Personal Passive Air Samplers for Chlorinated Gases Generated from the Use of Consumer Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Personal Passive Air Sampler (PPAS) Preparation
2.3. Measurement of Chlorine Concentration Using Proton Transfer Reaction/Selective Reagent Ionization Mass Spectrometer (PTR/SRI-MS)
2.4. Chamber Study for Estimating Passive Sampler-Derived Chlorine Gas Equivalent Exposure
3. Results and Discussions
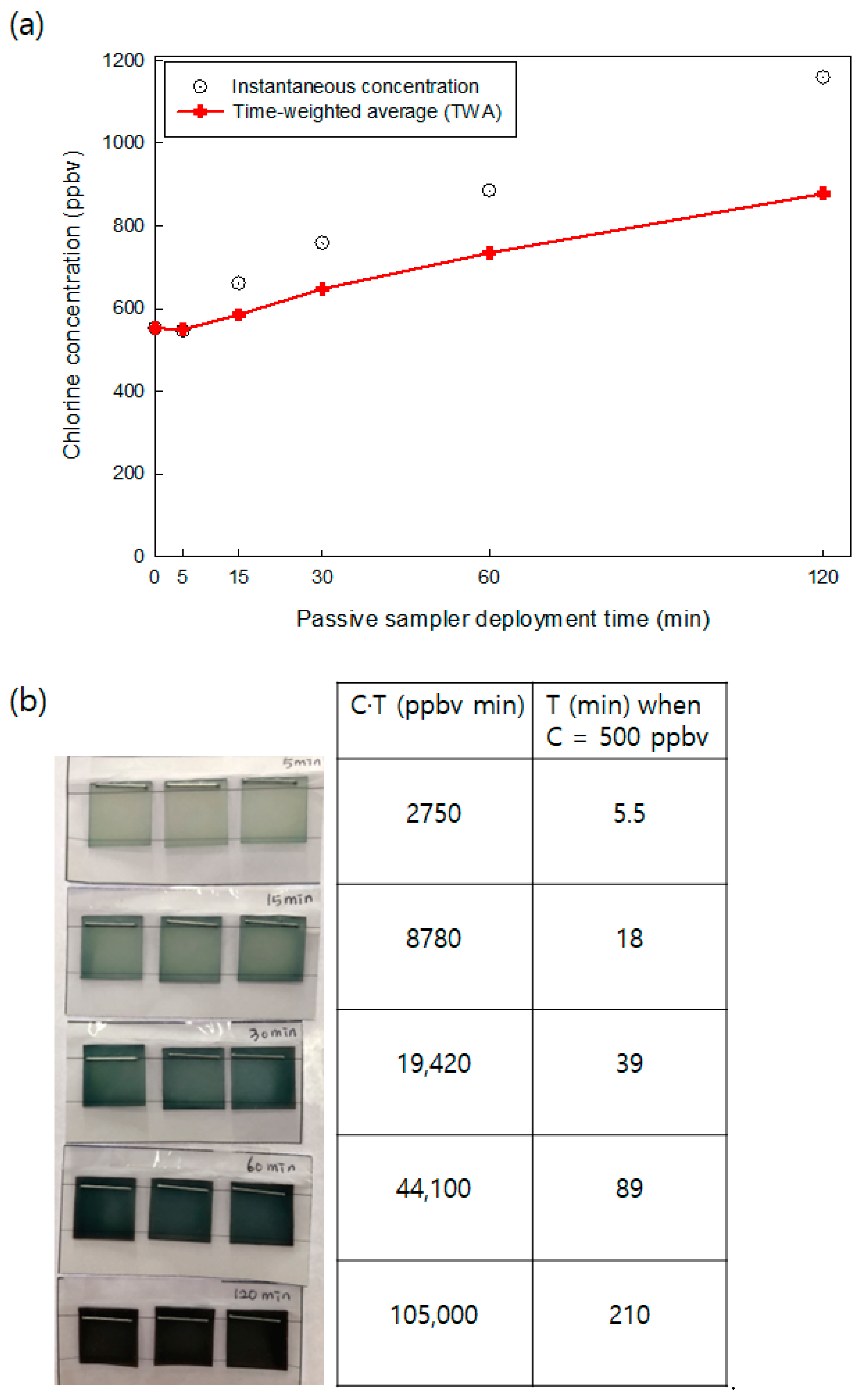
3.1. Color Changes of the Passive Sampler According to Chlorine Exposure Concentration and Time
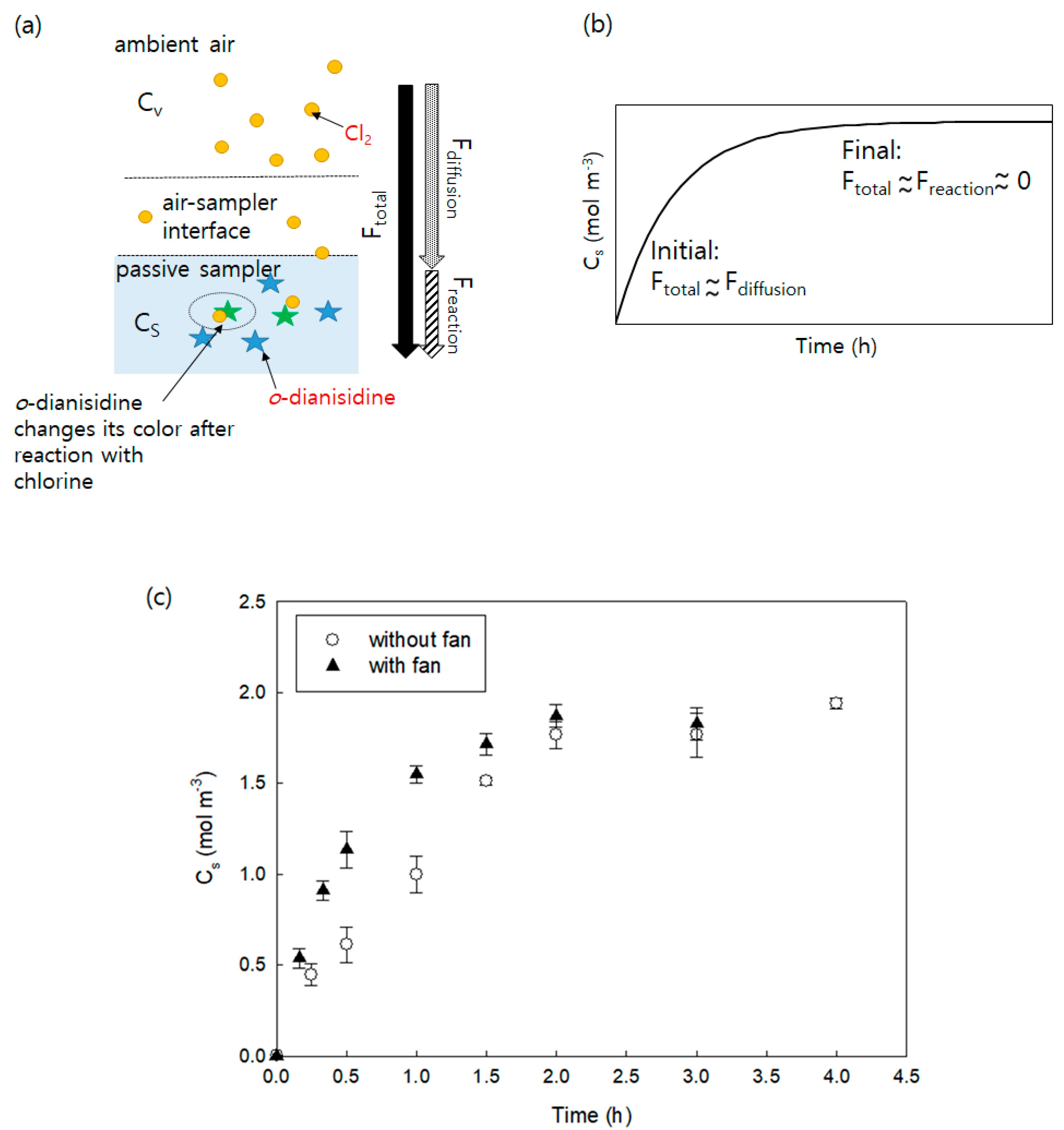
3.2. Uptake and Reaction Rate of the Passive Samplers
3.3. Effects of Disinfectant Types on the Chlorine Gas Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. COVID-19: How to Protect Yourself & Others. Atlanta, GA: US Department of Health and Human Services, CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 1 June 2021).
- WHO. Preventing and Mitigating COVID-19 at Work; WHO: Geneva, Switzerland. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-workplace-actions-policy-brief-2021-1 (accessed on 1 June 2021).
- Klemeš, J.J.; Van Fan, Y.; Jiang, P. The energy and environmental footprints of COVID-19 fighting measures–PPE, disinfection, supply chains. Energy 2020, 211, 118701. [Google Scholar] [CrossRef]
- EPA. List N Tool: COVID-19 Disinfectants. Available online: https://cfpub.epa.gov/wizards/disinfectants/ (accessed on 7 June 2021).
- MOE. Guidelines for Disinfection of COVID-19 and Detailed Guidelines for Safe use of Disinfection Products (Written in Korean). Available online: https://ecolife.me.go.kr/ecolife/bbs/notice/show/2897 (accessed on 7 June 2021).
- Rutala, W.A.; Weber, D.J. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 1997, 10, 597. [Google Scholar] [CrossRef]
- Wong, J.; Carslaw, N.; Zhao, R.; Zhou, S.; Abbatt, J. Observations and impacts of bleach washing on indoor chlorine chemistry. Indoor Air 2017, 27, 1082–1090. [Google Scholar] [CrossRef]
- Mattila, J.M.; Lakey, P.S.; Shiraiwa, M.; Wang, C.; Abbatt, J.P.; Arata, C.; Goldstein, A.H.; Ampollini, L.; Katz, E.F.; DeCarlo, P.F. Multiphase chemistry controls inorganic chlorinated and nitrogenated compounds in indoor air during bleach cleaning. Environ. Sci. Technol. 2020, 54, 1730–1739. [Google Scholar] [CrossRef] [Green Version]
- Achanta, S.; Jordt, S.-E. Toxic effects of chlorine gas and potential treatments: A literature review. Toxicol. Mech. Methods 2019, 31, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Carbonnelle, S.; de Burbure, C.; Michel, O.; Nickmilder, M. Chlorinated pool attendance, atopy, and the risk of asthma during childhood. Environ. Health Perspect. 2006, 114, 1567–1573. [Google Scholar] [CrossRef] [Green Version]
- Racioppi, F.; Daskaleros, P.; Besbelli, N.; Borges, A.; Deraemaeker, C.; Magalini, S.; Arrifta, R.M.; Pulce, C.; Ruggerone, M.; Vlachos, P. Household bleaches based on sodium hypochlorite: Review of acute toxicology and poison control center experience. Food Chem. Toxicol. 1994, 32, 845–861. [Google Scholar] [CrossRef]
- Gorguner, M.; Aslan, S.; Inandi, T.; Cakir, Z. Reactive airways dysfunction syndrome in housewives due to a bleach–hydrochloric acid mixture. Inhal. Toxicol. 2004, 16, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Schnall, A.H.; Law, R.; Bronstein, A.C.; Marraffa, J.M.; Spiller, H.A.; Hays, H.L.; Funk, A.R.; Mercurio-Zappala, M.; Calello, D.P. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19—national poison data system, United States, january 1, 2020–march 31, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 496. [Google Scholar] [CrossRef] [Green Version]
- Gharpure, R.; Hunter, C.M.; Schnall, A.H.; Barrett, C.E.; Kirby, A.E.; Kunz, J.; Berling, K.; Mercante, J.W.; Murphy, J.L.; Garcia--Williams, A.G. Knowledge and practices regarding safe household cleaning and disinfection for COVID-19 prevention—United States, May 2020. Wiley Online Libr. 2020, 2946–2950. [Google Scholar] [CrossRef]
- Ha, Y.; Kim, Y.; Song, E.; Yoo, H.J.; Kwon, J.H. Development of a personal passive air sampler for estimating exposure to effective chlorine while using chlorine-based disinfectants. Indoor Air 2021, 31, 557–565. [Google Scholar] [CrossRef]
- Tromp, P.C.; Beeltje, H.; Okeme, J.O.; Vermeulen, R.; Pronk, A.; Diamond, M.L. Calibration of polydimethylsiloxane and polyurethane foam passive air samplers for measuring semi volatile organic compounds using a novel exposure chamber design. Chemosphere 2019, 227, 435–443. [Google Scholar] [CrossRef]
- Bohlin, P.; Audy, O.; Škrdlíková, L.; Kukučka, P.; Vojta, Š.; Přibylová, P.; Prokeš, R.; Čupr, P.; Klánová, J. Evaluation and guidelines for using polyurethane foam (PUF) passive air samplers in double-dome chambers to assess semi-volatile organic compounds (SVOCs) in non-industrial indoor environments. Environ. Sci. Process. Impacts 2014, 16, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, S.; Górecki, T. Polydimethylsiloxane-based permeation passive air sampler. part I: Calibration constants and their relation to retention indices of the analytes. J. Chromatogr. A 2011, 1218, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Safety, O.; Administration, H. Method ID-101: Chlorine in workplace atmospheres. In Analytical Methods Manual; OSHA: Washington, DC, USA, 1981. [Google Scholar]
- Council, N.R. Chlorine: Acute Exposure Guideline Levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 4; National Academies Press (US): Washington, DC, USA, 2004. [Google Scholar]
- Tuduri, L.; Harner, T.; Hung, H. Polyurethane foam (PUF) disks passive air samplers: Wind effect on sampling rates. Environ. Pollut. 2006, 144, 377–383. [Google Scholar] [CrossRef]
- Zhang, X.; Brown, T.N.; Ansari, A.; Yeun, B.; Kitaoka, K.; Kondo, A.; Lei, Y.D.; Wania, F. Effect of wind on the chemical uptake kinetics of a passive air sampler. Environ. Sci. Technol. 2013, 47, 7868–7875. [Google Scholar] [CrossRef]
- Bartkow, M.E.; Booij, K.; Kennedy, K.E.; Müller, J.F.; Hawker, D.W. Passive air sampling theory for semivolatile organic compounds. Chemosphere 2005, 60, 170–176. [Google Scholar] [CrossRef]
- Bartkow, M.E.; Hawker, D.W.; Kennedy, K.E.; Müller, J.F. Characterizing uptake kinetics of PAHs from the air using polyethylene-based passive air samplers of multiple surface area-to-volume ratios. Environ. Sci. Technol. 2004, 38, 2701–2706. [Google Scholar] [CrossRef]
- McEneff, G.L.; Murphy, B.; Webb, T.; Wood, D.; Irlam, R.; Mills, J.; Green, D.; Barron, L.P. Sorbent film-coated passive samplers for explosives vapour detection part A: Materials optimisation and integration with analytical technologies. Sci. Rep. 2018, 8, 5815. [Google Scholar] [CrossRef]
- Wang, S.; Romanak, K.A.; Stubbings, W.A.; Arrandale, V.H.; Hendryx, M.; Diamond, M.L.; Salamova, A.; Venier, M. Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environ. Int. 2019, 132, 105104. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Gravel, S.; Labrèche, F.; Bakhiyi, B.; Verner, M.-A.; Zayed, J.; Jantunen, L.M.; Arrandale, V.H.; Diamond, M.L. Can Silicone Passive Samplers be Used for Measuring Exposure of e-Waste Workers to Flame Retardants? Environ. Sci. Technol. 2020, 54, 15277–15286. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.; Koo, Y.; Kwon, J.-H. Personal Passive Air Samplers for Chlorinated Gases Generated from the Use of Consumer Products. Int. J. Environ. Res. Public Health 2021, 18, 8940. https://doi.org/10.3390/ijerph18178940
Ha Y, Koo Y, Kwon J-H. Personal Passive Air Samplers for Chlorinated Gases Generated from the Use of Consumer Products. International Journal of Environmental Research and Public Health. 2021; 18(17):8940. https://doi.org/10.3390/ijerph18178940
Chicago/Turabian StyleHa, Yeonjeong, Yerim Koo, and Jung-Hwan Kwon. 2021. "Personal Passive Air Samplers for Chlorinated Gases Generated from the Use of Consumer Products" International Journal of Environmental Research and Public Health 18, no. 17: 8940. https://doi.org/10.3390/ijerph18178940
APA StyleHa, Y., Koo, Y., & Kwon, J.-H. (2021). Personal Passive Air Samplers for Chlorinated Gases Generated from the Use of Consumer Products. International Journal of Environmental Research and Public Health, 18(17), 8940. https://doi.org/10.3390/ijerph18178940





