Effects of Intradialytic Exercise on Dialytic Parameters, Health-Related Quality of Life, and Depression Status in Hemodialysis Patients: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
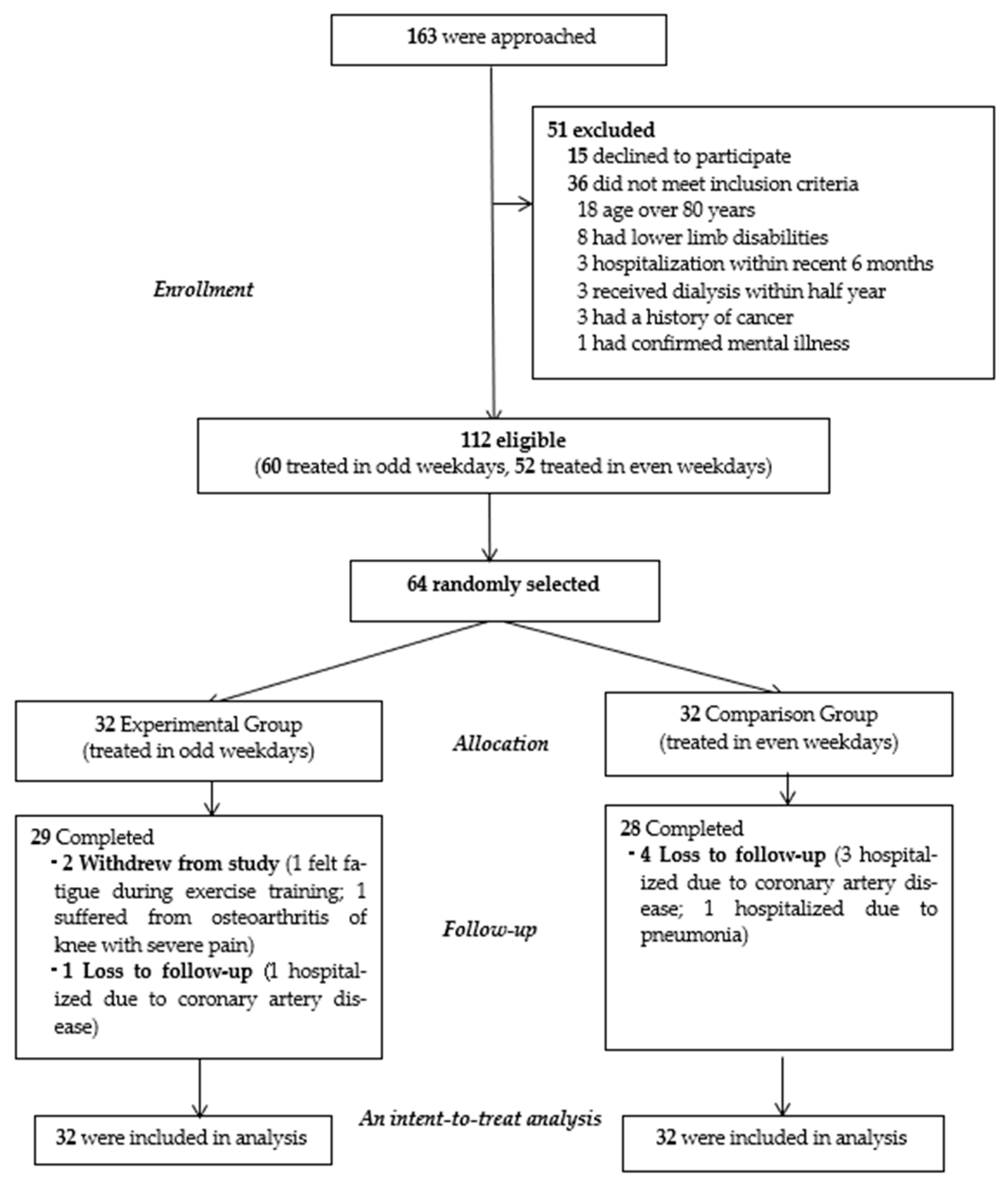
2.2. Participants
2.3. Study Cohorts and Interventions
2.4. Measures
2.5. Dialytic Parameters
2.6. Cardiometabolic Factors
2.7. Health-Related Quality of Life
2.8. Depression Status
2.9. Ethical Consideration
2.10. Data Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Outcome Evaluation
3.2.1. Dialytic Parameters
3.2.2. Cardiometabolic Factor
3.2.3. Health-Related Quality of Life
3.2.4. Depression Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US renal data system 2019 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2020, 75 (Suppl. 1), A6–A7. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Larive, B.; Painter, P.; Craig, A.; Lindsay, R.M.; Rocco, M.V.; Daugirdas, J.T.; Schulman, G.; Chertow, G.M. Baseline physical performance, health, and functioning of participants in the Frequent Hemodialysis Network (FHN) trial. Am. J. Kidney Dis. 2011, 57, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, R.; Matsunaga, A.; Wang, G.; Kutsuna, T.; Ishii, A.; Abe, Y.; Takagi, Y.; Yoshida, A.; Takahira, N. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 2010–2016. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Sarnak, M.J.; Tighiouart, H.; Drew, D.A.; Kantor, A.L.; Lou, K.V.; Shaffi, K.; Scott, T.M.; Weiner, D.E. Depression and all-cause mortality in hemodialysis patients. Am. J. Nephrol. 2014, 40, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.C.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Hedayati, S.S.; et al. Association between depression and death in people with CKD: A meta-analysis of cohort studies. Am. J. Kidney Dis. 2013, 62, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.; Clark, L.; Olausson, J. Physical function and physical activity assessment and promotion in the hemodialysis clinic: A qualitative study. Am. J. Kidney Dis. 2014, 64, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Kaysen, G.A.; Dalrymple, L.S.; Grimes, B.A.; Glidden, D.V.; Anand, S.; Chertow, G.M. Association of physical activity with survival among ambulatory patients on dialysis: The comprehensive dialysis study. Clin. J. Am. Soc. Nephrol. 2013, 8, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Heiwe, S.; Jacobson, S.H. Exercise training for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2011, 10, CD003236. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lv, A.; Wang, J.; Xu, N.; Ma, G.; Zhai, Z.; Zhang, B.; Gao, J.; Ni, C. Exercise training and outcomes in hemodialysis patients: Systematic review and meta-analysis. Am. J. Nephrol. 2019, 50, 240–254. [Google Scholar] [CrossRef]
- MacKinnon, H.J.; Wilkinson, T.J.; Clarke, A.L.; Gould, D.W.; O’Sullivan, T.F.; Xenophontos, S.; Watson, E.L.; Singh, S.J.; Smith, A.C. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: A systematic review. Ther. Adv. Chronic Dis. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Ouzouni, S.; Kouidi, E.; Sioulis, A.; Grekas, D.; Deligiannis, A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin. Rehabil. 2009, 23, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avesani, C.M.; Trolonge, S.; Deleaval, P.; Baria, F.; Mafra, D.; Faxen-Irving, G.; Chauveau, P.; Teta, D.; Kamimura, M.A.; Cuppari, L.; et al. Physical activity and energy expenditure in haemodialysis patients: An international survey. Nephrol. Dial. Transpl. 2012, 27, 2430–2434. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Shapiro, B.B.; Zhang, M.; Li, Y.; Porszasz, J.; Bross, R.; Feroze, U.; Upreti, R.; Kalantar-Zadeh, K.; Kopple, J.D. Daily physical activity and physical function in adult maintenance hemodialysis patients. J. Cachexia Sarcopenia Muscle 2014, 5, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, C.; Johansen, K.L. Barriers to exercise participation among dialysis patients. Nephrol. Dial. Transpl. 2012, 27, 1152–1157. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.; Bronas, U.G. Barriers to exercise for patients with renal disease: An integrative review. J. Nephrol. 2017, 30, 729–741. [Google Scholar] [CrossRef]
- Wang, X.X.; Lin, Z.H.; Wang, Y.; Xu, M.C.; Kang, Z.M.; Zeng, W.; Ma, Y.C. Motivators for and barriers to exercise rehabilitation in hemodialysis centers: A multicenter cross-sectional survey. Am. J. Phys. Med. Rehabil. 2019. [Google Scholar] [CrossRef]
- McKenna, C.F.; Salvador, A.F.; Hendriks, F.K.; Harris, A.P.; van Loon, L.J.; Burd, N.A. Exercising to offset muscle mass loss in hemodialysis patients: The disconnect between intention and intervention. Semin. Dial. 2019, 32, 379–385. [Google Scholar] [CrossRef]
- Coll-Fernández, R.; Coll, R.; Muñoz-Torrero, J.F.; Aguilar, E.; Ramón Álvarez, L.; Sahuquillo, J.C.; Yeste, M.; Jiménez, P.E.; Mujal, A.; Monreal, M. Supervised versus non-supervised exercise in patients with recent myocardial infarction: A propensity analysis. Eur. J. Prev. Cardiol. 2016, 23, 245–252. [Google Scholar] [CrossRef]
- Matarán-Peñarrocha, G.A.; Lara Palomo, I.C.; Antequera Soler, E.; Gil-Martínez, E.; Fernández-Sánchez, M.; Aguilar-Ferrándiz, M.E.; Castro-Sánchez, A.M. Comparison of efficacy of a supervised versus non-supervised physical therapy exercise program on the pain, functionality and quality of life of patients with non-specific chronic low-back pain: A randomized controlled trial. Clin. Rehabil. 2020, 34, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.A.; Mangrum, J.M.; Passman, R. Sudden cardiac death and dialysis patients. Semin. Dial. 2008, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Choi, H.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, C.D.; Ryu, D.R.; Kim, Y.L. Dialysis modality-related disparities in sudden cardiac death: Hemodialysis versus peritoneal dialysis. Kidney Res. Clin. Pract. 2019, 38, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Genovesi, S.; Boriani, G.; Covic, A.; Vernooij, R.W.M.; Combe, C.; Burlacu, A.; Davenport, A.; Kanbay, M.; Kirmizis, D.; Schneditz, D.; et al. Sudden cardiac death in dialysis patients: Different causes and management strategies. Nephrol. Dial. Transpl. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pun, P.H.; Smarz, T.R.; Honeycutt, E.F.; Shaw, L.K.; Al-Khatib, S.M.; Middleton, J.P. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009, 76, 652–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salhab, N.; Karavetian, M.; Kooman, J.; Fiaccadori, E.; El Khoury, C.F. Effects of intradialytic aerobic exercise on hemodialysis patients: A systematic review and meta-analysis. J. Nephrol. 2019, 32, 549–566. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.C.; Yeh, M.L.; Liu, Y.M. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: A systematic review and meta-analysis of randomised controlled trials. J. Clin. Nurs. 2017, 26, 1801–1813. [Google Scholar] [CrossRef]
- Pu, J.; Jiang, Z.; Wu, W.; Li, L.; Zhang, L.; Li, Y.; Liu, Q.; Ou, S. Efficacy and safety of intradialytic exercise in haemodialysis patients: A systematic review and meta-analysis. BMJ Open 2019, 9, e020633. [Google Scholar] [CrossRef] [Green Version]
- Gomes Neto, M.; de Lacerda, F.F.R.; Lopes, A.A.; Martinez, B.P.; Saquetto, M.B. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: Systematic review and meta-analysis. Clin. Rehabil. 2018, 47, 1189–1202. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [Green Version]
- Chiang, S.-L.; Shen, C.-L.; Chen, L.-C.; Lo, Y.-P.; Lin, C.-H.; Lin, C.-H. Effectiveness of a home-based telehealth exercise training program for patients with cardiometabolic multimorbidity: A randomized controlled trial. J. Cardiovasc. Nurs. 2020, 35, 491–501. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chiang, S.-L.; Heitkemper, M.M.; Hung, Y.-J.; Lee, M.-S.; Tzeng, W.-C.; Chiang, L.-C. Effects of telephone-based motivational interviewing in lifestyle modification program on reducing metabolic risks in middle-aged and older women with metabolic syndrome: A randomized controlled trial. Int. J. Nurs. Stud. 2016, 60, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.; Kosinski, M.; Keller, S. SF-36 Physical & Mental Health Summary Scales: A Manual for Users; QualityMetric; Cornell University: Ithaca, NY, USA, 2001. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yuan, C.-M.; Huang, J.; Li, Z.-Z.; Chen, J.; Zhang, H.-Y.; Fang, Y.-R.; Xiao, Z.-P. Reliability and validity of the Chinese version of Beck Depression Inventory-II among depression patients. Chin. Mental Health J. 2011, 25, 476–480. [Google Scholar]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuire, S.; Horton, E.J.; Renshaw, D.; Jimenez, A.; Krishnan, N.; McGregor, G. Hemodynamic instability during dialysis: The potential role of intradialytic exercise. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef]
- Makhlough, A.; Ilali, E.; Mohseni, R.; Shahmohammadi, S. Effect of intradialytic aerobic exercise on serum electrolytes levels in hemodialysis patients. Iran. J. Kidney Dis. 2012, 6, 119–123. [Google Scholar]
- Zhang, L.; Wang, Y.; Xiong, L.; Luo, Y.; Huang, Z.; Yi, B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: Evidence from a meta-analysis. BMC Nephrol. 2019, 20, 398. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, P.; Chen, L.; Cheng, J.; Wu, C.; Chen, J. Intradialytic exercise in hemodialysis patients: A systematic review and meta-analysis. Am. J. Nephrol. 2014, 40, 478–490. [Google Scholar] [CrossRef]
- Ookawara, S.; Miyazawa, H.; Ito, K.; Ueda, Y.; Kaku, Y.; Hirai, K.; Hoshino, T.; Mori, H.; Yoshida, I.; Morishita, Y.; et al. Blood volume changes induced by low-intensity intradialytic exercise in long-term hemodialysis patients. ASAIO J. 2016, 62, 190–196. [Google Scholar] [CrossRef]
- Battista, F.; Ermolao, A.; van Baak, M.A.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Encantado, J.; Dicker, D.; Farpour-Lambert, N.; et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat-A systematic review and meta-analysis. Obes. Rev. 2021, e13269. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Strijcker, D.; Lapauw, B.; Ouwens, D.M.; Van de Velde, D.; Hansen, D.; Petrovic, M.; Cuvelier, C.; Tonoli, C.; Calders, P. High intensity interval training is associated with greater impact on physical fitness, insulin sensitivity and muscle mitochondrial content in males with overweight/obesity, as opposed to continuous endurance training: A randomized controlled trial. J. Musculoskelet. Neuronal Interact 2018, 18, 215–226. [Google Scholar] [PubMed]
- Andrade, F.P.; de Souza Rezende, P.; de Souza Ferreira, T.; Borba, G.C.; Müller, A.M.; Rovedder, P.M.E. Effects of intradialytic exercise on cardiopulmonary capacity in chronic kidney disease: Systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.Y.; Hu, R.J.; Diao, Y.S.; Chen, L.; Jiang, X.L. Effects of exercise training on restless legs syndrome, depression, sleep quality, and fatigue among hemodialysis patients: A systematic review and meta-analysis. J. Pain Symptom. Manag. 2018, 55, 1184–1195. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Peredo, G.B. Functional dependence and the mental dimension of quality of life in Hemodialysis patients: The PROHEMO study. Health Qual. Life Outcomes 2020, 18, 234. [Google Scholar] [CrossRef]
- Lopes, A.A.; Bragg, J.; Young, E.; Goodkin, D.; Mapes, D.; Combe, C.; Piera, L.; Held, P.; Gillespie, B.; Port, F.K.; et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002, 62, 199–207. [Google Scholar] [CrossRef] [Green Version]
| Variables | EG | CG | t/x2 | p |
|---|---|---|---|---|
| n = 32 | n = 32 | |||
| Sociodemographic Characteristics | ||||
| Age (year), mean (SD) | 62.0 (9.5) | 62.1 (12.3) | 0.03 | 0.97 |
| Gender (male), n (%) | 22 (68.8) | 19 (59.4) | 0.61 | 0.60 |
| Marital status (married), n (%) | 28 (87.5) | 23 (71.9) | 2.41 | 0.12 |
| Education (more than high school), n (%) | 21 (65.6) | 25 (78.1) | 1.24 | 0.27 |
| Currently employed, n (%) | 10 (31.2) | 7 (21.9) | 0.72 | 0.40 |
| Body mass index (Kg/m2), mean (SD) | 23.4 (3.7) | 23.4 (4.5) | −0.06 | 0.95 |
| Duration of hemodialysis (year), mean (SD) | 6.7 (5.7) | 6.2 (5.1) | 0.39 | 0.70 |
| Comorbidities | ||||
| Hypertension, n (%) | 28 (87.5) | 24 (75.0) | 1.64 | 0.34 |
| Type 2 diabetes, n (%) | 14 (43.8) | 19 (59.4) | 1.56 | 0.21 |
| Hyperlipidemia, n (%) | 5 (15.6) | 6 (18.8) | 0.11 | 0.74 |
| Cardiovascular disease, n (%) | 4 (12.5) | 11 (34.4) | 4.27 | 0.08 |
| Metabolic syndrome, n (%) | 6 (18.8) | 7 (21.9) | 0.10 | 0.76 |
| Lifestyle Factors | ||||
| Smoking, n (%) | 3 (9.4) | 0 (0) | 3.15 | 0.24 |
| Drinking, n (%) | 0 (0) | 1 (3.1) | 1.02 | 1.00 |
| Variables | EG (n = 32) | CG (n = 32) | Baseline | 12-Week | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Week | p | Baseline | 12-Week | p | EG vs. CG | EG vs. CG | |||
| t | p | t | p | |||||||
| Dialytic parameters | ||||||||||
| Serum chemistries | ||||||||||
| Red blood cell (×10^6/uL) | 3.5 (0.6) | 3.4 (0.6) | 0.58 | 3.6 (0.7) | 3.6 (0.9) | 1.00 | −0.46 | 0.647 | −0.83 | 0.41 |
| Hemoglobin (g/dL) | 10.1 (1.4) | 9.9 (1.1) | 0.51 | 10.3 (1.3) | 10.4 (1.3) | 0.90 | −0.676 | 0.50 | −2.17 | 0.11 |
| Hematocrit (%) | 30.5 (4.2) | 29.6 (3.6) | 0.37 | 31.4 (4.4) | 31.6 (5.6) | 0.82 | −0.827 | 0.411 | −1.74 | 0.09 |
| Mean corpuscular volume (fL) | 87.9 (9.4) | 88.1 (9.6) | 0.95 | 89.0 (8.6) | 93.1 (17.9) | 0.31 | −0.45 | 0.654 | −1.35 | 0.18 |
| Albumin (g/dL) | 3.9 (0.3) | 4.0 (0.3) | 0.65 | 3.9 (0.3) | 3.8 (0.2) | 0.20 | 0.65 | 0.52 | 2.43 | 0.02 |
| GPT (IU/L) | 13.2 (4.2) | 14.2 (4.5) | 0.37 | 14.7 (7.1) | 14.5 (7.4) | 0.89 | −1.05 | 0.30 | −0.18 | 0.86 |
| GOT (IU/L) | 13.3 (4.9) | 15.0 (6.8) | 0.25 | 14.2 (6.1) | 15.4 (6.6) | 0.45 | −0.68 | 0.50 | −0.26 | 0.80 |
| BUN (mg/dL) | 65.6 (18.1) | 70.4 (20.6) | 0.33 | 65.8 (16.5) | 70.5 (25.6) | 0.39 | −0.04 | 0.97 | −0.02 | 0.99 |
| Cr (mg/dL) | 10.6 (2.2) | 10.4 (2.3) | 0.72 | 9.7 (2.0) | 10.1 (2.1) | 0.43 | 1.69 | 0.10 | 0.49 | 0.63 |
| Serum electrolytes | ||||||||||
| Na (mEq/L) | 137.6 (3.0) | 137.5 (2.4) | 0.92 | 137.4 (2.8) | 142.0 (24.5) | 0.29 | 0.31 | 0.76 | −1.04 | 0.30 |
| K (mEq/L) | 4.8 (0.8) | 4.6 (0.6) | 0.29 | 4.6 (0.7) | 4.7 (1.1) | 0.66 | 1.11 | 0.27 | −0.40 | 0.69 |
| Ca (mg/dL) | 9.2 (1.2) | 9.3 (1.1) | 0.91 | 9.3 (1.0) | 9.0 (0.9) | 0.23 | −0.13 | 0.90 | 1.16 | 0.25 |
| P (mg/dL) | 5.2 (1.4) | 4.9 (1.2) | 0.48 | 4.8 (1.2) | 5.0 (1.3) | 0.69 | 1.05 | 0.30 | −0.06 | 0.95 |
| IPTH (pg/mL) | 416.3 (351.6) | 438.7 (293.9) | 0.79 | 330.9 (335.0) | 321.8 (289.2) | 0.93 | 0.81 | 0.42 | 1.47 | 0.15 |
| eGFR (mL/min1.73m2) | 5.0 (1.1) | 5.1 (1.2) | 0.67 | 5.4 (1.5) | 5.1 (1.3) | 0.38 | −1.415 | 0.162 | −0.10 | 0.92 |
| Cardiometabolic factors | ||||||||||
| Systolic blood pressure (mmHg) | 141.5 (20.8) | 136.5 (14.4) | 0.27 | 145.1 (8.0) | 152.2 (25.5) | 0.30 | −0.58 | 0.562 | −3.03 | 0.004 |
| Diastolic blood pressure (mmHg) | 75.6 (12.2) | 71.7 (7.4) | 0.13 | 73.1 (13.8) | 74.8 (14.4) | 0.62 | 0.76 | 0.451 | −1.09 | 0.28 |
| Resting heart rate (beat/min) | 71.6 (8.3) | 71.4 (8.5) | 0.93 | 71.4 (9.8) | 70.8 (9.7) | 0.79 | 0.08 | 0.935 | 0.29 | 0.77 |
| Fasting blood glucose (mg/dL) | 105.4 (29.1) | 103.6 (28.3) | 0.81 | 125.8 (86.5) | 116.8 (47.2) | 0.61 | −1.26 | 0.21 | −1.36 | 0.18 |
| Cholesterol (mg/dL) | 156.6 (32.2) | 153.8 (31.3) | 0.73 | 156.5 (29.9) | 157.4 (32.3) | 0.92 | 0.01 | 0.99 | −0.42 | 0.68 |
| Triglyceride (mg/dL) | 124.6 (127.6) | 125.8 (116.2) | 0.97 | 112.3 (62.9) | 119.2 (86.5) | 0.75 | 0.43 | 0.67 | 0.24 | 0.82 |
| Uric acid (mg/dL) | 6.4 (1.7) | 5.7 (1.3) | 0.16 | 6.2 (1.2) | 6.0 (1.4) | 0.64 | 0.60 | 0.55 | −0.53 | 0.60 |
| HRQL | ||||||||||
| Total mean score | 62.8 (17.5) | 81.0 (18.7) | <0.001 | 64.1 (16.9) | 58.1 (16.1) | 0.15 | 0.30 | 0.77 | 5.27 | <0.001 |
| Bodily pain | 74.4 (22.5) | 90.8 (18.9) | 0.003 | 79.9 (26.6) | 79.9 (26.6) | 1.00 | 0.89 | 0.38 | 1.89 | 0.06 |
| General health | 44.1 (17.6) | 58.6 (20.6) | 0.004 | 48.1 (18.8) | 42.7 (19.8) | 0.26 | 0.89 | 0.38 | 3.16 | 0.002 |
| Mental health | 63.8 (16.8) | 79.8 (18.2) | 0.001 | 70.6 (15.7) | 67.4 (14.4) | 0.39 | 1.69 | 0.10 | 3.02 | 0.004 |
| Physical function | 72.5 (21.0) | 88.3 (15.7) | 0.001 | 75.0 (19.3) | 73.9 (19.1) | 0.82 | 0.50 | 0.62 | 3.29 | 0.002 |
| Role-emotional | 78.1 (37.5) | 90.6 (29.6) | 0.14 | 63.5 (40.9) | 44.8 (42.8) | 0.08 | −1.49 | 0.14 | 4.98 | <0.001 |
| Role-physical | 46.9 (39.0) | 85.9 (31.7) | <0.001 | 50.0 (43.1) | 32.0 (39.3) | 0.09 | 0.30 | 0.76 | 6.04 | <0.001 |
| Social functioning | 71.9 (24.4) | 88.7 (22.8) | 0.01 | 72.3 (22.6) | 70.7 (22.1) | 0.78 | 0.07 | 0.95 | 3.20 | 0.002 |
| Vitality | 50.6 (20.6) | 65.6 (21.1) | 0.01 | 53.0 (19.1) | 53.4 (18.1) | 0.92 | 0.47 | 0.64 | 2.48 | 0.02 |
| Depression status | 12.8 (9.3) | 5.0 (6.8) | <0.001 | 11.2 (9.8) | 12.5 (9.2) | 0.58 | 0.65 | 0.52 | −3.73 | <0.001 |
| Variables | Within Group | Between Group | Interaction Group (EG) × Time | Interaction a Group (EG) × Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref: Baseline | Ref: CG | Reference Group: (CG) × Time | Reference Group: (CG) × Time | |||||||||
| ß | p | ß | p | ß | 95% C.I. | p | ß | 95% C.I. | p-Adjusted | |||
| Lower | Upper | Lower | Upper | |||||||||
| Dialytic parameters | ||||||||||||
| Serum chemistries | ||||||||||||
| Red blood cell (×10^6/uL) | 0.001 | 1.00 | −0.08 | 0.65 | −0.08 | −0.58 | 0.42 | 0.76 | −0.03 | −0.47 | 0.42 | 0.90 |
| Hemoglobin (g/dL) | 0.05 | 0.89 | −0.22 | 0.51 | −0.27 | −1.13 | 0.59 | 0.54 | −0.23 | −1.03 | 0.58 | 0.58 |
| Hematocrit (%) | 0.29 | 0.82 | −0.89 | 0.40 | −1.17 | −4.27 | 1.92 | 0.46 | −0.96 | −3.86 | 1.95 | 0.52 |
| Mean corpuscular volume (fL) | 4.07 | 0.28 | −1.09 | 0.64 | −3.90 | −12.6 | 4.83 | 0.38 | −3.82 | −11.6 | 3.96 | 0.34 |
| Albumin (g/dL) | −0.08 | 0.18 | 0.04 | 0.51 | 0.12 | −0.07 | 0.30 | 0.21 | 0.11 | −0.07 | 0.29 | 0.21 |
| GPT (IU/L) | −0.25 | 0.89 | −1.53 | 0.29 | 1.25 | −2.85 | 5.35 | 0.55 | 1.42 | −2.16 | 5.01 | 0.44 |
| GOT (IU/L) | 1.22 | 0.44 | −0.94 | 0.49 | 0.50 | −3.70 | 4.70 | 0.82 | 0.69 | −3.26 | 4.63 | 0.73 |
| BUN (mg/dL) | 4.66 | 0.38 | −0.19 | 0.97 | 0.09 | −13.9 | 14.1 | 0.99 | 0.93 | −12.2 | 14.1 | 0.89 |
| Cr (mg/dL) | 0.41 | 0.42 | 0.88 | 0.09 | −0.61 | −2.07 | 0.85 | 0.41 | −0.53 | −1.75 | 0.69 | 0.40 |
| Serum electrolytes | ||||||||||||
| Na (mEq/L) | 4.68 | 0.28 | 0.22 | 0.76 | −4.74 | −13.3 | 3.77 | 0.28 | −4.71 | −12.8 | 3.34 | 0.25 |
| K (mEq/L) | 0.10 | 0.65 | 0.20 | 0.26 | −0.29 | −0.84 | 0.26 | 0.31 | −0.27 | −0.80 | 0.25 | 0.31 |
| Ca (mg/dL) | −0.29 | 0.21 | −0.03 | 0.90 | 0.33 | −0.39 | 1.04 | 0.37 | 0.31 | −0.38 | 1.00 | 0.38 |
| P (mg/dL) | 0.13 | 0.69 | 0.34 | 0.28 | −0.36 | −1.23 | 0.52 | 0.42 | −0.30 | −1.14 | 0.54 | 0.49 |
| IPTH (pg/mL) | −9.09 | 0.93 | 85.38 | 0.40 | 31.49 | −220.2 | 283.2 | 0.81 | 3.5 | −227.5 | 234.5 | 0.98 |
| eGFR (mL/min1.73m2) | −0.31 | 0.37 | −0.47 | 0.15 | 0.44 | −0.44 | 1.32 | 0.33 | 0.40 | −0.38 | 1.18 | 0.32 |
| Cardiometabolic factors | ||||||||||||
| Systolic blood pressure (mmHg) | −7.06 | 0.28 | −15.7 | 0.002 | 12.1 | −3.44 | 27.6 | 0.13 | −12.5 | −26.5 | 1.37 | 0.08 |
| Diastolic blood pressure (mmHg) | 1.75 | 0.61 | 2.47 | 0.44 | −5.59 | −14.0 | 2.77 | 0.19 | −5.80 | −13.2 | 1.59 | 0.12 |
| Resting heart rate (beat/min) | −0.66 | 0.79 | 0.19 | 0.93 | 0.47 | −5.74 | 6.68 | 0.88 | 0.54 | −5.29 | 6.37 | 0.86 |
| Fasting blood glucose (mg/dL) | −8.94 | 0.60 | −20.3 | 0.20 | 7.16 | −29.2 | 43.5 | 0.70 | 5.80 | −27.2 | 38.8 | 0.73 |
| Cholesterol (mg/dL) | 0.86 | 0.92 | 0.11 | 0.99 | −3.67 | −26.8 | 19.4 | 0.76 | −8.69 | −29.4 | 12.0 | 0.41 |
| Triglyceride (mg/dL) | 6.95 | 0.74 | 12.3 | 0.63 | −5.72 | −78.4 | 67.0 | 0.88 | −4.18 | −70.3 | 61.9 | 0.90 |
| Uric acid (mg/d) | −0.22 | 0.64 | 0.22 | 0.25 | −0.54 | −1.83 | 0.75 | 0.41 | −0.57 | −1.78 | 0.65 | 0.36 |
| HRQL | ||||||||||||
| Total mean score | −6.0 | 0.14 | −1.3 | 0.76 | 24.2 | 12.4 | 36.0 | <0.001 | 22.6 | 11.2 | 34.0 | <0.001 |
| Bodily pain | 0.0 | 1.00 | −5.5 | 0.37 | 16.4 | 0.1 | 32.7 | 0.05 | 12.8 | −2.0 | 27.5 | 0.09 |
| General health | −5.5 | 0.25 | −4.1 | 0.36 | 20.0 | 6.9 | 33.1 | 0.003 | 19.2 | 6.3 | 32.1 | 0.004 |
| Mental health | −3.3 | 0.38 | −6.9 | 0.09 | 19.3 | 8.1 | 30.4 | 0.001 | 17.7 | 7.5 | 27.8 | 0.001 |
| Physical function | −1.1 | 0.82 | −2.5 | 0.61 | 16.9 | 4.0 | 29.7 | 0.01 | 14.5 | 2.1 | 27.0 | 0.02 |
| Role-emotional | −18.8 | 0.07 | 14.6 | 0.13 | 31.3 | 5.3 | 57.2 | 0.02 | 28.9 | 2.1 | 55.8 | 0.04 |
| Role-physical | −18.0 | 0.08 | −3.1 | 0.76 | 57.0 | 30.8 | 83.3 | <0.001 | 63.7 | 36.3 | 91.1 | <0.001 |
| Social functioning | −1.6 | 0.78 | −0.4 | 0.95 | 18.4 | 2.7 | 34.0 | 0.02 | 13.9 | −1.4 | 29.1 | 0.08 |
| Vitality | 0.5 | 0.92 | −2.3 | 0.63 | 14.5 | 1.1 | 28.0 | 0.04 | 10.2 | −3.4 | 23.7 | 0.14 |
| Depression status | 1.3 | 0.57 | 1.6 | 0.51 | −9.1 | −15.1 | −3.1 | 0.003 | −7.5 | −13.8 | −1.3 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Hsu, Y.-J.; Hsu, P.-H.; Lee, Y.-L.; Lin, C.-H.; Lee, M.-S.; Chiang, S.-L. Effects of Intradialytic Exercise on Dialytic Parameters, Health-Related Quality of Life, and Depression Status in Hemodialysis Patients: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 9205. https://doi.org/10.3390/ijerph18179205
Lin C-H, Hsu Y-J, Hsu P-H, Lee Y-L, Lin C-H, Lee M-S, Chiang S-L. Effects of Intradialytic Exercise on Dialytic Parameters, Health-Related Quality of Life, and Depression Status in Hemodialysis Patients: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(17):9205. https://doi.org/10.3390/ijerph18179205
Chicago/Turabian StyleLin, Chia-Huei, Yu-Juei Hsu, Pi-Hsiu Hsu, Yi-Ling Lee, Chueh-Ho Lin, Meei-Shyuan Lee, and Shang-Lin Chiang. 2021. "Effects of Intradialytic Exercise on Dialytic Parameters, Health-Related Quality of Life, and Depression Status in Hemodialysis Patients: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 17: 9205. https://doi.org/10.3390/ijerph18179205
APA StyleLin, C.-H., Hsu, Y.-J., Hsu, P.-H., Lee, Y.-L., Lin, C.-H., Lee, M.-S., & Chiang, S.-L. (2021). Effects of Intradialytic Exercise on Dialytic Parameters, Health-Related Quality of Life, and Depression Status in Hemodialysis Patients: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(17), 9205. https://doi.org/10.3390/ijerph18179205







