North America. EcoHealth 2005, 2, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, A.J.; Moore, S.M.; Sampson, K.M.; Beard, C.B.; Eisen, R.J. Climate change influences on the annual onset of Lyme disease in the United States. Ticks Tick-Borne Dis. 2015, 6, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Subak, S. Effects of climate on variability in Lyme disease incidence in the northeastern United States. Am. J. Epidemiol. 2003, 157, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Fischhoff, I.R.; Keesing, F.; Ostfeld, R.S. Risk Factors for Bites and Diseases Associated With Black-Legged Ticks: A Meta-Analysis. Am. J. Epidemiol. 2019, 188, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Sajanti, E.; Virtanen, M.; Helve, O.; Kuusi, M.; Lyytikäinen, O.; Hytönen, J.; Sane, J. Lyme borreliosis in Finland, 1995–2014. Emerg. Infect. Dis. 2017, 23, 1282. [Google Scholar] [CrossRef] [PubMed]
- Institut de la Statistique du Québec. Le Québec Chiffres en Main. Available online: http://www.stat.gouv.qc.ca/quebec-chiffre-main/pdf/qcm2018_fr.pdf (accessed on 2 June 2019).
- CIRANO. Répartition de la Population Active Selon le Secteur D’activité et le Sexe, Québec. 2017. Available online: https://qe.cirano.qc.ca/theme/marche-travail/population-active/tableau-repartition-population-active-selon-secteur-dactivite-sexe-quebec-2017 (accessed on 2 June 2019).
- Chaire de Tourisme Transat ESG UQAM. Étude des Clientèles, des Lieux de Pratique et Des Retombées Économiques et Sociales des Activités Physiques de Plein Air. Available online: https://chairedetourisme.uqam.ca/upload/files/%C3%89tude_Plein_air_rapport_final.pdf (accessed on 5 July 2019).
- Bron, G.M.; Lee, X.; Paskewitz, S.M. Do-It-Yourself Tick Control: Granular Gamma-Cyhalothrin Reduces Ixodes scapularis (Acari: Ixodidae) Nymphs in Residential Backyards. J. Med. Entomol. 2021, 58, 749–755. [Google Scholar] [CrossRef]
- Crowder, L.A.; Yedlin, V.A.; Weinstein, E.R.; Kortte, K.B.; Aucott, J.N. Lyme disease and post-treatment Lyme disease syndrome: The neglected disease in our own backyard. Public Health 2014, 128, 784–791. [Google Scholar] [CrossRef]
- Mead, P.; Hook, S.; Niesobecki, S.; Ray, J.; Meek, J.; Delorey, M.; Prue, C.; Hinckley, A. Risk factors for tick exposure in suburban settings in the Northeastern United States. Ticks Tick-Borne Dis. 2018, 9, 319–324. [Google Scholar] [CrossRef]
- Falco, R.C.; Fish, D.; Piesman, J. Duration of tick bites in a Lyme disease-endemic area. Am. J. Epidemiol. 1996, 143, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Ogden, N.H.; Leighton, P.A.; Lindsay, L.R.; Thivierge, K. Analysis of the human population bitten by Ixodes scapularis ticks in Quebec, Canada: Increasing risk of Lyme disease. Ticks Tick-Borne Dis. 2016, 7, 1075–1081. [Google Scholar] [CrossRef]
- Ogden, N.H.; Gasmi, S.; Koffi, J.K.; Barton, M.; Lindsay, L.R.; Langley, J.M. Lyme disease in children: Data from the Canadian Paediatric Surveillance Program. Ticks Tick-Borne Dis. 2020, 11, 101347. [Google Scholar] [CrossRef] [PubMed]
- Aenishaenslin, C.; Bouchard, C.; Koffi, J.K.; Pelcat, Y.; Ogden, N.H. Evidence of rapid changes in Lyme disease awareness in Canada. Ticks Tick-Borne Dis. 2016, 7, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.O.; Nelder, M.P.; Russell, C.; Li, Y.; Badiani, T.; Sander, B.; Sider, D.; Patel, S.N. Clinical manifestations of reported Lyme disease cases in Ontario, Canada: 2005–2014. PLoS ONE 2018, 13, e0198509. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Ogden, N.H.; Leighton, P.A.; Adam-Poupart, A.; Milord, F.; Lindsay, L.R.; Barkati, S.; Thivierge, K. Practices of Lyme disease diagnosis and treatment by general practitioners in Quebec, 2008–2015. BMC Fam. Pract. 2017, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- INSPQ. Plan D’analyse de Surveillance Intégrée de la Maladie de Lyme. Available online: https://www.inspq.qc.ca/sites/default/files/publications/2257_plan_analyse_surveillance_lyme.pdf (accessed on 2 April 2018).
- Lloyd, V.K.; Hawkins, R.G. Under-Detection of Lyme Disease in Canada. Healthcare 2018, 6, 125. [Google Scholar] [CrossRef] [PubMed]

| Case Definition | |||
|---|---|---|---|
| Scenarios | Date of Symptom Onset | Date of Notification | |
| 1 | First case for each municipality between 2006–2018 | A1 | B1 |
| 2 | Municipalities with at least two cases between 2013–2018 | A2 | B2 |
| 3 | Municipalities with at least two cases between 2006–2018 | A3 | B3 |
| All Years | 2006–2012 | 2013–2018 | |
|---|---|---|---|
| Total cases | 727 | 25 | 702 |
| Number of municipalities with at least 1 case | 138 | 20 | 136 |
| Male (%) | 413 (56.8%) | 17 (68.0%) | 396 (56.4%) * |
| Number of cases per age group | |||
| 1–9 | 77 | 2 | 75 |
| 10–19 | 59 | 2 | 57 |
| 20–29 | 49 | 1 | 48 |
| 30–39 | 79 | 3 | 76 |
| 40–49 | 81 | 4 | 77 |
| 50–59 | 140 | 5 | 135 |
| 60–69 | 171 | 6 | 165 |
| 70–79 | 62 | 2 | 60 |
| 80–89 | 9 | 0 | 9 |
| Average time from symptom onset to medical consultation (days) | 59 § | 57 † | 59 ‡ |
| Model A1 * | Model B1 † | |
| Northern spread (km/year) § | 32 km/year | 18 km/year |
| Average velocity (km/year deg) | 34 km/year 341° NNW | 21 km/year 327° NWN |
| Range (km/year) | 2–568 km/year | 14.5–2,949 km/year |
| SD | 67 km/year | 258 km/year |
| Model A2 ** | Model B2 †† | |
| Northern spread (km/year) § | 40 km/year | 46 km/year |
| Average velocity (km/year deg) | 41 km/year 16° NE | 47 km/year 15° NE |
| Range (km/year) | 21–107 km/year | 13–107 km/year |
| SD | 22 km/year | 19 km/year |
| Model A3 *** | Model B3 ††† | |
| Northern spread (km/year) § | 46 km/year | 44 km/year |
| Average velocity (km/year deg) | 46 km/year 357° N | 44 km/year 11° NE |
| Range (km/year) | 13–82 km/year | 12–103 km/year |
| SD | 16 km/year | 17 km/year |
| Clusters | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Radius (km) | 20.80 | 24.81 | 20.41 | 23.97 | 23.67 | 14.05 | 12.33 |
| Time period | 2013–2018 | 2013–2018 | 2013–2018 | 2015–2018 | 2015–2018 | 2015–2018 | 2016–2018 |
| Municipalities at-risk | 15 | 14 | 8 | 14 | 4 | 2 | 7 |
| Population at-risk | 52,675 | 26,423 | 15,645 | 100,670 | 7239 | 2074 | 109,448 |
| Declared cases | 282 | 75 | 40 | 88 | 6 | 5 | 48 |
| Expected cases | 4.68 | 2.35 | 1.39 | 5.96 | 0.43 | 0.12 | 4.86 |
| Annual cases/100,000 | 89.2 | 47.3 | 42.6 | 21.9 | 20.7 | 60.3 | 14.6 |
| Relative risk | 97.80 | 35.51 | 30.39 | 16.65 | 14.10 | 40.96 | 10.49 |
| p-value | <0.001 | <0.001 | <0.01 | <0.001 | 0.022 | 0.0013 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutt-Guérette, M.-A.; Yuan, M.; Szaroz, D.; McKinnon, B.; Kestens, Y.; Guillot, C.; Leighton, P.; Zinszer, K. Modelling Spatiotemporal Patterns of Lyme Disease Emergence in Québec. Int. J. Environ. Res. Public Health 2021, 18, 9669. https://doi.org/10.3390/ijerph18189669
Tutt-Guérette M-A, Yuan M, Szaroz D, McKinnon B, Kestens Y, Guillot C, Leighton P, Zinszer K. Modelling Spatiotemporal Patterns of Lyme Disease Emergence in Québec. International Journal of Environmental Research and Public Health. 2021; 18(18):9669. https://doi.org/10.3390/ijerph18189669
Chicago/Turabian StyleTutt-Guérette, Marc-Antoine, Mengru Yuan, Daniel Szaroz, Britt McKinnon, Yan Kestens, Camille Guillot, Patrick Leighton, and Kate Zinszer. 2021. "Modelling Spatiotemporal Patterns of Lyme Disease Emergence in Québec" International Journal of Environmental Research and Public Health 18, no. 18: 9669. https://doi.org/10.3390/ijerph18189669
APA StyleTutt-Guérette, M.-A., Yuan, M., Szaroz, D., McKinnon, B., Kestens, Y., Guillot, C., Leighton, P., & Zinszer, K. (2021). Modelling Spatiotemporal Patterns of Lyme Disease Emergence in Québec. International Journal of Environmental Research and Public Health, 18(18), 9669. https://doi.org/10.3390/ijerph18189669







