The Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.2.1. Study Selection
2.2.2. Data Collection Process
2.2.3. Statistical Analysis
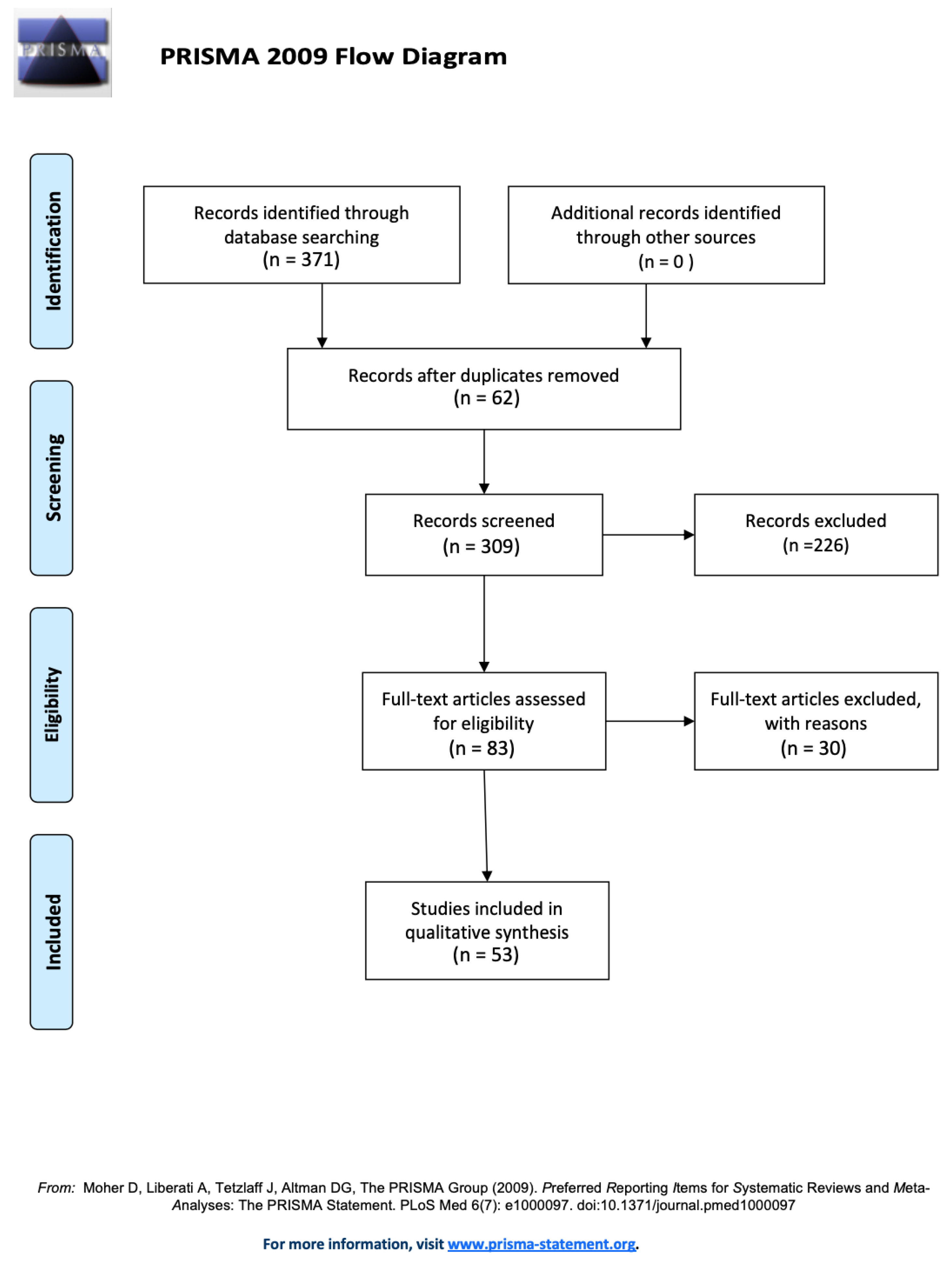
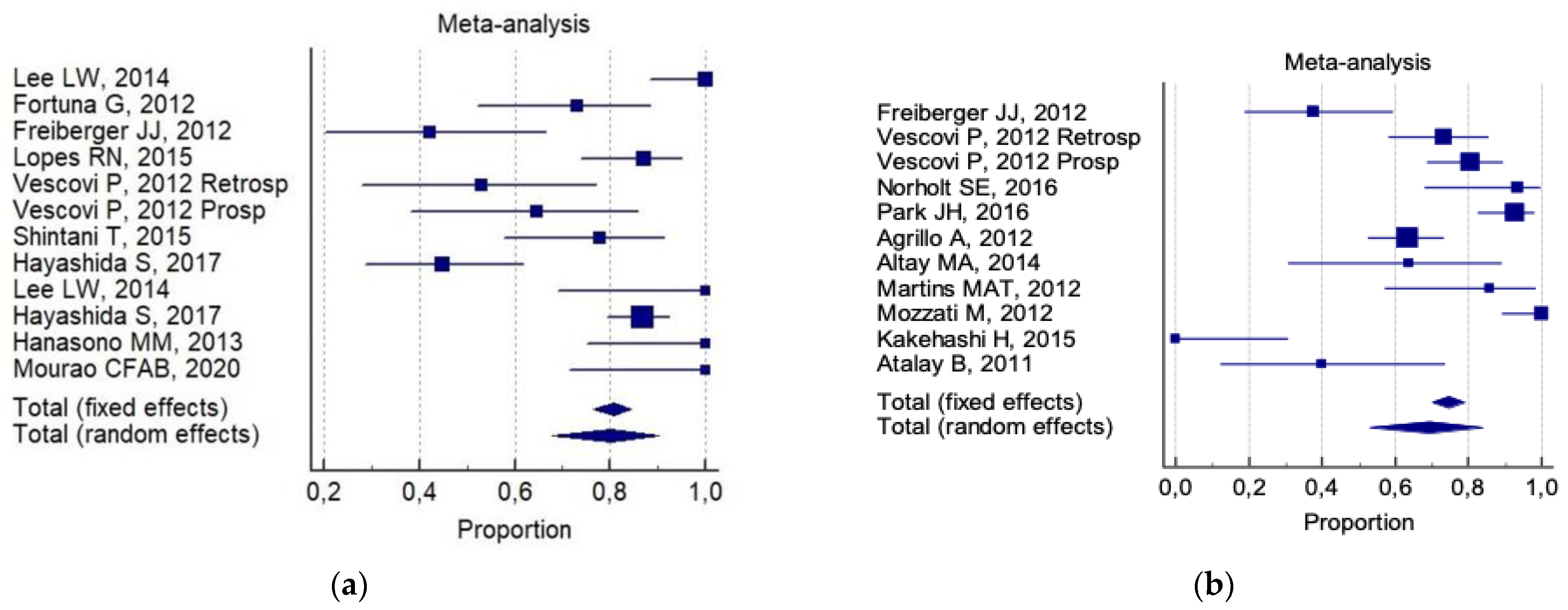
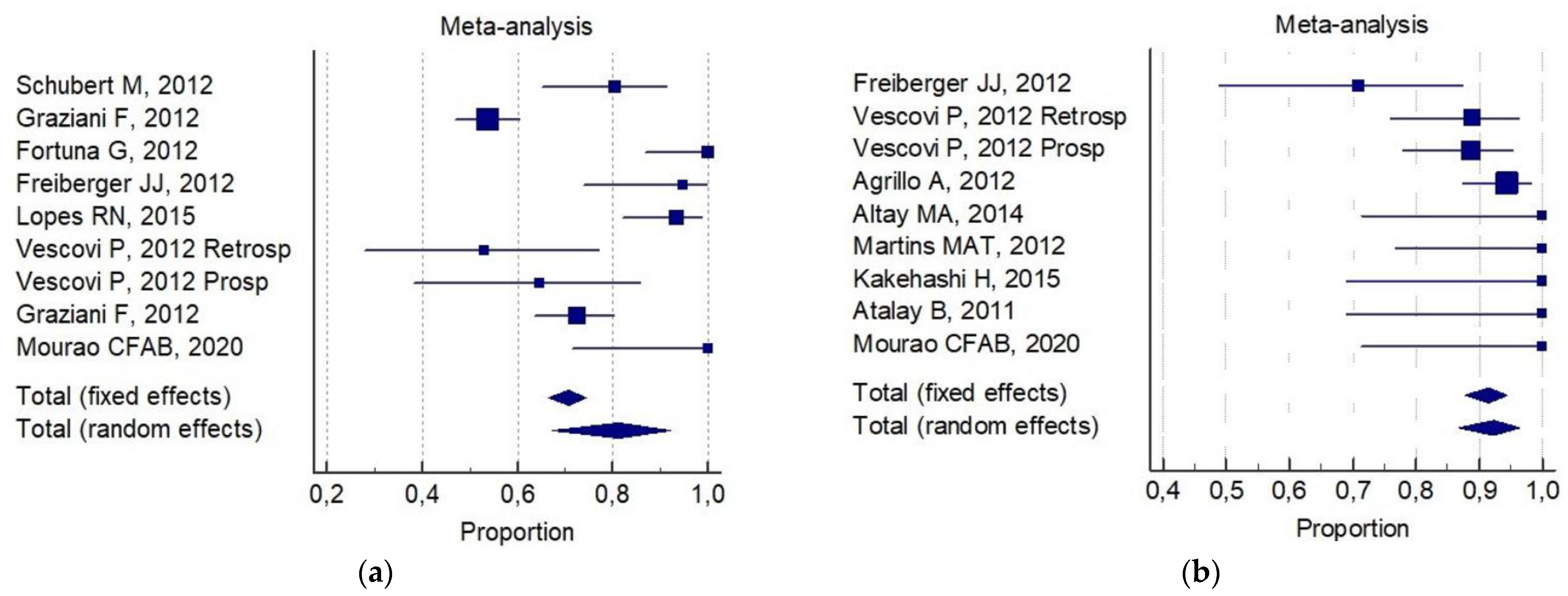
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campisi, G.; Bedogni, A.; Fusco, V. Raccomandazioni Clinico-Terapeutiche Sull’osteonecrosi Delle Ossa Mascellari (ONJ) Farmaco-Relata e Sua Prevenzione; NDF, S., Ed.; Palermo University Press: Palermo, Italy, 2020. [Google Scholar]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Sawase, T. Medication-Related Osteonecrosis of the Jaw: A Literature Review. J. Oral Biosciences. Jpn. Assoc. Oral Biology 2019, 61, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Schiødt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Wyngaert, T.V.D. Medication-related osteonecrosis of the jaw: Definition and best practice for prevention, diagnosis, and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef]
- Campisi, G.; Fedele, S.; Fusco, V.; Pizzo, G.; di Fede, O.; Bedogni, A. Epidemiology, clinical manifestations, risk reduction and treatment strategies of jaw osteonecrosis in cancer patients exposed to antiresorptive agents. Futur. Oncol. 2014, 10, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Herlofson, B.B.; Ristow, O.; Kofod, T. Workshop of European task force on medication-related osteonecrosis of the jaw—Current challenges. Oral Dis. 2019, 25, 1815–1821. [Google Scholar] [CrossRef]
- Gómez-Moreno, G.; Arribas-Fernández, M.C.; Fernández-Guerrero, M.; Castro, A.B.; Aguilar-Salvatierra, A.; Guardia, J.; Botticelli, D.; Calvo-Guirado, J.L. Bisphosphonate-associated osteonecrosis of the jaw 2 years after teeth extractions: A case report solved with non-invasive treatment. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1391–1397. [Google Scholar]
- Moretti, F.; Pelliccioni, G.A.; Montebugnoli, L.; Marchetti, C. A prospective clinical trial for assessing the efficacy of a minimally invasive protocol in patients with bisphosphonate-associated osteonecrosis of the jaws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Saia, G.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Ferronato, G.; Nocini, P.F.; Blandamura, S. Long-term outcomes of surgical resection of the jaws in cancer patients with bisphosphonate-related osteonecrosis. Oral Oncol. 2011, 47, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2017, 10, CD012432. [Google Scholar] [CrossRef]
- Laimer, J.; Steinmassl, O.; Hechenberger, M.; Rasse, M.; Pikula, R.; Bruckmoser, E. Intraoral Vacuum-Assisted Closure Therapy—A Pilot Study in Medication-Related Osteonecrosis of the Jaw. J. Oral Maxillofac. Surg. 2017, 75, 2154–2161. [Google Scholar] [CrossRef]
- Owosho, A.A.; Estilo, C.L.; Huryn, J.M.; Yom, S.K. Pentoxifylline and tocopherol in the management of cancer patients with medication-related osteonecrosis of the jaw: An observational retrospective study of initial case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 455–459. [Google Scholar] [CrossRef]
- Magremanne, M.; Reychler, H. Pentoxifylline and Tocopherol in the Treatment of Yearly Zoledronic Acid–Related Osteonecrosis of the Jaw in a Corticosteroid-Induced Osteoporosis. J. Oral Maxillofac. Surg. 2014, 72, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, G.; Amosso, E.; Scarpella, R.; Carini, F. Doxycycline fluorescence-guided Er:YAG laser ablation combined with Nd:YAG/diode laser biostimulation for treating bisphosphonate-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Luomanen, M.; Alaluusua, S. Treatment of bisphosphonate-induced osteonecrosis of the jaws with Nd:YAG laser biostimulation. Lasers Med. Sci. 2011, 27, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Heggendorn, F.L.; Leite, T.C.; Cunha, K.S.G.; Júnior, A.S.; Gonçalves, L.S.; Da Costa, K.B.F.F.; Dias, E.P. Bisphosphonate-related osteonecrosis of the jaws: Report of a case using conservative protocol. Spéc. Care Dent. 2016, 36, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yoshiga, D.; Yamashita, Y.; Nakamichi, I.; Tanaka, T.; Yamauchi, K.; Yamamoto, N.; Nogami, S.; Kaneuji, T.; Mitsugi, S.; Sakurai, T.; et al. Weekly teriparatide injections successfully treated advanced bisphosphonate-related osteonecrosis of the jaws. Osteoporos. Int. 2013, 24, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, Y.; Miyake, M.; Sawai, F.; Minami, Y.; Iwasaki, A.; Matsui, Y. Adjunct teriparatide therapy with monitoring of bone turnover markers and bone scintigraphy for bisphosphonate-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e31–e37. [Google Scholar] [CrossRef]
- Yamachika, E.; Matsubara, M.; Ikeda, A.; Matsumura, T.; Moritani, N.; Iida, S. Treatment of Osteonecrosis of the Jaw. J. Craniofacial Surg. 2015, 26, e575–e577. [Google Scholar] [CrossRef] [PubMed]
- Thumbigere-Math, V.; Michalowicz, B.S.; Hodges, J.S.; Tsai, M.L.; Swenson, K.K.; Rockwell, L.; Gopalakrishnan, R. Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J. Periodontol. 2014, 85, 226–233. [Google Scholar] [CrossRef]
- Mauceri, R.; Panzarella, V.; Maniscalco, L.; Bedogni, A.; Licata, M.E.; Albanese, A.; Toia, F.; Cumbo, E.M.G.; Mazzola, G.; Di Fede, O.; et al. Conservative Surgical Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw with Er,Cr:YSGG Laser and Platelet-Rich Plasma: A Longitudinal Study. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Min. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, S.; Troeltzsch, M.; Hafner, S.; Kaeppler, G.; Mast, G.; Otto, S. Surgical treatment of medication-related osteonecrosis of the upper jaw: Case series. Oral Dis. 2019, 25, 497–507. [Google Scholar] [CrossRef]
- Schiodt, M.; Vadhan-Raj, S.; Chambers, M.S.; Nicolatou-Galitis, O.; Politis, C.; Coropciuc, R.; Fedele, S.; Jandial, D.; Zhang, J.; Ma, H.; et al. A multicenter case registry study on medication-related osteonecrosis of the jaw in patients with advanced cancer. Support. Care Cancer 2018, 26, 1905–1915. [Google Scholar] [CrossRef]
- Ristow, O.; Rückschloß, T.; Müller, M.; Berger, M.; Kargus, S.; Pautke, C.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Cranio-Maxillofacial Surg. 2019, 47, 491–499. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons Position Paper on Bisphosphonate-Related Osteonecrosis of the Jaws—2009 Update. J. Oral Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Mozzati, M.; Gallesio, G.; Arata, V.; Pol, R.; Scoletta, M. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: A report of 32 cases. Oral Oncol. 2012, 48, 469–474. [Google Scholar] [CrossRef]
- Bocanegra-Pérez, S.; Vicente-Barrero, M.; Knezevic, M.; Castellano-Navarro, J.; Bocanegra, E.R.; Rodríguez-Millares, J.; Pérez-Plasencia, D.; Ramos-Macías, A. Use of platelet-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Int. J. Oral Maxillofac. Surg. 2012, 41, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, J.-W.; Kim, S.-J. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J. Oral Maxillofac. Surg. 2017, 75, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Begoña, L.; Orive, G. Treatment of hemimandibular paresthesia in a patient with bisphosphonate-related osteonecrosis of the jaw (BRONJ) by combining surgical resection and PRGF-Endoret. Br. J. Oral Maxillofac. Surg. 2013, 51, e272–e274. [Google Scholar] [CrossRef]
- Pelaz, A.; Junquera, L.; Gallego, L.; Garcia-Consuegra, L.; Gomez, C. Alternative treatments for oral bisphosphonate-related osteonecrosis of the jaws: A pilot study comparing fibrin rich in growth factors and teriparatide. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e320–e326. [Google Scholar] [CrossRef] [PubMed]
- Dincă, O.; Zurac, S.; Stăniceanu, F.; Bucur, M.B.; Bodnar, D.C.; Vlădan, C.; Bucur, A. Clinical and histopathological studies using fibrin-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2014, 55, 961–964. [Google Scholar]
- Nørholt, S.; Hartlev, J. Surgical treatment of osteonecrosis of the jaw with the use of platelet-rich fibrin: A prospective study of 15 patients. Int. J. Oral Maxillofac. Surg. 2016, 45, 1256–1260. [Google Scholar] [CrossRef]
- Soydan, S.S.; Uckan, S. Management of Bisphosphonate-Related Osteonecrosis of the Jaw With a Platelet-Rich Fibrin Membrane: Technical Report. J. Oral Maxillofac. Surg. 2014, 72, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-L.; Huang, Y.-F.; Chang, Y.-C. Treatment of bisphosphonate-related osteonecrosis of the jaw with platelet-rich fibrin. J. Formos. Med. Assoc. 2016, 115, 585–586. [Google Scholar] [CrossRef]
- Maluf, G.; de Pinho, M.C.; da Cunha SR de, B.; Santos PS da, S.; Fregnani, E.R. Surgery combined with lprf in denosumab osteonecrosis of the jaw: Case report. Braz. Dent. J. 2016, 27, 353–358. [Google Scholar] [CrossRef]
- Gönen, Z.B.; Asan, C.Y. Treatment of bisphosphonate-related osteonecrosis of the jaw using platelet-rich fibrin. CRANIO 2016, 35, 332–336. [Google Scholar] [CrossRef]
- de Castro, M.S.; Ribeiro, N.V.; de Carli, M.L.; Pereira, A.A.C.; Sperandio, F.F.; Hanemann, J.A.C. Photodynamically dealing with bisphosphonate-related osteonecrosis of the jaw: Successful case reports. Photodiagnosis Photodyn Ther. 2016, 16, 72–75. [Google Scholar] [CrossRef]
- Lee, J.-J.; Cheng, S.-J.; Jeng, J.-H.; Chiang, C.-P.; Lau, H.-P.; Kok, S.-H. Successful treatment of advanced bisphosphonate-related osteonecrosis of the mandible with adjunctive teriparatide therapy. Head Neck 2010, 33, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yoo, H.-Y.; Kim, G.-T.; Lee, J.-W.; Lee, Y.-A.; Kim, D.-Y.; Kwon, Y.-D. Short-Term Teriparatide and Recombinant Human Bone Morphogenetic Protein-2 for Regenerative Approach to Medication-Related Osteonecrosis of the Jaw: A Preliminary Study. J. Bone Miner. Res. 2017, 32, 2445–2452. [Google Scholar] [CrossRef]
- Gonzálvez-García, M.; Rodríguez-Lozano, F.J.; Villanueva, V.; Segarra-Fenoll, D.; Rodriguez-Gonzalez, M.A.; Oñate-Sánchez, R.; Blanquer, M.; Moraleda, J.M. Cell Therapy in Bisphosphonate-Related Osteonecrosis of the Jaw. J. Craniofacial Surg. 2013, 24, e226–e228. [Google Scholar] [CrossRef]
- Da Guarda, M.G.; Paraguassú, G.M.; Cerqueira, N.S.; Cury, P.R.; Farias, J.G.; Ramalho, L.M.P. Laser GaAlAs (λ860nm) photobiomodulation for the treatment of bisphosphonate-induced osteonecrosis of the jaw. Photomed. Laser Surg. 2012, 30, 293–297. [Google Scholar] [CrossRef]
- Altay, M.A.; Tasar, F.; Tosun, E.; Kan, B. Low-Level Laser Therapy Supported Surgical Treatment of Bisphosphonate Related Osteonecrosis of Jaws: A Retrospective Analysis of 11 Cases. Photomed. Laser Surg. 2014, 32, 468–475. [Google Scholar] [CrossRef]
- Atalay, B.; Yalcin, S.; Emes, Y.; Aktas, I.; Aybar, B.; Issever, H.; Mandel, N.M.; Cetin, O.; Oncu, B. Bisphosphonate-related osteonecrosis: Laser-assisted surgical treatment or conventional surgery? Lasers Med. Sci. 2011, 26, 815–823. [Google Scholar] [CrossRef]
- Vescovi, P.; Manfredi, M.; Merigo, E.; Guidotti, R.; Meleti, M.; Pedrazzi, G.; Fornaini, C.; Bonanini, M.; Ferri, T.; Nammour, S. Early Surgical Laser-Assisted Management of Bisphosphonate-Related Osteonecrosis of the Jaws (BRONJ): A Retrospective Analysis of 101 Treated Sites with Long-Term Follow-Up. Photomed. Laser Surg. 2012, 30, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; Merigo, E.; Meleti, M.; Manfredi, M.; Guidotti, R.; Nammour, S. Bisphosphonates-related osteonecrosis of the jaws: A concise review of the literature and a report of a single-centre experience with 151 patients. J. Oral Pathol. Med. 2011, 41, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; Giovannacci, I.; Otto, S.; Manfredi, M.; Merigo, E.; Fornaini, C.; Nammour, S.; Meleti, M. Medication-Related Osteonecrosis of the Jaw: An Autofluorescence-Guided Surgical Approach Performed with Er:YAG Laser. Photomed. Laser Surg. 2015, 33, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.T.; Martins, M.D.; LaScala, C.A.; Curi, M.M.; Migliorati, C.A.; Tenis, C.A.; Marques, M. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: A preliminary study. Oral Oncol. 2012, 48, 79–84. [Google Scholar] [CrossRef]
- Agrillo, A.; Filiaci, F.; Ramieri, V.; Riccardi, E.; Quarato, D.; Rinna, C.; Gennaro, P.; Cascino, F.; Mitro, V.; Ungari, C. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): 5 year experience in the treatment of 131 cases with ozone therapy. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1741–1747. [Google Scholar]
- Al-Zoman, K.H.; Albazie, S.; Robert, A.A.; Baskaradoss, J.K.; Alsuwyed, A.S.; Ciancio, S.; Al-Mubarak, S. Surgical management of bisphosphonate-related osteonecrosis of the jaw: Report of three cases. J. Palliat. Care 2013, 29, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Fatema, C.N.; Sato, J.; Yamazaki, Y.; Hata, H.; Hattori, N.; Shiga, T.; Tamaki, N.; Kitagawa, Y. FDG-PET may predict the effectiveness of hyperbaric oxygen therapy in a patient with bisphosphonate-related osteonecrosis of the jaw: Report of a case. Odontology 2013, 103, 105–108. [Google Scholar] [CrossRef]
- Freiberger, J.J.; Padilla-Burgos, R.; McGraw, T.; Suliman, H.B.; Kraft, K.H.; Stolp, B.W.; Moon, R.E.; Piantadosi, C.A. What Is the Role of Hyperbaric Oxygen in the Management of Bisphosphonate-Related Osteonecrosis of the Jaw: A Randomized Controlled Trial of Hyperbaric Oxygen as an Adjunct to Surgery and Antibiotics. J. Oral Maxillofac. Surg. 2012, 70, 1573–1583. [Google Scholar] [CrossRef]
- Ripamonti, C.; Maniezzo, M.; Boldini, S.; Pessi, M.; Mariani, L.; Cislaghi, E. Efficacy and tolerability of medical ozone gas insufflations in patients with osteonecrosis of the jaw treated with bisphosphonates—Preliminary data: Medical ozone gas insufflation in treating ONJ lesions. J. Bone Oncol. 2012, 1, 81–87. [Google Scholar] [CrossRef]
- Brozoski, M.A.; Traina, A.A.; Deboni, M.C.Z.; Marques, M.M.; Naclério-Homem M da, G. Bisphosphonate-related osteonecrosis of the jaw. Rev. Bras. Reumatol. 2020, 52, 265–270. [Google Scholar] [CrossRef]
- Kwon, Y.-D.; Lee, D.-W.; Choi, B.-J.; Lee, J.-W.; Kim, D.-Y. Short-term teriparatide therapy as an adjunctive modality for bisphosphonate-related osteonecrosis of the jaws. Osteoporos. Int. 2012, 23, 2721–2725. [Google Scholar] [CrossRef]
- Doh, R.-M.; Park, H.-J.; Rhee, Y.; Kim, H.S.; Huh, J.; Park, W. Teriparatide Therapy for Bisphosphonate-Related Osteonecrosis of the Jaw Associated With Dental Implants. Implant. Dent. 2015, 24, 222–226. [Google Scholar] [CrossRef]
- Kakehashi, H.; Ando, T.; Minamizato, T.; Nakatani, Y.; Kawasaki, T.; Ikeda, H.; Kuroshima, S.; Kawakami, A.; Asahina, I. Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: Preliminary findings. Int. J. Oral Maxillofac. Surg. 2015, 44, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Rahim, I.; Salt, S.; Heliotis, M. Successful long-term mandibular reconstruction and rehabilitation using non-vascularised autologous bone graft and recombinant human BMP-7 with subsequent endosseous implant in a patient with bisphosphonate-related osteonecrosis of the jaw. Br. J. Oral Maxillofac. Surg. 2015, 53, 870–874. [Google Scholar] [CrossRef]
- Montebugnoli, L.; Felicetti, L.; Gissi, D.B.; Pizzigallo, A.; Pelliccioni, G.A.; Marchetti, C. Biphosphonate-associated osteonecrosis can be controlled by nonsurgical management. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, 473–477. [Google Scholar] [CrossRef] [PubMed]
- de Souza Póvoa, R.C.; Marlierè, D.A.A.; da Silveira, H.M.; Pires, F.R. Denosumab-related osteonecrosis of the jaws: Successful management with a conservative surgical approach. Spec. Care Dent. 2016, 36, 231–236. [Google Scholar] [CrossRef]
- Ribeiro, N.R.B.; Silva, L.D.F.; Santana, D.M.; Nogueira, R.L.M. Bisphosphonate-Related Osteonecrosis of the Jaw After Tooth Extraction. J. Craniofacial Surg. 2015, 26, e606–e608. [Google Scholar] [CrossRef]
- de Souza Faloni, A.P.; Queiroz, T.P.; Lia RC, C.; Cerri, P.S.; Margonar, R.; de Souza Rastelli, A.N.; Marcantonio, E. Accurate approach in the treatment of oral bisphosphonate-related jaw osteonecrosis. J. Craniofac. Surg. 2011, 22, 2185–2190. [Google Scholar] [CrossRef]
- Lee, L.-W.; Hsiao, S.-H.; Chen, L.-K. Clinical treatment outcomes for 40 patients with bisphosphonates-related osteonecrosis of the jaws. J. Formos. Med. Assoc. 2014, 113, 166–172. [Google Scholar] [CrossRef]
- Graziani, F.; Vescovi, P.; Campisi, G.; Favia, G.; Gabriele, M.; Gaeta, G.M.; Gennai, S.; Goia, F.; Miccoli, M.; Peluso, F.; et al. Resective Surgical Approach Shows a High Performance in the Management of Advanced Cases of Bisphosphonate-Related Osteonecrosis of the Jaws: A Retrospective Survey of 347 Cases. J. Oral Maxillofac. Surg. 2012, 70, 2501–2507. [Google Scholar] [CrossRef]
- Duarte, L.F.M.; Alonso, K.; Basso, E.C.; Dib, L.L. Surgical Treatment of Bisphosphonate-Related Osteonecrosis of the Jaws with the Use of Buccal Fat Pad: Case Report. Braz. Dent. J. 2015, 26, 317–320. [Google Scholar] [CrossRef][Green Version]
- Berrone, M.; Florindi, F.U.; Carbone, V.; Aldiano, C.; Pentenero, M. Stage 3 Medication-Related Osteonecrosis of the Posterior Maxilla: Surgical Treatment Using a Pedicled Buccal Fat Pad Flap: Case Reports. J. Oral Maxillofac. Surg. 2015, 73, 2082–2086. [Google Scholar] [CrossRef]
- Lopes, R.N.; Rabelo, G.; Rocha, A.C.; Carvalho, P.A.G.; Alves, F.A. Surgical Therapy for Bisphosphonate-Related Osteonecrosis of the Jaw: Six-Year Experience of a Single Institution. J. Oral Maxillofac. Surg. 2015, 73, 1288–1295. [Google Scholar] [CrossRef]
- Hewson, I.; Syme, D.; Bruscino-Raiola, F. Radical surgical treatment of bisphosphonate related osteonecrosis of the jaw. Aust. Dent. J. 2012, 57, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, N.; Collyer, J.; Tighe, J. Hemimandibulectomy and vascularized fibula flap in bisphosphonate-induced mandibular osteonecrosis with polycythaemia rubra vera. Int. J. Oral Maxillofac. Surg. 2013, 42, 120–123. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Militsakh, O.; Richmon, J.D.; Rosenthal, E.L.; Wax, M.K. Mandibulectomy and Free Flap Reconstruction for Bisphosphonate-Related Osteonecrosis of the Jaws. JAMA Otolaryngol. Neck Surg. 2013, 139, 1135–1142. [Google Scholar] [CrossRef]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO clinical practice guideline. J. Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Wilde, F.; Heufelder, M.; Winter, K.; Hendricks, J.; Frerich, B.; Schramm, A.; Hemprich, A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 111, 153–163. [Google Scholar] [CrossRef]
- Carlson, E.R.; Basile, J.D. The Role of Surgical Resection in the Management of Bisphosphonate-Related Osteonecrosis of the Jaws. J. Oral Maxillofac. Surg. 2009, 67, 85–95. [Google Scholar] [CrossRef]
- Shintani, T.; Hayashido, Y.; Mukasa, H.; Akagi, E.; Hoshino, M.; Ishida, Y.; Hamana, T.; Okamoto, K.; Kanda, T.; Koizumi, K.; et al. Comparison of the prognosis of bisphosphonate-related osteonecrosis of the jaw caused by oral and intravenous bisphosphonates. Int. J. Oral Maxillofac. Surg. 2015, 44, 840–844. [Google Scholar] [CrossRef]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, e62. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Mourão, C.F.D.A.B.; Calasans-Maia, M.D.; Del Fabbro, M.; Vieira, F.L.D.; Machado, R.; Capella, R.; Miron, R.J.; Alves, G.G. The use of Platelet-rich Fibrin in the management of medication-related osteonecrosis of the jaw: A case series. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 84–89. [Google Scholar] [CrossRef]
- Antonelli, A.; Giudice, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar] [CrossRef]
- Schubert, M.; Klatte, I.; Linek, W.; Müller, B.; Döring, K.; Eckelt, U.; Hemprich, A.; Berger, U.; Hendricks, J. The Saxon Bisphosphonate Register–Therapy and prevention of bisphosphonate-related osteonecrosis of the jaws. Oral Oncol. 2012, 48, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pechalova, P.; Bakardjiev, A.; Zaprianov, Z.; Vladimirov, B.; Poriazova, E.; Zheleva, A.; Hadjigeorgiev, G.; Goranova-Marinova, V.; Goranov, S. Bisphosphonate-associated osteonecrosis of the jaws-report of three cases in bulgaria and review of the literature. Acta Clin. Croatica. 2011, 50, 273–279. [Google Scholar]
- Gallego, L.; Junquera, L.; Pelaz, A.; Hernando, J.; Megias, J. The use of pedicled buccal fat pad combined with sequestrectomy in bisphosphonate-related osteonecrosis of the maxilla. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e236–e241. [Google Scholar] [CrossRef]
- Fortuna, G.; Ruoppo, E.; Pollio, A.; Aria, M.; Adamo, D.; Leuci, S.; Orabona, G.D.; Mignogna, M.D. Multiple myeloma vs. breast cancer patients with bisphosphonates-related osteonecrosis of the jaws: A comparative analysis of response to treatment and predictors of outcome. J. Oral Pathol. Med. 2011, 41, 222–228. [Google Scholar] [CrossRef]
- De Santis, G.C.; Macedo, L.; Orellana, M.D.; Innocentini, L.M.A.R.; Ferrari, T.C.; Ricz, H.M.A.; Caruso, S.R.; Fernandes, T.R.; Covas, D.T. Mesenchymal stromal cells administration for osteonecrosis of the jaw caused by bisphosphonate: Report of two cases. Acta Oncol. 2020, 59, 789–792. [Google Scholar] [CrossRef]
- Bouland, C.; Meuleman, N.; Widelec, J.; Keiani-Mothlagh, K.; Voisin, C.; Lagneaux, L.; Philippart, P. Case reports of medication-related osteonecrosis of the jaw (MRONJ) treated with uncultured stromal vascular fraction and L-PRF. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, S.; Denaro, M.; Cosola, S.; Gabriele, M.; Toti, P.; Mijiritsky, E.; Proietti, A.; Basolo, F.; Giammarinaro, E.; Covani, U. Myofibroblast Gene Expression Profile after Tooth Extraction in the Rabbit. Materials 2019, 12, 3697. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, S.; Giammarinaro, E.; Cosola, S.; Genovesi, A.M.; Covani, U. Mandibular Osteonecrosis Associated with Antacid Therapy (Esomeprazole). Eur. J. Case Rep. Intern. Med. 2019, 6. [Google Scholar] [CrossRef]
- Bedogni, A.; Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br. J. Oral Maxillofac. Surg. 2014, 52, 603–608. [Google Scholar] [CrossRef]
| “Osteonecrosis”[Mesh] AND “Jaw Diseases”[Mesh] |
▪ AND (“Conservative Treatment”[Mesh] |
○ OR “Drug Therapy”[Mesh] |
○ OR “Therapeutics”[Mesh] |
○ OR “therapy” [Subheading] |
○ OR “Surgical Procedures, Operative”[Mesh] |
○ OR “drug therapy” [Subheading]) |
▪ AND “Ozone”[Mesh] |
▪ AND “Teriparatide”[Mesh] |
▪ AND (“Laser Therapy”[Mesh] |
○ OR “Low-Level Light Therapy”[Mesh]) |
▪ AND “Pentoxifylline”[Mesh] |
▪ AND “Hyperbaric Oxygenation”[Mesh] |
▪ AND “Tocopherols”[Mesh] |
▪ AND “Platelet-Rich Plasma”[Mesh] |
▪ AND “Bone Morphogenetic Proteins”[Mesh] |
▪ AND “Parathyroid Hormone”[Mesh]) |
| Treatment | Study | Study Type | Pts | Intervention | Outcome | Follow-Up |
|---|---|---|---|---|---|---|
| Conservative Surgery | De Souza Povoa et al., 2016 | Case report | N = 1 Onc Stage 1 | Removal of the exposed necrotic bone and primary wound closure | Complete healing and new bone formation in the surgical site | 26 months |
| Ribeiro et al., 2015 | Case report | N = 1 Ost Stage unspecified | Surgical removal of whole necrotic bone, extraction of all compromised teeth | Complete healing | 12 months | |
| De Souza Faloni et al., 2011 | Case report | N = 1 Ost Stage 2 | Conservative debridement of the necrotic bone and of part of the surrounding healthy bone, as a margin of safety | Complete healing | 8 months | |
| Pechalova et al., 2011 | Case series | N = 3 Onc Stage unspecified | Conservative surgical debridement | Complete healing | Average of 4 months | |
| Martins et al., 2012 | Retrospective clinical study | N = 5 Onc Stage 1,2 | Sequestrectomy and/or ostectomy and/or osteoplasty until bone marrow bleeding | 60% patients completely healed | 6 months | |
| Jung et al., 2017 | Case series | N = 7 Ost Stage 2,3 | Patient underwent conventional surgery, and the bone defects were filled with absorbable collagen plugs. | Complete healing and new bone formation in the surgical site | 3 months | |
| Atalay et al., 2011 | Retrospective clinical study | N = 10 Onc Stage | The affected bony tissues were curetted from the surface of the bone using bone curettes and round tungsten carbide burs. The necrotic bone was completely removed until the vital bone tissues and vessel spots appeared | 40% patients completely healed | 6 months | |
| Vescovi et al., 2012 | Retrospective clinical study | N = 17 Onc + Ost Stage 1,2,3 | Conservative surgical treatments consisted of sequestrectomy of necrotic bone, superficial debridement/curettage, or corticotomy/surgical removal of alveolar and/or cortical bone | 53% patients completely healed | 9 months | |
| Vescovi et al., 2011 | Prospective clinical study | N = 17 Onc + Ost Stage 1,2,3 | Conservative surgical treatments included sequestrectomies, superficial debridement/curettage and corticotomies/surgical removal of surrounding alveolar and/or cortical bone | 65% patients completely healed | 12 months | |
| Freiberger et al., 20125 | Randomized control trial | N = 19 Onc + Ost Stage 1,2,3 | Surgical debridement of the necrotic bone | 33% patients completely healed | 24 months | |
| Fortuna et al., 2012 | Single-center prospective open-label clinical trial | N = 26 Onc Stage 2,3 | Systemic and topical antibiotic therapy following by sequestrectomy | 73% patients completely healed | Average of 10 months | |
| Lee et al., 2014 | Case series | N = 30 Ost + Onc Stage 1,2,3 | Minor surgical debridement was performed after irrigation, in which the necrotic bone fragments were removed | Complete healing | Average of 16 months | |
| Schubert et al., 2012 | Prospective study | N = 54 Onc + Ost Stage 1,2,3 | Complete electrical or manual removal of the osteonecrosis until points of bleeding from the bone can be macroscopically detected. | 88.8% patients completely healed | 6 months (72%) | |
| Graziani et al., 2012 | Retrospective cohort multicenter study | N = 227 Ost + Onc Stage 1,2,3 | Local debridement was comprised of all surgical interventions, such as sequestrectomy, soft tissue debridement and curettage, that did not require bone surgery beyond the regular margins | 49% patients completely healed | 6 months | |
| Conservative Surgery with Buccal Fat Pad Closure | Duarte et al., 2015 | Case report | N = 1 Onc Stage 2 | The extensive necrotic bone area was surgically removed, resulting in oral sinus communication. A buccal fat pad was used to cover the defect | Complete healing | 3 months |
| Gallego et al., 2012 | Case series | N = 3 Onc + Ost Stage 1,2,3 | Sequestrectomy and bone debridement. The overlying mucosa was sutured over the defect with reconstruction with buccal fat pad. | Complete healing | Average of 12 months | |
| Berrone et al., 2015 | Case series | N = 5 Onc Stage 3 | Removal of the necrotic bone and primary closure of the oroantral communication using a buccal fat pad flap. | Complete healing | Average of 12 months | |
| Lopes et al., 2015 | Retrospective observational cohort study | N = 46 Onc + Ost Stage 2,3 | Removal of all necrotic bone until bleeding was obtaining at the bony margins, conscious smoothing of all sharp bone edges and primary closure of the wound. | 87% patients completely healed | 10 months | |
| Hayashida et al., 2017 | Multicenter retrospective study | N = 38 Onc + Ost Stage 1,2,3 | One group received conservative surgery, removal of only the necrotic bone and extensive surgery, defined as removal of the necrotic and surrounding bone (marginal mandibulectomy or partial maxillectomy). | 76.7% patients completely healed | Average of 15 months | |
| Aggressive Surgery | Hewson et al., 2012 | Case report | N = 1 Onc Stage 3 | Radical surgical excision of all diseased bone and nasio-labial flap reconstruction. | Complete healing | 6 months |
| Ghazali et al., 2013 | Case report | N = 1 Ost Stage 3 | Hemimandibulectomy and an osteocutaneous fibula flap reconstruction | Complete healing | 24 months | |
| Shintani et al., 2015 | Cohort study | N = 4 Ost + Onc Stage 1,2,3 | Segmental resection and immediate reconstruction with a reconstruction plate were performed. | 3/4 patients completely healed | 12 months | |
| Lee et al., 2014 | Case report | N = 10 Ost + Onc Stage 1,2,3 | Large necrotic bone segment was removed by an ultrasonic bone saw. A bone file or rongeur was used for rounding the sharp bone edge. Then, the bone defect was closed by sutures or COE pack. | Complete healing | Average of 8 months | |
| Hanasono et al., 2013 | Case series | N = 13 Onc Stage2, 3 | Segmental mandibulectomy and microvascular free flap reconstruction. | Complete healing | Average of 15 months | |
| Graziani et al., 2012 | Retrospective cohort multicenter study | N = 120 Ost + Onc Stage 1,2,3 | Re-sective procedures were defined as corticotomy, surgical removal of the lesion and extended bone removal without prejudice for the continuity of the mandible/maxilla. | 68% patients completely healed | 6 months | |
| Hayashida et al., 2017 | Multicenter retrospective study | N = 121 Onc + Ost Stage 1,2,3 | Extensive surgery, defined as removal of the necrotic and surrounding bone (marginal mandibulectomy or partial maxillectomy). | 86.8% patients completely healed | Average of 15 months |
| Study | Study Type | Population | Intervention | Outcome | Follow-Up | |
|---|---|---|---|---|---|---|
| Conservative surgery plus (+) non-invasive procedures | ||||||
| 1. Surgery + Blood Component | Gönen et al., 2017 | Case report | N = 1 Onc Stage 3 | Sequestrectomy + PRF | Complete resolution | 18 months |
| Soydan et al., 2014 | Case report | N = 1 Onc Stage unspecified | Curettage + PRF | Complete resolution | 6 months | |
| Maluf et al., 2016 | Case series | N = 2 Onc Stage 2 | Resection of the necrotic tissues, curettage and osteotomy + L-PRF | Partial healing | 6 months | |
| Dincă et al., 2014 | Retrospective clinical study | N = 10 Onc Stage 2 | Sequestrectomy or curettage + PRF | Complete resolution | 1 month | |
| Nørholt et al., 2016 | Prospective study | N = 15 Onc + Ost Stage 2,3 | Curettage + L-PRF | 93.3% patients completely healed | 20 months | |
| Anitua et al., 2013 | Case report | N = 1 Onc Stage unspecified | Curettage + PRGF | Complete resolution | 12 months | |
| Bocanegra-Pérez et al., 2012 | Prospective descriptive study | N = 8 Onc + Ost Stage 2 | Curettage + PRP | Complete resolution | 14 months | |
| Mozzati et al., 2012 | Retrospective clinical study | N = 32 Onc Stage 2 | Conservative surgery + PRFG | Complete resolution | From 48 to 50 months | |
| Tsai et al., 2016 | Case report | N = 1 Ost Stage 3 | Surgical debridement, sequestrectomy + PRF | Complete resolution | 10 months | |
| Pelaz et al., 2014 | Cohort study | N = 5 Ost Stage 3 | Sequestrectomy and curettage + PRF | Complete resolution | An average of 20 months | |
| Park et al., 2017 | Prospective study | N = 25 Onc + Ost Stage 1,2,3 | Conservative surgery + L-PRF | 36% patients completely healed | 4 months | |
| Fernando de Almeida Barros Mourao C et al., 2020 | Case series | N = 11 Ost Stage 2 | Surgical removal of necrotic bone + PRF membranes | Complete healing | 24 months | |
| Giudice A et al., 2020 | Case report | N = 1 Ost Stage 3 | Surgical removal of necrotic bone + PRF membranes | Complete healing | 60 months | |
| Bouland C et al., 2020 | Case report | N = 2 Ost + Onc Stage 2 and 3 | Surgical removal of necrotic bone + SVF and L-PRF membranes | Complete healing | 18 months | |
| 2. Surgery + Blood Component + Photodynamic Therapy | De Castro et al., 2016 | Case series | N = 2 Ost Stage 2,3 | Surgical debridement + PDT + PRF | Complete resolution | An average of 12 months |
| 3. Surgery + Blood Component + Bone Morphogenetic Protein | Park et al., 2017 | Prospective study | N = 30 Onc + Ost Stage 1,2,3 | Conservative surgery + combined L-PRF and recombinant human BMP-2 (rhBMP-2) | 60% patients completely healed | 4 months |
| 4. Surgery + Teriparatide | Lee et al., 2010 | Case report | N = 1 Ost Stage 2 | Sequestrectomy + teriparatide | Complete resolution | 6 months |
| 5. Surgery + Teriparatide + Bone Morphogenetic Protein | Jung et al., 2017 | Cohort study | N = 6 Ost Stage 2,3 | Conservative surgery and absorbable collagen plugs soaked by rhBMP-2 into the bone defect plus daily subcutaneous injection of 20 mg teriparatide for 1–4 months. | Complete resolution | 3 months |
| 6. Surgery + Bone Morphogenetic Protein | Jung et al., 2017 | Cohort study | N = 4 Ost Stage 2,3 | Conservative surgery and absorbable collagen plugs soaked by rhBMP-2 into the bone defect. | Complete resolution | 3 months |
| 7. Surgery + Blood Component + Autolugus Bone Marrow Stem Cells | Gonzálvez-García et al., 2013 | Case report | N = 1 Onc Stage 2 | Removal of the necrotic bone+ bone marrow stem cells + beta tricalcium phosphate + demineralized bone matrix + PRP | Complete resolution | 6 months |
| De Santis et al., 2020 | Case report | N = 2 Onc Stage 2 | Debridement of the exposed necrotic bone followed by bone marrow stem cells injection | Complete healing and new bone formation in the surgical site. | 13 months | |
| 8. Surgery + LLLT | Da Guarda et al., 2012 | Case report | N = 1 Onc Stage unspecified | GaAlAs diode laser every 48 h for 10 days + antibiotic therapy + curettage | Complete resolution | 6 months |
| 9. Surgery + Blood Component + Laser Phototherapy | Altay et al., 2014 | Retrospective clinical study | N = 11 Onc Stage2,3 | Pre- and post-operative antibiotic administrations + GaA-lAs diode laser | Complete resolution | 12 months |
| Atalay et al., 2011 | Retrospective clinical study | N = 10 Onc Stage 1,2 | Conservative surgery + low-level laser therapy application (Er:YAG and Nd:YAG) | 70% patients completely healed | 12 months | |
| Vescovi et al., 2012 | Retrospective clinical study | N = 45 Onc + Ost Stage 1,2,3 | Conservative surgery + laser Nd:YAG | 89% patients completely healed | 6 months | |
| Vescovi et al., 2011 | Prospective clinical study | N = 62 Onc + Ost Stage 1,2,3 | Conservative surgery + laser LLLT | 73% patients completely healed | 17 months. | |
| Martins et al., 2012 | Retrospective clinical study | N = 14 Onc Stage 1,2,3 | Conservative surgery + continuous indium-gallium-aluminum-phosphide diode laser. The LPT treatment started on the first visit and continued daily until mucosal healing was observed. | 86% patients completely healed | 12 months | |
| 10. Surgery + Ozone | Agrillo et al., 2012 | Retrospective study | N = 94 Onc + Ost Stage unspecified | Curettage or sequestrectomy + Ozone therapy (3 min sessions 2/week) + pharmacological therapy | 90% patients completely healed | An average of 6 months |
| 11. HBO + Surgery * | Fatema et al., 2013 | Case report | N = 1 Onc Stage 2 | Antibiotics therapy, irrigation, pre-operative HBO therapy for 20 sessions, conservative minor surgical debridement and again post-operative HBO therapy for ten sessions. | Complete resolution | Unspecified |
| Al-Zoman et al., 2013 | Case series | N = 3 Onc Stage2,3 | HBO therapy, oral/parenteral antibiotic, analgesics, conservative surgery (debridement of bone sequestra) and daily rinsing with chlorhexidine mouthwash. | Complete resolution | 12 months | |
| Freiberger et al., 2012 | Randomized control trial | N = 24 Onc + Ost Stage 1,2,3 | 40 HBO treatments at 2.0 atm for 2 h twice per day and conservative surgical debridement of the necrotic bone. | 52% patients completely healed | 24 months | |
| 12. Ozone + Surgery* | Ripamonti et al., 2012 | Case report | N = 1 Onc Stage unspecified | Antibiotic + antimycotic therapy for 10 days. Local ozone gas (total of 15 applications). Conservative surgery (sequestrectomy). | Complete resolution | 36 months |
| Brozoski et al., 2020 | Case series | N = 2 Onc + Ost Stage 2 | Weekly irrigation with aqueous ozone solution on bone-exposed region + daily mouthwashes of ozone solution. After 3 and 6 months: conservative surgery (debridement and sequestrectomy) | Complete resolution | An average of 24 months | |
| 13. Teriparatide + Surgery * | Doh et al., 2015 | Case report | N = 1 Ost Stage 2 | After 4 months of daily teriparatide therapy conservative surgery (sequestrectomy). The TPTD therapy was terminated 6 months after the initial treatment. | Complete resolution | 20 months |
| Kwon et al., 2012 | Case series | N = 6 Ost Stage 2,3 | Daily Teriparatide (20 μg/day) 1–3 months + conservative sequestrectomy/marginal/aggressive segmental resection | Complete resolution | 3 months | |
| Kakehashi et al., 2015 | Case series | N = 10 Ost Stage 2,3 | Daily teriparatide (20 μg/day) ranged from 4 to 24 months. In some cases, surgery was performed to obtain the healing. | Partial resolution | From 4 to 24 months (duration of teriparatide therapy until mucosal healing) | |
| Aggressive surgery plus non-invasive procedures | ||||||
| 1. Surgery + Bone Graft + Bone Morphogenetic Protein | Rahim I 2015 | Case report | N = 1 Ost Stage 3 | Partial mandibulectomy + bone graft from the iliac crest + rhBMP-7 | Complete resolution | 60 months |
| 2. AF-Guided Surgery + LLLT | Vescovi P 2015 | Case report | N = 1 Onc Stage 3 | Osteotomy with Er:YAG laser + AF visualization to guide the osteoplasty. Intraoral irrigations with povidone iodine solution + application of Nd:YAG laser + weekly applications of LLLT for 3 weeks after intervention | Complete resolution | 7 months |
| 6-Month Total Resolution Rate (a) | 6-Month Improvement Rate (b) | |
|---|---|---|
| Conservative surgery alone | 67% (IC 95%; 50–83%) | 82% (IC 95%, 65–95%) |
| Aggressive surgery alone | 93% (IC 95%; 82–99%) | 72% (IC 95%; 64–80%) |
| Conservative surgery plus non-invasive procedures | 75% (IC 95%; 60–87%) | 91% (IC95%; 87–96%) |
| Aggressive plus non-invasive procedures | not assessable | not assessable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fede, O.; Canepa, F.; Panzarella, V.; Mauceri, R.; Del Gaizo, C.; Bedogni, A.; Fusco, V.; Tozzo, P.; Pizzo, G.; Campisi, G.; et al. The Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols. Int. J. Environ. Res. Public Health 2021, 18, 8432. https://doi.org/10.3390/ijerph18168432
Di Fede O, Canepa F, Panzarella V, Mauceri R, Del Gaizo C, Bedogni A, Fusco V, Tozzo P, Pizzo G, Campisi G, et al. The Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols. International Journal of Environmental Research and Public Health. 2021; 18(16):8432. https://doi.org/10.3390/ijerph18168432
Chicago/Turabian StyleDi Fede, Olga, Federica Canepa, Vera Panzarella, Rodolfo Mauceri, Carmine Del Gaizo, Alberto Bedogni, Vittorio Fusco, Pietro Tozzo, Giuseppe Pizzo, Giuseppina Campisi, and et al. 2021. "The Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols" International Journal of Environmental Research and Public Health 18, no. 16: 8432. https://doi.org/10.3390/ijerph18168432
APA StyleDi Fede, O., Canepa, F., Panzarella, V., Mauceri, R., Del Gaizo, C., Bedogni, A., Fusco, V., Tozzo, P., Pizzo, G., Campisi, G., & Galvano, A. (2021). The Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols. International Journal of Environmental Research and Public Health, 18(16), 8432. https://doi.org/10.3390/ijerph18168432














