Understanding the Impact of Perfluorinated Compounds on Cardiovascular Diseases and Their Risk Factors: A Meta-Analysis Study
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Inclusion Criteria
- Publication Type. Research and review articles were eligible. Conference Proceedings was excluded.
- Typesof studies. Randomised controlled trials (RCTs), cohort and cross-sectional studies were eligible. A narrative review and systematic review were excluded.
- Types of participants. Studies of adults (older than 18 years), adolescents (aged 10–18), children (aged 2–9) and infants. Animal and plant were excluded.
- Types of interventions. Studies that compared PFCs exposure with healthy or non-healthy humans.A selection was made for the PFCs exposure only. No restrictions were made regarding concentrations, duration of exposure, type of CVDs and type of CVDs risks.
- Types of outcomes. Studies were eligible if they assessed either (1) comparing non-diseased and CVDs patients (2) comparing healthy and CVDs risk population (3) comparing healthy and metabolic syndrome population (4) ascertained the prevalence of one or more manifestation of CVDs or CVDs risks diagnosis (5) provided quantitative estimation on the association between PFCs exposure and CVDs/their risk outcomes, including odds ratios (ORs) with 95% confidence intervals (CIs) or mean, standard deviation (SD) or difference in mean and sample size in healthy and non-healthy population.
2.3. Data Extraction
2.4. Data Analysis
2.4.1. Assessment of Overall Effect Size
2.4.2. Assessment of Heterogeneity
2.4.3. Subgroup Analysis
2.4.4. Risk of Bias across Studies
3. Results
3.1. Analysis of Overall Effect and Heterogeneity
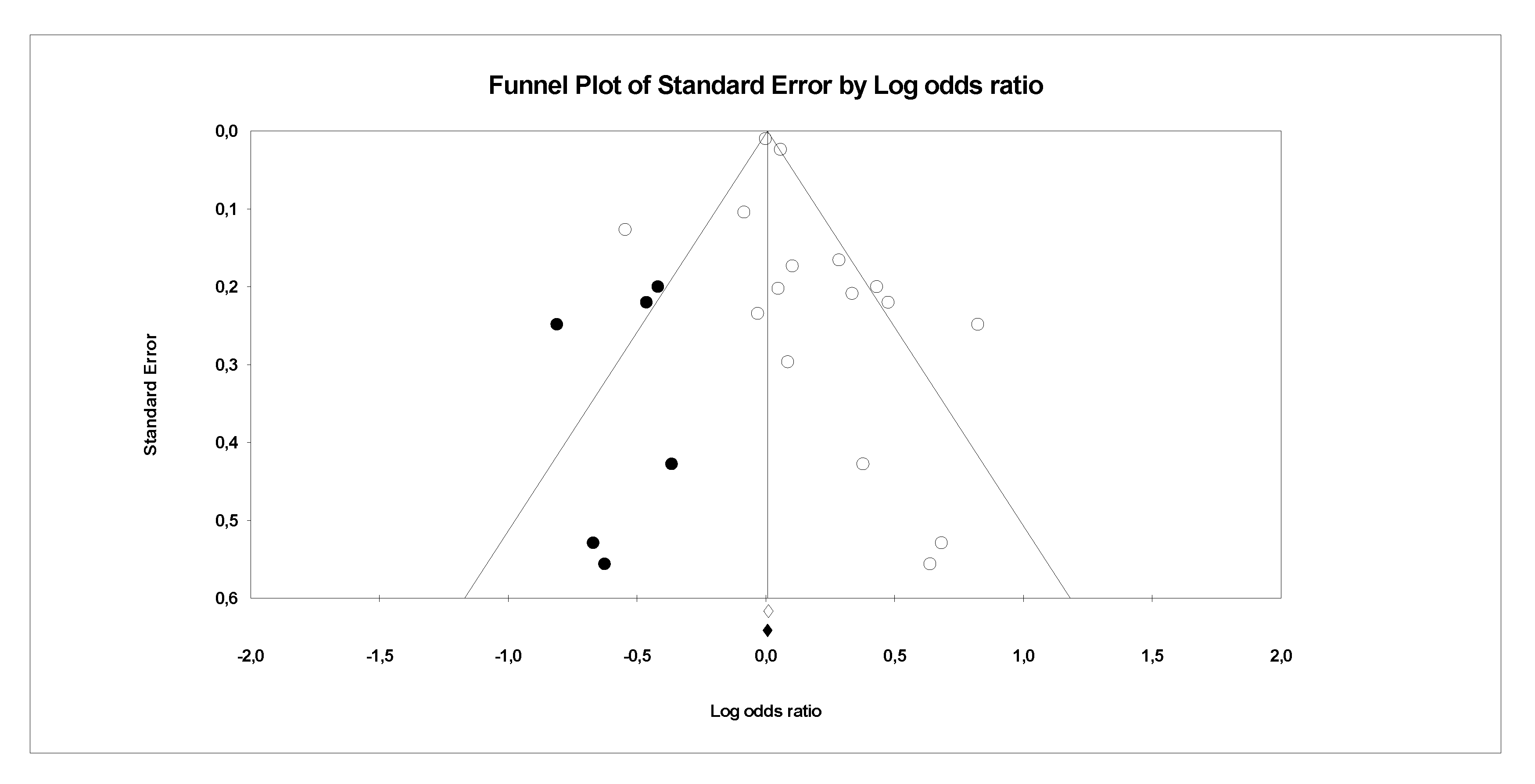
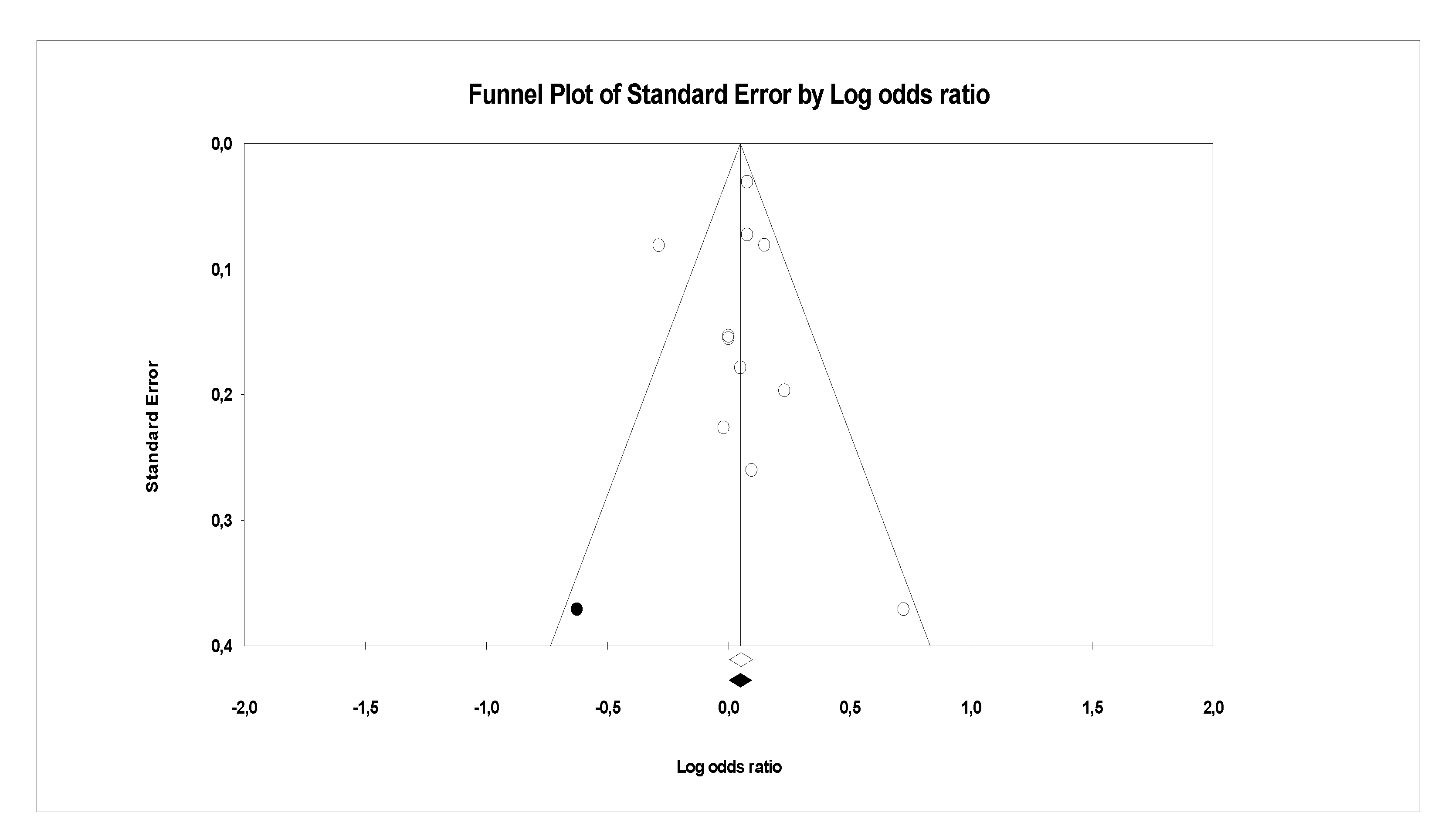
3.2. Analysis of Publication Bias
3.3. Association of Specific PFC with Cardiometabolic Diseases Based on Epidemiological Studies
3.4. Conflicting Data on the Association of Specific Type PFCs with CVDs from Epidemiological Studies
4. Discussion
Limitations
5. Conclusions
The Implication of the Key Findings
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctane sulfonic acid |
| PFDA | Perfluorodecanoic acid |
| PFDO | Perfluorododecanoic acid |
| PFHP | Perfluoroheptanoic acid |
| PFHxA | Perfluorohexanoic acid |
| PFHxS | Perfluorohexane sulfonic acid |
| PFNA | Perfluorononanoic acid |
| PFSA | Perfluorooctane sulfonamide |
| PFUA | Perfluoroundecanoic acid |
| PFBS | Perfluorobutane sulfonate |
| EPAH | 2-(N-ethyl-perfluorooctane sulfonamido) acetate |
| MPAH | 2-(N-methyl-perfluorooctane sulfonamido) actetate |
| FTOH | Fluorotolemer alcohols |
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Corsini, E.; Luebke, R.W.; Germolec, D.R.; DeWitt, J.C. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicol. Lett. 2014, 230, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Wang, T.; Liu, S.; Jones, K.C.; Sweetman, A.J.; Lu, Y. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ. Int. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the Ability of Perfluorohexane Sulfonate (PFHxS) Bioaccumulation by Two Pseudomonas sp. Strains Isolated from PFAS-Contaminated Environmental Matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, A.J.; Webster, T.F.; Watkins, D.J.; Strynar, M.J.; Kato, K.; Calafat, A.M.; Vieira, V.M.; McClean, M.D. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum. Environ. Int. 2013, 60, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Adachi, N.; Saha, M.; Morita, C.; Takada, H. Levels, temporal trends, and tissue distribution of perfluorinated surfactants in freshwater fish from Asian countries. Arch. Environ. Contam. Toxicol. 2011, 61, 631–641. [Google Scholar] [CrossRef]
- Zainuddin, K.; Zakaria, M.P.; Al-Odaini, N.A.; Bakhtiari, A.R.; Latif, P.A. Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) in Surface Water from the Langat River, Peninsular Malaysia. Environ. Forensics 2012, 13, 82–92. [Google Scholar] [CrossRef]
- Tao, L.; Ma, J.; Kunisue, T.; Libelo, E.L.; Tanabe, S.; Kannan, K. Perfluorinated Compounds in Human Breast Milk from Several Asian Countries, and in Infant Formula and Dairy Milk from the United States. Environ. Sci. Technol. 2008, 42, 8597–8602. [Google Scholar] [CrossRef] [PubMed]
- Lupton, S.J.; Huwe, J.K.; Smith, D.J.; Dearfield, K.L.; Johnston, J.J. Distribution and excretion of perfluorooctane sulfonate (PFOS) in beef cattle (Bos taurus). J. Agric. Food Chem. 2014, 62, 1167–1173. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. Perfluoroalkyl sulfonic and carboxylic acids: A critical review of physicochemical properties, levels and patterns in waters and wastewaters, and treatment methods. J. Environ. Sci. Health A Tox Hazard Subst. Env. Eng. 2009, 44, 1145–1199. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, Z.; Möller, A.; Sturm, R.; Tang, J.; Zhang, G.; Ebinghaus, R. Distribution and long-range transport of polyfluoroalkyl substances in the Arctic, Atlantic Ocean and Antarctic coast. Environ. Pollut. 2012, 170, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, H. Understanding Long-Range Transport Mechanisms of Perfluoroalkyl Substances. Master’s Thesis, Memorial University of Newfoundland, St. John’s, Canada, 2017. [Google Scholar]
- Calafat, A.M.; Wong, L.Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Sunantha, G.; Namasivayam, V. Impacts of Perfluorinated Compounds on Human Health. Bull. Env. Pharmacol. Life Sci. 2015, 4, 183–191. [Google Scholar]
- Seo, S.H.; Son, M.H.; Choi, S.D.; Lee, D.H.; Chang, Y.S. Influence of exposure to perfluoroalkyl substances (PFASs) on the Korean general population: 10-year trend and health effects. Environ. Int. 2018, 113, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zeng, F. Distribution and Bioaccumulation of Perfluoroalkyl Acids in Xiamen Coastal Waters. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Perez, F.; Nadal, M.; Navarro-Ortega, A.; Fabrega, F.; Domingo, J.L.; Barcelo, D.; Farre, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, B.; Hu, Y.; Rood, J.; Liang, L.; Qi, L.; Bray, G.A.; DeJonge, L.; Coull, B.; Grandjean, P.; et al. Associations of Perfluoroalkyl substances with blood lipids and Apolipoproteins in lipoprotein subspecies: The POUNDS-lost study. Environ. Health 2020, 19, 5. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Song, B.; Xiao, R.; Zeng, G.; Gong, J.; Chen, M.; Xu, P.; Zhang, P.; Shen, M.; Yi, H. Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. Environ. Int. 2019, 126, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Birkholz, D. Biomonitoring and elimination of perfluorinated compounds and polychlorinated biphenyls through perspiration: Blood, Urine, and Sweat Study. ISRN Toxicol. 2013, 2013, 483832. [Google Scholar] [CrossRef] [Green Version]
- Worley, R.R.; Moore, S.M.; Tierney, B.C.; Ye, X.; Calafat, A.M.; Campbell, S.; Woudneh, M.B.; Fisher, J. Per-and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ. Int. 2017, 106, 135–143. [Google Scholar] [CrossRef]
- Harada, K.; Inoue, K.; Morikawa, A.; Yoshinaga, T.; Saito, N.; Koizumi, A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ. Res. 2005, 99, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.H.; Hashida, S.; Kaneko, T.; Takenaka, K.; Minata, M.; Inoue, K.; Saito, N.; Koizumi, A. Biliary excretion and cerebrospinal fluid partition of perfluorooctanoate and perfluorooctane sulfonate in humans. Environ. Toxicol. Pharmacol. 2007, 24, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Fai Tse, W.K.; Li, J.W.; Kwan Tse, A.C.; Chan, T.F.; Hin Ho, J.C.; Sun Wu, R.S.; Chu Wong, C.K.; Lai, K.P. Fatty liver disease induced by perfluorooctane sulfonate: Novel insight from transcriptome analysis. Chemosphere 2016, 159, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.T.; Zhao, Y.G.; Wei, X.; Hui, K.Y.; Giesy, J.P.; Wong, C.K.C. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 1092–1101. [Google Scholar] [CrossRef]
- Butenhoff, J.L.; Kennedy, G.L.; Frame, S.R.; O’Connor, J.C.; York, R.G. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rats. Toxicology 2004, 196, 95. [Google Scholar] [CrossRef]
- Li, W.; He, Q.-Z.; Wu, C.-Q.; Pan, X.-Y.; Wang, J.; Tan, Y.; Shan, X.-Y.; Zeng, H.-C. PFOS Disturbs BDNF-ERK-CREB Signalling in Association with Increased MicroRNA-22 in SH-SY5Y Cells. Biomed. Res. Int. 2015, 2015, 302653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genuis, S.J.; Kyrillos, E. The chemical disruption of human metabolism. Toxicol. Mech. Methods 2017, 27, 477–500. [Google Scholar] [CrossRef]
- Tang, L.L.; Wang, J.D.; Xu, T.T.; Zhao, Z.; Zheng, J.J.; Ge, R.S.; Zhu, D.Y. Mitochondrial toxicity of perfluorooctane sulfonate in mouse embryonic stem cell-derived cardiomyocytes. Toxicology 2017, 382, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Xiao, J.; Ducatman, A. Perfluorooctanoic Acid and Cardiovascular Disease in US Adults Perfluorooctanoic Acid and CVD in Adults. JAMA Intern. Med. 2012, 172, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, P.M.; Salihovic, S.; van Bavel, B.; Lind, L. Circulating levels of perfluoroalkyl substances (PFASs) and carotid artery atherosclerosis. Environ. Res. 2017, 152, 157–164. [Google Scholar] [CrossRef]
- Osorio-Yáñez, C.; Sanchez-Guerra, M.; Cardenas, A.; Lin, P.D.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.F.; Fleisch, A.F.; Calafat, A.M.; et al. Per- and polyfluoroalkyl substances and calcifications of the coronary and aortic arteries in adults with prediabetes: Results from the diabetes prevention program outcomes study. Environ. Int. 2021, 151, 106446. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; p. 176. [Google Scholar]
- Seacat, A.M.; Thomford, P.J.; Hansen, K.J.; Clemen, L.A.; Eldridge, S.R.; Elcombe, C.R.; Butenhoff, J.L. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 2003, 183, 117–131. [Google Scholar] [CrossRef]
- Sanchez-Gomez, M.C.; Garcia-Mejia, K.A.; Perez-Diaz Conti, M.; Diaz-Rosas, G.; Palma-Lara, I.; Sanchez-Urbina, R.; Klunder-Klunder, M.; Botello-Flores, J.A.; Balderrabano-Saucedo, N.A.; Contreras-Ramos, A. MicroRNAs Association in the Cardiac Hypertrophy Secondary to Complex Congenital Heart Disease in Children. Pediatric Cardiol. 2017, 38, 991–1003. [Google Scholar] [CrossRef]
- Qian, Y.; Ducatman, A.; Ward, R.; Leonard, S.; Bukowski, V.; Lan Guo, N.; Shi, X.; Vallyathan, V.; Castranova, V. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: Role in endothelial permeability. J. Toxicol. Environ. Health A 2010, 73, 819–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circ. Res. 2006, 99, 692–705. [Google Scholar] [CrossRef] [Green Version]
- Rappazzo, K.M.; Coffman, E.; Hines, E.P. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int. J. Environ. Res. Public Health 2017, 14, 691. [Google Scholar] [CrossRef]
- Cordner, A.; De La Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Health Canada. Health Canada’s Drinking Water Screening Values for Perfluoroalkylated Substances. 2016. Available online: http://scottreid.ca/wp-content/uploads/2016/03/Health-Canada-PFAS-Screening-Values-Fact-Sheet-EN.pdf (accessed on 24 July 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Predieri, B.; Iughetti, L.; Guerranti, C.; Bruzzi, P.; Perra, G.; Focardi, S.E. High Levels of Perfluorooctane Sulfonate in Children at the Onset of Diabetes. Int. J. Endocrinol. 2015, 2015, 234358. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical notes for clinical researchers: Effect size. Restor. Dent. Endod. 2015, 40, 328–331. [Google Scholar] [CrossRef]
- Borghese, M.M.; Walker, M.; Helewa, M.E.; Fraser, W.D.; Arbuckle, T.E. Association of perfluoroalkyl substances with gestational hypertension and preeclampsia in the MIREC study. Environ. Int. 2020, 141, 105789. [Google Scholar] [CrossRef]
- Hutcheson, R.; Innes, K.; Conway, B. Perfluoroalkyl substances and likelihood of stroke in persons with and without diabetes. Diabetes Vasc. Dis. Res. 2020, 17, 1479164119892223. [Google Scholar] [CrossRef]
- Liao, S.; Yao, W.; Cheang, I.; Tang, X.; Yin, T.; Lu, X.; Zhou, Y.; Zhang, H.; Li, X. Association between perfluoroalkyl acids and the prevalence of hypertension among US adults. Ecotoxicol. Environ. Saf. 2020, 196, 110589. [Google Scholar] [CrossRef] [PubMed]
- Pitter, G.; Zare Jeddi, M.; Barbieri, G.; Gion, M.; Fabricio, A.S.C.; Daprà, F.; Russo, F.; Fletcher, T.; Canova, C. Perfluoroalkyl substances are associated with elevated blood pressure and hypertension in highly exposed young adults. Environ. Health 2020, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, R.; Chu, X.; Wang, F.; Sun, X.; Wang, Y.; Pang, L. Overexpression of microRNA-23a-5p induces myocardial infarction by promoting cardiomyocyte apoptosis through inhibited of PI3K/AKT signalling pathway. Cell Biochem. Funct. 2020, 38, 1047–1055. [Google Scholar] [CrossRef]
- Sun, Q.; Zong, G.; Valvi, D.; Nielsen, F.; Coull, B.; Grandjean, P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ. Health Perspect. 2018, 126, 037001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.S.; Wen, L.L.; Chu, P.L.; Lin, C.Y. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ. Pollut. 2018, 232, 73–79. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Du, H.; Xu, L.; Liu, S.; Yi, J.; Qian, X.; Chen, Y.; Jiang, Q.; He, G. Perfluoroalkyl substances, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: A repeat measurement-based prospective study. Environ. Int. 2018, 114, 12–20. [Google Scholar] [CrossRef]
- Huang, M.; Jiao, J.; Zhuang, P.; Chen, X.; Wang, J.; Zhang, Y. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ. Int. 2018, 119, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Santander, N.; Valvi, D.; Lopez-Espinosa, M.-J.; Manzano-Salgado, C.B.; Ballester, F.; Ibarluzea, J.; Santa-Marina, L.; Schettgen, T.; Guxens, M.; Sunyer, J.; et al. Exposure to Perfluoroalkyl Substances and Metabolic Outcomes in Pregnant Women: Evidence from the Spanish INMA Birth Cohorts. Environ. Health Perspect. 2017, 125, 117004. [Google Scholar] [CrossRef]
- Su, T.-C.; Kuo, C.-C.; Hwang, J.-J.; Lien, G.-W.; Chen, M.-F.; Chen, P.-C. Serum perfluorinated chemicals, glucose homeostasis and the risk of diabetes in working-aged Taiwanese adults. Environ. Int. 2016, 88, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Rignell-Hydbom, A.; Holmberg, S.; Thelin, A.; Jonsson, B.A.; Lindh, C.H.; Sehlstedt, A.; Rylander, L. Levels of perfluoroalkyl substances and risk of coronary heart disease: Findings from a population-based longitudinal study. Environ. Res. 2015, 142, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sundaram, R.; Maisog, J.; Calafat, A.M.; Barr, D.B.; Buck Louis, G.M. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil. Steril. 2015, 103, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Lind, L.; Zethelius, B.; Salihovic, S.; van Bavel, B.; Lind, P.M. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia 2014, 57, 473–479. [Google Scholar] [CrossRef]
- Mora, A.M.; Fleisch, A.F.; Rifas-Shiman, S.L.; Woo Baidal, J.A.; Pardo, L.; Webster, T.F.; Calafat, A.M.; Ye, X.; Oken, E.; Sagiv, S.K. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ. Int. 2018, 111, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Koshy, T.T.; Attina, T.M.; Ghassabian, A.; Gilbert, J.; Burdine, L.K.; Marmor, M.; Honda, M.; Chu, D.B.; Han, X.; Shao, Y.; et al. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ. Int. 2017, 109, 128–135. [Google Scholar] [CrossRef]
- Skuladottir, M.; Ramel, A.; Rytter, D.; Haug, L.S.; Sabaredzovic, A.; Bech, B.H.; Henriksen, T.B.; Olsen, S.F.; Halldorsson, T.I. Examining confounding by diet in the association between perfluoroalkyl acids and serum cholesterol in pregnancy. Environ. Res. 2015, 143, 33–38. [Google Scholar] [CrossRef]
- Zeng, X.W.; Qian, Z.; Emo, B.; Vaughn, M.; Bao, J.; Qin, X.D.; Zhu, Y.; Li, J.; Lee, Y.L.; Dong, G.H. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci. Total. Environ. 2015, 512, 364–370. [Google Scholar] [CrossRef]
- Geiger, S.D.; Xiao, J.; Ducatman, A.; Frisbee, S.; Innes, K.; Shankar, A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere 2014, 98, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, C.A.; Rossing, L.I.; Grontved, A.; Ried-Larsen, M.; Dalgard, C.; Andersen, L.B.; Grandjean, P.; Nielsen, F.; Svendsen, K.D.; Scheike, T.; et al. Adiposity and glycemic control in children exposed to perfluorinated compounds. J. Clin. Endocrinol. Metab. 2014, 99, E608–E614. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, K.; Raaschou-Nielsen, O.; McLaughlin, J.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Sørensen, M. Association between Plasma PFOA and PFOS Levels and Total Cholesterol in a Middle-Aged Danish Population. PLoS ONE 2013, 8, e56969. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Simon, N.; Fletcher, T.; Luster, M.I.; Steenland, K.; Calafat, A.M.; Kato, K.; Armstrong, B. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology 2013, 24, 569–576. [Google Scholar] [CrossRef]
- Frisbee, S.J.; Shankar, A.; Knox, S.S.; Steenland, K.; Savitz, D.A.; Fletcher, T.; Ducatman, A.M. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 Health Project. Arch. Pediatrics Adolesc. Med. 2010, 164, 860–869. [Google Scholar] [CrossRef]
- Nelson, J.W.; Hatch, E.E.; Webster, T.F. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect. 2010, 118, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, G.; Sartori, S.; Consonni, D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J. Occup. Environ. Med. 2009, 51, 364–372. [Google Scholar] [CrossRef]
- Higgins, J.P. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration. 2011. Available online: www.cochrane-handbook.org. (accessed on 14 July 2021).
- Geiger, S.D.; Yao, P.; Vaughn, M.G.; Qian, Z. PFAS exposure and overweight/obesity among children in a nationally representative sample. Chemosphere 2021, 268, 128852. [Google Scholar] [CrossRef]
- Sutton, A.J.; Duval, S.J.; Tweedie, R.L.; Abrams, K.R.; Jones, D.R. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000, 320, 1574–1577. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot–Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Huang, R.; Chen, Q.; Zhang, L.; Luo, K.; Chen, L.; Zhao, S.; Feng, L.; Zhang, J. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ. Health 2019, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Lin, L.Y.; Wen, T.W.; Lien, G.W.; Chien, K.L.; Hsu, S.H.; Liao, C.C.; Sung, F.C.; Chen, P.C.; Su, T.C. Association between levels of serum perfluorooctane sulfate and carotid artery intima-media thickness in adolescents and young adults. Int. J. Cardiol. 2013, 168, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- De Toni, L.; Radu, C.M.; Sabovic, I.; Di Nisio, A.; Dall’acqua, S.; Guidolin, D.; Spampinato, S.; Campello, E.; Simioni, P.; Foresta, C. Increased cardiovascular risk associated with chemical sensitivity to perfluoro–octanoic acid: Role of impaired platelet aggregation. Int. J. Mol. Sci. 2020, 21, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-Y.; Chen, P.-C.; Lin, Y.-C.; Lin, L.-Y. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 2009, 32, 702–707. [Google Scholar] [CrossRef] [Green Version]
- Roep, B.O. The role of T-cells in the pathogenesis of Type 1 diabetes: From cause to cure. Diabetologia 2003, 46, 305–321. [Google Scholar] [CrossRef]
- Kannan, K.; Corsolini, S.; Falandysz, J.; Fillmann, G.; Kumar, K.S.; Loganathan, B.G.; Mohd, M.A.; Olivero, J.; Wouwe, N.V.; Yang, J.H.; et al. Perfluorooctanesulfonate and Related Fluorochemicals in Human Blood from Several Countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.G. Developmental Exposure to Endocrine Disrupting Chemicals and Type 1 Diabetes Mellitus. Front. Endocrinol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Miller, M.; Nasir, K.; McEvoy, J.W.; Herrington, D.; Blumenthal, R.S.; Blaha, M.J. Primary Low Level of High-Density Lipoprotein Cholesterol and Risks of Coronary Heart Disease, Cardiovascular Disease, and Death: Results From the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 2016, 183, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Babaev, V.R.; Fazio, S.; Gleaves, L.A.; Carter, K.J.; Semenkovich, C.F.; Linton, M.F. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J. Clin. Investig. 1999, 103, 1697–1705. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, M.; Levin, M.; Skalen, K.; Perman, J.; Friden, V.; Jirholt, P.; Olofsson, S.O.; Fazio, S.; Linton, M.F.; Semenkovich, C.F.; et al. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: Evidence for a role of lipoprotein lipase. Circ. Res. 2007, 101, 777–783. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Buekers, J.; Colles, A.; Cornelis, C.; Morrens, B.; Govarts, E.; Schoeters, G. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int. J. Environ. Res. Public Health 2018, 15, 2818. [Google Scholar] [CrossRef] [Green Version]
- Cornelis, C.; D’Hollander, W.; Roosens, L.; Covaci, A.; Smolders, R.; Van Den Heuvel, R.; Govarts, E.; Van Campenhout, K.; Reynders, H.; Bervoets, L. First assessment of population exposure to perfluorinated compounds in Flanders, Belgium. Chemosphere 2012, 86, 308–314. [Google Scholar] [CrossRef]
- Haug, L.S.; Thomsen, C.; Brantsaeter, A.L.; Kvalem, H.E.; Haugen, M.; Becher, G.; Alexander, J.; Meltzer, H.M.; Knutsen, H.K. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int. 2010, 36, 772–778. [Google Scholar] [CrossRef]
- Joensen, U.N.; Veyrand, B.; Antignac, J.P.; Blomberg Jensen, M.; Petersen, J.H.; Marchand, P.; Skakkebaek, N.E.; Andersson, A.M.; Le Bizec, B.; Jorgensen, N. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum. Reprod. 2013, 28, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Karnes, C.; Winquist, A.; Steenland, K. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ. Res. 2014, 128, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.F.; van den Bosch, M.T.; Hunter, R.W.; Sakamoto, K.; Poole, A.W.; Hers, I. Dual regulation of glycogen synthase kinase 3 (GSK3)alpha/beta by protein kinase C (PKC)alpha and Akt promotes thrombin-mediated integrin alphaIIbbeta3 activation and granule secretion in platelets. J. Biol. Chem. 2013, 288, 3918–3928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blann, A.D.; Nadar, S.K.; Lip, G.Y.H. The adhesion molecule P-selectin and cardiovascular disease. Eur. Heart J. 2003, 24, 2166–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, H.; Maeno, T.; Fukumoto, S.; Shoji, T.; Yamane, T.; Yokoyama, H.; Emoto, M.; Shoji, T.; Tahara, H.; Inaba, M.; et al. Platelet P-Selectin Expression Is Associated with Atherosclerotic Wall Thickness in Carotid Artery in Humans. Circulation 2003, 108, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Salihovic, S.; Stubleski, J.; Kärrman, A.; Lind, L. Changes in plasma levels of perfluoroalkyl substances (PFASs) are related to increase in carotid intima-media thickness over 10 years-a longitudinal study. Environ. Health A Glob. Access Sci. Source 2018, 17, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Jurkovic-Mlakar, S.; Li, Y.; Wahlberg, K.; Scott, K.; Pineda, D.; Lindh, C.H.; Jakobsson, K.; Engström, K. Association between serum concentrations of perfluoroalkyl substances (PFAS) and expression of serum microRNAs in a cohort highly exposed to PFAS from drinking water. Environ. Int. 2020, 136, 105446. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Bruzzi, P.; Bigi, E.; Ciancia, S.; Madeo, S.F.; Lucaccioni, L.; Iughetti, L. Endocrine Disrupting Chemicals and Type 1 Diabetes. Int. J. Mol. Sci. 2020, 21, 2937. [Google Scholar] [CrossRef]

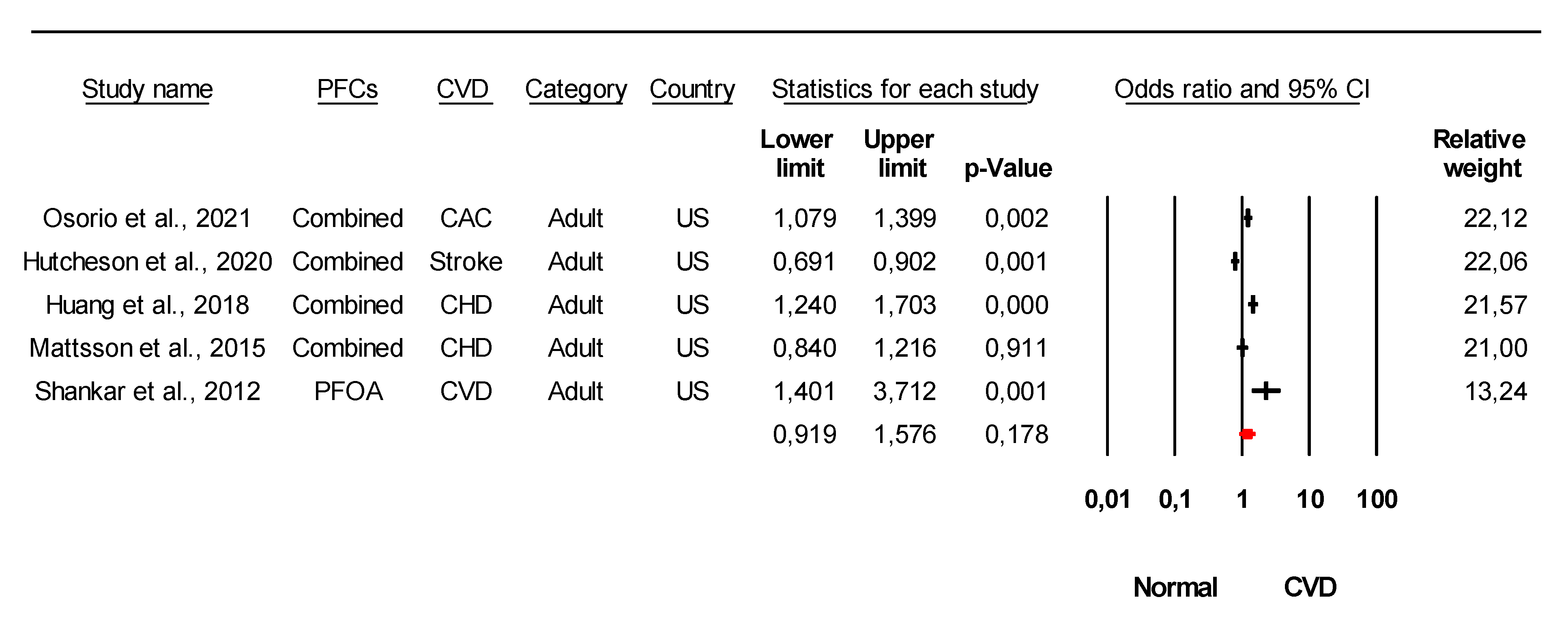

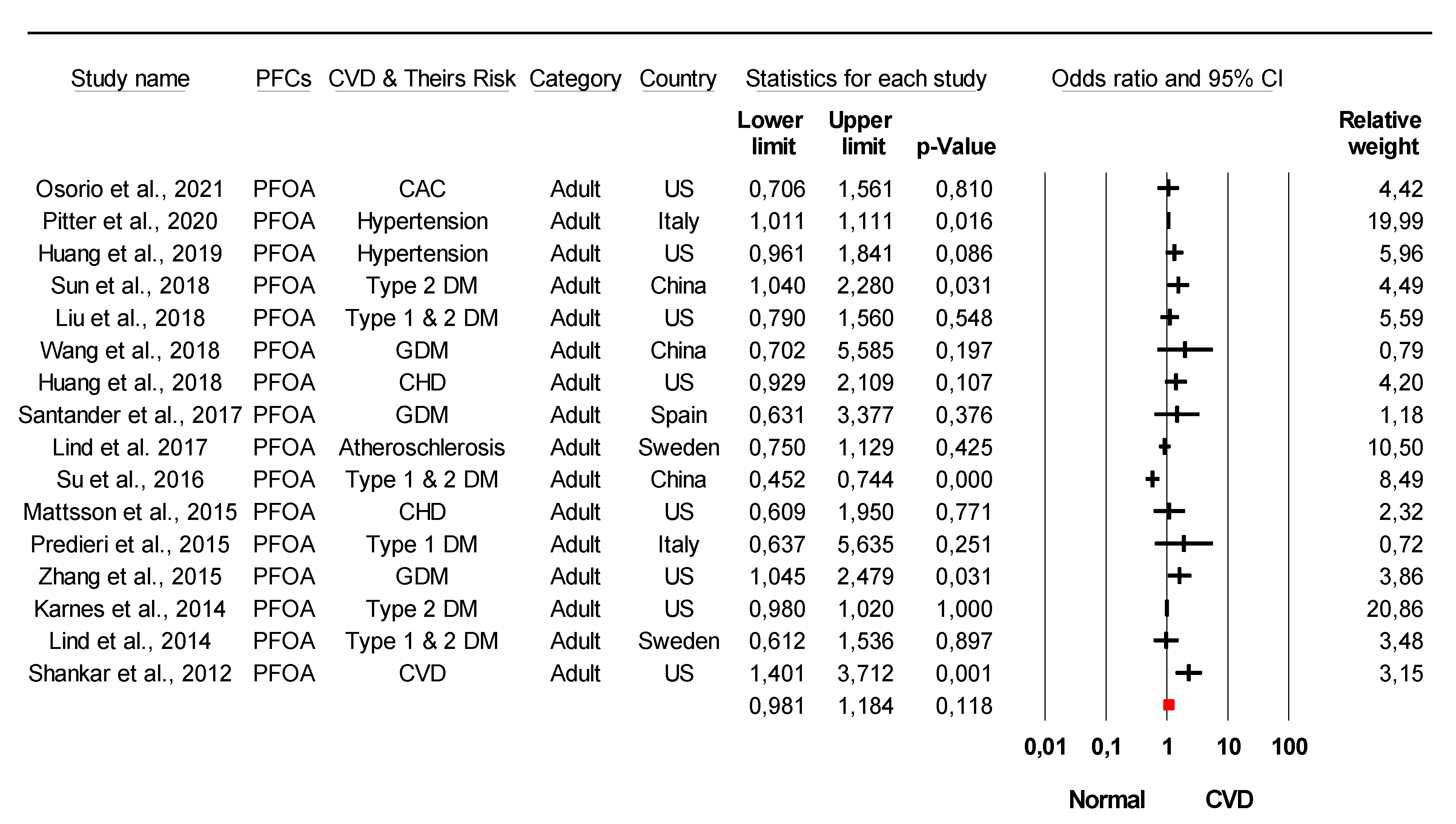
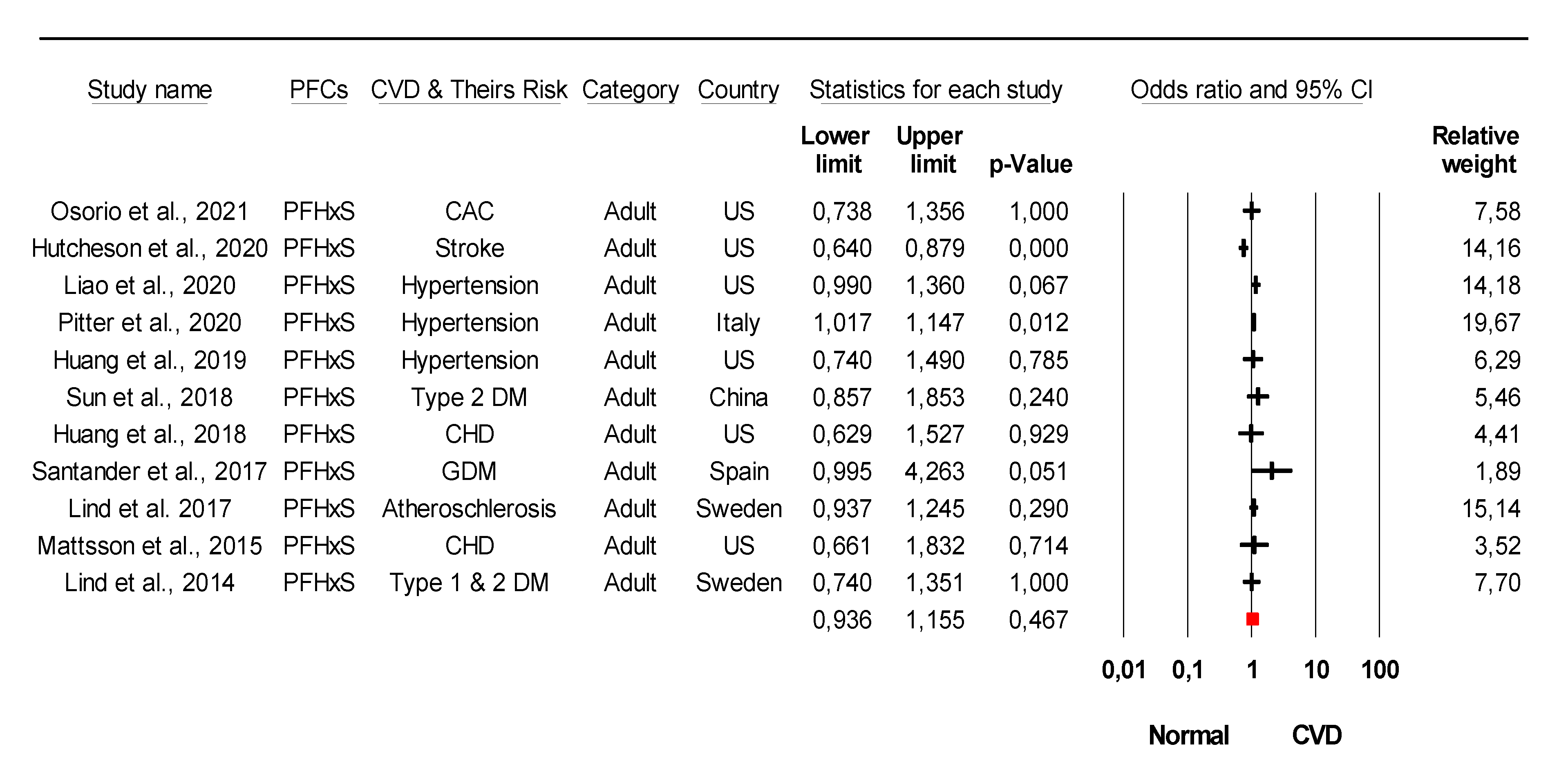

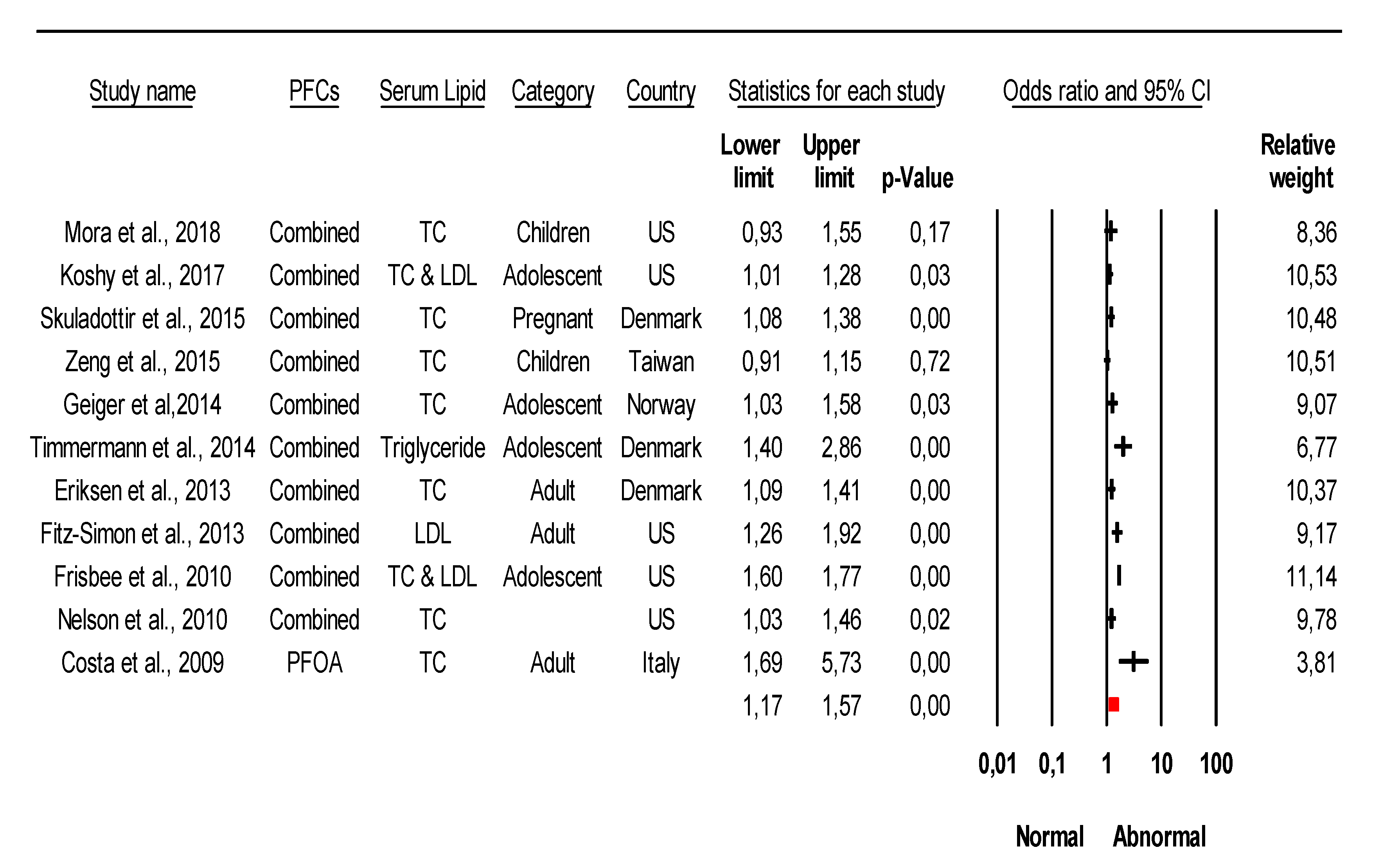



| Study | PFCs Association with CVDs and Their Risk | Country | Ref. |
|---|---|---|---|
| Osorio et al., 2021 | PFOA, PFOS, PFHxS, PFNA, EPAH, MPAH | US | [33] |
| Borghese et al., 2020 | PFOA, PFOS, PFHxS | Canada | [47] |
| Hutcheson et. al, 2020 | PFOA, PFOS, PFHxS, PFNA | US | [48] |
| Liao et al., 2020 | PFOA, PFOS, PFHxS, PFNA | US | [49] |
| Pitter et al., 2020 | PFOA, PFOS, PFHxS, PFNA | Italy | [50] |
| Huang et al., 2019 | PFOS, PFOA, PFHxS, PFNA, PFHP, PFDE, PFDO, PFBS, PFSA, PFUA | US | [51] |
| Sun et al., 2018 | PFOA, PFOS, PFHxS, PFNA, PFUA | China | [52] |
| Liu et al., 2018 | PFOA, PFOS | US | [53] |
| Wang et al., 2018 | PFOS, PFOA | China | [54] |
| Huang et al., 2018 | PFOA, PFOS, PFHxS, PFNA, PFHP, PFDO, PFBS | US | [55] |
| Santander et al., 2017 | PFOA, PFOA, PFHxS, PFNA | Spain | [56] |
| Lind et al., 2017 | PFOA, PFHxS, PFNA, PFHP, PFSA, PFUA, PFDA | Sweden | [32] |
| Su et al., 2016 | PFOA, PFOS, PFNA, PFUA | China | [57] |
| Mattsson et al., 2015 | PFOA, PFOS, PFHxS, PFNA, PFHP, PFDO, PFUA, PFDA | US | [58] |
| Predieri et al., 2015 | PFOA, PFOS | Italy | [44] |
| Zhang et al., 2015 | PFOA, PFOS, PFNA, PFSA, | US | [59] |
| Lind et al., 2014 | PFOA, PFOS, PFHxS, PFNA, PFSA, PFUA PFHP | Sweden | [60] |
| Shankar et al., 2012 | PFOA | US | [31] |
| Study | PFCs Associate with CVDs Risk | Serum Lipid | Country | Ref. |
|---|---|---|---|---|
| Mora et al., 2018 | PFOA, PFOS, PFHxS, PFDA | TC | US | [61] |
| Koshy et al., 2017 | PFOA, PFOS, PFHxS, PFNA, PFDA, PFUA | TC and LDL | US | [62] |
| Skuladottir et al., 2015 | PFOA, PFOS | TC | Denmark | [63] |
| Zeng et al., 2015 | PFOA, PFOS, PFHxS, PFNA, PFBS, PFHxA, PFHxS, PFDO | TC | Taiwan | [64] |
| Geiger et al.,2014 | PFOA, PFOS | TC | Norway | [65] |
| Timmermann et al., 2014 | PFOA, PFOS | TG | Denmark | [66] |
| Eriksen et al., 2013 | PFOA, PFOS | TC | Denmark | [67] |
| Fitz-Simon et al., 2013 | PFOA, PFOS | LDL | US | [68] |
| Frisbee et al., 2010 | PFOA, PFOS | TC and LDL | US | [69] |
| Nelson et al., 2010 | PFOA, PFOS, PFHxS, PFNA | TC | US | [69,70] |
| Costa et al., 2009 | PFOA | TC | Italy | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah Soheimi, S.S.; Abdul Rahman, A.; Abd Latip, N.; Ibrahim, E.; Sheikh Abdul Kadir, S.H. Understanding the Impact of Perfluorinated Compounds on Cardiovascular Diseases and Their Risk Factors: A Meta-Analysis Study. Int. J. Environ. Res. Public Health 2021, 18, 8345. https://doi.org/10.3390/ijerph18168345
Abdullah Soheimi SS, Abdul Rahman A, Abd Latip N, Ibrahim E, Sheikh Abdul Kadir SH. Understanding the Impact of Perfluorinated Compounds on Cardiovascular Diseases and Their Risk Factors: A Meta-Analysis Study. International Journal of Environmental Research and Public Health. 2021; 18(16):8345. https://doi.org/10.3390/ijerph18168345
Chicago/Turabian StyleAbdullah Soheimi, Siti Suhana, Amirah Abdul Rahman, Normala Abd Latip, Effendi Ibrahim, and Siti Hamimah Sheikh Abdul Kadir. 2021. "Understanding the Impact of Perfluorinated Compounds on Cardiovascular Diseases and Their Risk Factors: A Meta-Analysis Study" International Journal of Environmental Research and Public Health 18, no. 16: 8345. https://doi.org/10.3390/ijerph18168345
APA StyleAbdullah Soheimi, S. S., Abdul Rahman, A., Abd Latip, N., Ibrahim, E., & Sheikh Abdul Kadir, S. H. (2021). Understanding the Impact of Perfluorinated Compounds on Cardiovascular Diseases and Their Risk Factors: A Meta-Analysis Study. International Journal of Environmental Research and Public Health, 18(16), 8345. https://doi.org/10.3390/ijerph18168345






