Abstract
Objectives: To investigate the present occurrence of stunting and explore the role of iodine deficiency disorders (IDDs) as a predictor of stunting among primary school children in the Aseer Region. Methods: In a cross-sectional investigation on school children in the Aseer region, thyroid enlargement was evaluated clinically. Urine was collected to evaluate iodine content. Results: The present study involved 3046 school-age pupils. The study disclosed a total goiter rate of 24.0% (95% CI: 22.5–25.5%). The median urinary iodine content (UIC) was 17.0 µg/L. A prevalence of stunting (height for age z score of less than −2) of 7.8% (95% CI: 6.9–8.8%) was found. In a logistic regression model, pupils having clinical goiter (aOR = 1.739; 95% CI: 1.222–2.475) and students having UIC of less than 17 µg/L (aOR = 1.934; 95% CI: 1.457–2.571) were considerably related with stunting. In the receiver operating characteristic (ROC) curve, urinary iodine content to forecast stunting was good (AUC = 0.611, 95% CI: 0.594–0.629). The curve recognized the optimum cutoff point of urinary iodine content to be ≤19.0 µg/L. The sensitivity was 59.66% (95% CI: 53.1–66.0) and the specificity was 57.62% (95% CI: 55.8–59.5). Conclusion: The present study showed that stunting among school-aged children presents a mild public health problem. On the other hand, a severe iodine deficiency situation was revealed among school children in the Aseer region. Continuous monitoring of iodine status among school children is therefore necessary. Concerted interventions that blend nutrition-sensitive with nutrition-specific approaches are expected to influence decreasing stunting significantly.
1. Introduction
Linear development malfunction in early years is the utmost predominant type of undernutrition universally. An anticipated 165 million persons under five years are stunted, with a height-for-age z-score (HAZ) lower than −2 (i.e., more than two standard deviations below the population median). However, a more significant amount of young people with HAZ -2 still have insufficient linear development and are consequently suffering from stunting [1,2]. Stunting more persistently delays the developmental capacity and human resources of people owing to its prolonged term influence on intellectual function and adult financial efficiency; it is consequently considered the best proxy indicator of health disparities among young people [3] and stunting has been of considerable global health importance [4]. The WHO aims to decrease stunting by 40% by 2025 [5]. While remarkable improvement has been achieved in Asia, with a decrease in the percentage of stunted children from 49% to 28% between 1990 and 2010, in Africa, the stunting occurrence has continued stationary around 40% and, due to population expansion, the amount of stunted children is rising [1].
The effects of child stunting are both instantaneous and long-term. They involve: raised morbidity and mortality; inadequate child growth and learning capability; intensified risk of infections and non-communicable diseases; increased liability to store fat mainly in the central region of the body; reduced fat oxidation; lower energy expenditure; insulin resistance; a greater risk of acquiring diabetes, hypertension, and dyslipidemia; lowered working capacity; and unfavorable maternal reproductive outcomes in adulthood. Furthermore, stunted children who underwent rapid weight gain after two years have an increased risk of becoming overweight or obese later in life [6,7]. In an extensive analysis of ten studies in Asia, Africa, and South America, there was a sound dose-response association between HAZ and death. However, potential confounders were not appropriately considered [8].
The trace element iodine is a fundamental constituent of thyroid hormones. Iodine occurs biologically in soil, water, plants, and animals. Humans take up iodine via routine dietetic consumption. Iodine concentrations in water and soil vary extensively across the world. Mountain areas, delta, and flood regions are biologically predisposed to low iodine concentrations. Inadequate dietary iodine consumption and associated deficiencies in thyroid hormones cause a group of unfavorable consequences called iodine deficiency disorders (IDDs) [9]. IDDs are chief public health complications across the world. A projected 2 billion persons globally are at risk of IDDs; 266 million are school-aged children [9]. On the other hand, there is a strong biological foundation for the responsibility of iodine in child development. Development failure is the chief expression of undernutrition during gestation and in childhood and is recognized by a height or weight that is too little compared with that of an average-fed reference population (stunting and underweight) or a weight that is too low for a given height (wasting) [10]. Iodine deficiency damages the production of thyroid hormones, including growth hormone expression [11]. The effect of growth hormones is enabled by insulin-like growth factor I, which is commonly conjugated to insulin-like growth factor binding protein-3 in circulation [12].
The Aseer Region, with an overall population of 1,200,000, lies in southwestern Saudi Arabia. A nationwide epidemiological survey of school children for IDDs [13] identified a modest status of IDDs in all areas of the Kingdom with reasonably higher prevalence for the Aseer Region.
The hypothesis tested is that if a child is suffering from iodine deficiency disorder, then they may be stunted, with a height for age z score of less than −2. The objectives of the present work were to explore the existing frequency of stunting and explore the role of IDDs as a predictor of stunting among primary school children in the Aseer Region.
2. Materials and Methods
2.1. Design
Cross-sectional investigation.
2.2. Target Population
School-age pupils aged 8–10 years residing in the Aseer region (for their high susceptibility to IDDs) [14].
2.3. Sampling and Field Activities
Applying the WHO guidelines handbook [15], at 95% confidence interval with a conventional approximation of the expected proportion of 45% [13], and with an absolute precision of 2%, the required size is computed to be 2377 pupils. To counterweigh for a potential loss, a total of 3000 pupils was considered to be contained in the investigation.
The procedures applied included sampling using stratified proportional allocation. First, the sample was selected, bearing in mind gender, the magnitude of the people in each region, rural–urban disparities, height above sea level, and governmental and private differences.
Personal communications were sent to pupils’ guardians, clarifying the investigation reason and requesting their written permission. Inclusion criteria: All children aged 8–10 years old in the region. Exclusion criteria: Those children who were absent during the survey or those whose fathers were not willing to include their children in the study.
2.4. Clinical Goiter
Inspection and palpation procedures were used. Criteria of the WHO for grading were used [16]. Grades were: Grade “0—no palpable or visible goiter; Grade “1”—a bulk in the neck in harmony with an enlarged thyroid that is palpable but not visible when the neck is in the neutral position; it also moves up in the neck upon swallowing; and Grade “2”—a mass in the neck noticeable when the neck is in a neutral position and is in harmony with an enlarged thyroid when the neck is palpated. The total goiter rate (TGR) is the summation of grades 1 and 2. To prevent inter-observer dissimilarity, thorough training and standardized techniques were implemented.
2.5. Measurement of Urinary Iodine Concentration
Casual urine samples were obtained from the pupils. They were provided with 40-mL plastic containers and asked to half-fill the containers with urine. These containers were tightly closed with plastic screw caps to prevent leakage and evaporation and were labelled with stickers for identification.
Urine samples were examined for iodine concentration using ammonium persulfate to process the samples, followed by the Sandel–Kolthoff reaction [17]. Calibrators, urine samples, and urine controls were added to 16 × 100 mm glass tubes, followed by the addition of 1.0 mL of 1 mol/L ammonium persulfate to all tubes. All samples were oxidized for 30 min in a 91–95 °C heating block. The samples were then cooled to room temperature and 2.0 mL of arsenious acid (0.0253 mol/L), 1.0 mL of 1.25 mol/L H2SO4, and 1.0 mL water were sequentially added. The tubes were then placed in a 32 °C water bath and incubated for 10 min. The reaction was started by adding 0.5 mL of ceric ammonium sulfate to all tubes, which were incubated precisely for 10 min. At the end of incubation, the percent transmission was read at 420 nm in a 10 mm light path cuvette. Water was used to adjust the spectrophotometer to 100% transmission.
The WHO standards [18] for evaluating the severity of iodine nutrition grades based on the median urinary iodine concentration (UIC) were applied. Quality control of iodine measurements was performed.
2.6. Assessment of Stunting
Weight and standing height were evaluated by proficient workers using standard procedures. They were documented to the closest 0.5 kg for weight and the nearest 1 cm for height. Stunting was described as height for age and sex that is two standard deviations (SDs) or more under the WHO standard median (–2 z-score or below) [19]. z score was computed using “WHO AnthroPlus for Personal Computers” [20].
2.7. Data Analysis
Data were investigated using the SPSS Software, version 22 (IBM Corp., released 2013, IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA) and “MedCalc” statistical software version 16.4.3 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org, accessed on 21 March 2021). Frequency, percentage, arithmetic mean, median, standard deviation, and 95% confidence intervals were used to present the results. Suitable tests of significance were used at the 5% level. In addition, multivariable binary logistic regression analysis was computed to assess the adjusted odds ratio and the 95% confidence intervals to examine stunting associations.
A receiver operating characteristic (ROC) curve was generated through “MedCalc” software to predict the role of urinary iodine in identifying stunting. It showed the functioning of the cutoff points in terms of sensitivity versus 1-specificity. The area under the curve (AUC) is an assessment of the accuracy of the cutoff point. It lies between 0.5 and 1. Values < 0.6 indicate a weak classifier, and 1 shows an exceptional classifier.
3. Results
The current analysis involved 3046 pupils (1501 males and 1545 females).
3.1. Clinical Goiter Rate
Results disclosed that 18.5% (563) had grade I goiter, and 5.5% (167) had grade II goiter giving a total goiter rate (TGR) of 24.0% (95% CI: 22.5–25.5%).
3.2. Urinary Iodine Content
The average urinary iodine content (UIC) of the study sample of pupils was 19.03 ± 11.1 µg/L. The median UIC of the study sample was 17.0 µg/L.
3.3. Height-for-Age z Scores and Stunting
The height for age z score (HAZ) ranged from −4.75 to 3.5, with an average of −0.0588 ± 1.452 and a median of −0.09. The study showed that 238 school-aged children have HAZ of less than −2, giving a prevalence of stunting of 7.8% (95% CI: 6.9–8.8%).
3.4. Stunting Correlates
Table 1 shows that stunting was more frequent among females (8.3%, 129) than males (7.3%, 109). Yet it was not statistically significant (p = 0.17). On the other hand, stunting was significantly (p = 0.037) more frequent among children having clinical goiter (9.5%, 69). The median UIC was significantly (p = 0.001) lower among stunted pupils (17.0 µg/L) compared with normal children (22.0 µg/L). The proportion of stunted pupils with a UIC below the median of 17.0 µg/L (9.9%, 160) was significantly higher (p = 0.001) compared with those pupils with UIC above the median (5.5%, 78).

Table 1.
Sociodemographic, clinical, and biochemical correlates of stunting among school children (n = 3046).
Table 2 shows the univariate and multivariable analysis of factors associated with stunting. The table shows that in a logistic regression model, females (aOR = 1.348; 95%CI: 1.006–1.905), pupils having clinical goiter (aOR = 1.739; 95% CI: 1.222–2.475), and students having UIC of less than 17µg/L (aOR = 1.934; 95% CI: 1.457–2.571) were considerably connected to stunting.

Table 2.
Univariate and multivariable analysis for factors associated with stunting among school children (n = 3046).
Table 3 and Figure 1 show ROC curve analysis of the projection of urinary iodine content for stunting. The ability of urinary iodine content to predict stunting was good (AUC = 0.611, 95% CI: 0.594–0.629). The ROC curve identified the optimum cutoff point of urinary iodine content to be ≤19.0 µg/L. The sensitivity was 59.66% (95% CI: 53.1–66.0) and the specificity was 57.62% (95% CI: 55.8–59.5).

Table 3.
Results of receiver operating characteristics (ROC) for urinary iodine in µg/L to predict stunting among school children.
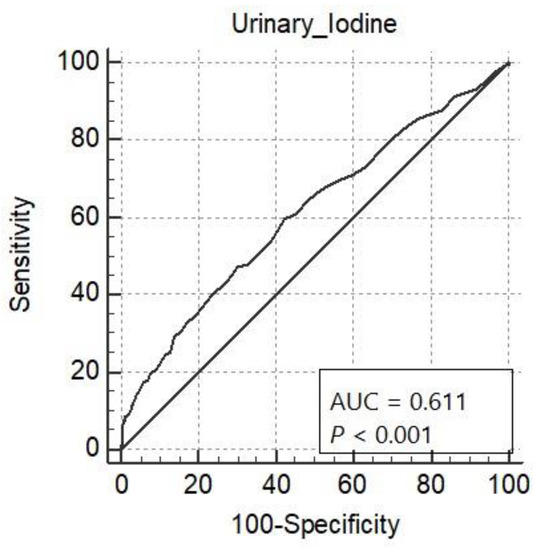
Figure 1.
Receiver operating characteristic curve analysis for urinary iodine in µg/L to predict stunting among school children.
4. Discussion
The present study showed a prevalence of stunting of 7.8% (95% CI: 6.9–8.8%) among school-aged children, indicating a mild public health problem (stunting prevalence of less than 20%) [21]. Similar figures of stunting were reported in Cameroon (7.5%) [22]. Lower figures of stunting were reported in Iran (1.6%) [23] and Guinea-Bissau (6.4%) [24]. On the other hand, higher figures of stunting were reported in South Africa (13.7%) [25], Haiti (14.1%) [26], Nigeria (15.4%) [27], Ethiopia (46.1%) [28], and India (64%) [29]. In Hail, Saudi Arabia, a recent study revealed a prevalence of 6.7% of stunting among school children [30]. A recent meta-analysis study including 65 low and middle-income counties using data from demographic health surveys found a pooled estimate of stunting of 29% [31].
Our results among pupils aged 8 to 10 years in the Aseer region of Saudi Arabia showed an overall prevalence of TGR of 24.0% by clinical examination. The study revealed moderate public health problems of IDD (20% to less than 30%) by WHO guidelines [32]. The Saudi countrywide study in 1995 found a goiter prevalence rate of 30% in the Aseer area. Alternatively, a recent study in 2012 described a goiter prevalence rate in Jazan (a southwestern region of Saudi Arabia) of 11% [33]. The median UIC (17.0 µg/L) of the current survey discovered a severe iodine deficiency situation [32] among school children (<20.0 µg/L).
A study in an iodine-deficient area in India (Gujarat, Western India) reported high stunting figures of 64% [29]. This study emphasized the significance of tackling the essential significance of iodine supplementation and the importance of focus on nutritional intake in general. Similarly, in primary school children in Obukpa, a rural Nigerian community, a stunning figure of 25% was found in a community of severe iodine deficiency (<20.0 µg/L) [27].
Our study disclosed that pupils having clinical goiter and students having UIC of less than 17µg/L were considerably connected with stunting. Similarly, the ability of urinary iodine content to predict stunting was good. The present study revealed that IDD was a predictor of stunting.
The role of goiter and IDD in developing stunting is most likely multifactorial. In stunted young people, iodide is less effectively captivated against the electrochemical gradient needing resources [29,34]. Iodine intensity of the thyroid gland diminishes due to low iodide clearance and uptake in stunting [35]. Stunting incidentally causes modifications in iodine metabolism that may lead to thyroid hyperplasia and decreases circulating thyroid hormone amounts. It may lead to goitrogenesis promptly via the absence of substrate accessibility, in particular the absence of essential amino acids such as tyrosine [36]. A meta-analyses study of the consequences of iodine repletion on growth displayed a convinced influence in moderate-to-mildly iodine-deficient schoolchildren on insulin-like growth factor-1 and insulin-like growth factor binding protein-3 [37].
Suitable diet and a healthful way of life are fundamental all through the life cycle to guarantee the most favorable health for both the person and future descendants. When a child ignores these, they may acquire stunting. Stunting is the second encountered form of malnutrition globally after anemia and a truly invalidating clinical condition. Stunting is the most apparent indication of a complex syndrome also involving reduced immune function, retarded growth, diminishing of cognitive function, and other metabolic disorders that might affect the individual either immediately or in the long term. The stunting syndrome is one of the leading forms of the extensive shortage of micronutrients in the diet, called “hidden hunger” [38].
Control of hidden hunger and its health-related effects are important public health concerns that can only be attained by safeguarding acceptable micronutrient consumptions in all clusters. Overall enhancement of living values is required, and dietary modification should be practiced in the long term. Immediate measures might include intensive marketing of exclusive breastfeeding up to 6 months and appropriate presentation of complementary foods [39]. Similarly, general availability of iodized salt is required for IDD elimination. Unfortunately, a recent study showed that the use of insufficient iodized salt in the Aseer region is still common [40]. Nevertheless, a systematic review paper showed that an iodine supplementation has only a marginal effect on growth, but especially in very short populations [41]. Finally, it should be noted that stunting is not a synonym of malnutrition [42]. Rather, interaction between the biology of human development and the social, economic, political, and emotional (SEPE) environment should be considered. SEPE factor regulation of human growth is shown to be a more inclusive justification for malleability in height than usual beliefs such as socioeconomic status and genetic factors [43].
Limitations of the study are mainly related to the lack of information on nutrition and differences in the diets of the study children.
5. Conclusions
The present study showed that stunting among school-aged children presents a mild public health problem. On the other hand, the study disclosed a severe iodine deficiency state among school children in the Aseer region. Continuous monitoring of iodine status among school children is therefore necessary. Multi-sectoral methodologies which join nutrition-sensitive with nutrition-specific approaches are expected to have a more significant influence on decreasing stunting. In the Aseer region, banning of non-iodized salt in the local markets should be strictly implemented.
Author Contributions
F.I.A., S.A.A. and A.A.M. revised the literature and designed the study. S.A.A.-E. and A.A.M. carried the data collection and clinical examination. A.A.P. carried out the laboratory analysis. A.A.M. and N.J.A. did the statistical analysis. A.A.M. drafted the manuscript. F.I.A., S.A.A., A.A.M., M.A.A., S.A.A., A.A.P., T.M.M., A.A.S. and N.J.A. contributed to the data interpretation and critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
King Abdulaziz City funded this study for Science and Technology, Saudi Arabia (APR-29-42). The funders approved the design, analysis, and reporting of the project. However, the authors alone are responsible for the content and writing of the paper.
Institutional Review Board Statement
The study was conducted by ethical standards and approved by King Khalid University Ethical Committee (ECM#2017-1204).
Informed Consent Statement
Written informed consent was taken from parents.
Data Availability Statement
Data are available on request from corresponding author.
Acknowledgments
The investigators wish to sincerely express their thanks and gratitude to the Director-General of Education of the Aseer Region, staff of the Boys and Girls School Health Units in Abha, Ahad Rufeida, Sarat Ebeida, and Muhayeel. The full cooperation and support of the study school directors and teachers are highly appreciated.
Conflicts of Interest
The authors report no conflict of interest.
References
- World Health Organization. Levels and Trends in Child Malnutrition; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child Health 2014, 34, 250–265. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, M.; Sahn, D.E.; Younger, S.D. Decomposing world health inequality. J. Health Econ. 2003, 22, 271–293. [Google Scholar] [CrossRef] [Green Version]
- UNICEF. Division of Communication. Tracking Progress on Child and Maternal Nutrition: A Survival and Development Priority; Unicef: New York, NY, USA, 2009. [Google Scholar]
- World Health Organization. Global Targets 2025 to Improve Maternal, Infant and Young Children Nutrition; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Dewey, K.G.; Begum, K. Long-term consequences of stunting in early life. Matern. Child Nutr. 2011, 7, 5–18. [Google Scholar] [CrossRef]
- Soliman, A.; De Sanctis, V.; Alaaraj, N.; Ahmed, S.; Alyafei, F.; Hamed, N.; Soliman, N. Early and long-term consequences of nutritional stunting: From childhood to adulthood. Acta Bio Med. Atenei Parm. 2021, 92, e2021168. [Google Scholar]
- Olofin, I.; McDonald, C.M.; Ezzati, M.; Flaxman, S.; Black, R.E.; Fawzi, W.W.; Caulfield, L.E.; Danaei, G.; Study, N.I.M. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: A pooled analysis of ten prospective studies. PLoS ONE 2013, 8, e64636. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Rogol, A.D.; Clark, P.A.; Roemmich, J.N. Growth and pubertal development in children and adolescents: Effects of diet and physical activity. Am. J. Clin. Nutr. 2000, 72, 521S–528S. [Google Scholar] [CrossRef]
- Ceda, G.P.; Fielder, P.J.; Donovan, S.M.; Rosenfeld, R.G.; Hoffman, A.R. Regulation of insulin-like growth factor-binding protein expression by thyroid hormone in rat GH3 pituitary tumor cells. Endocrinology 1992, 130, 1483–1489. [Google Scholar]
- Ferry, R.J., Jr.; Cerri, R.W.; Cohen, P. Insulin-like growth factor binding proteins: New proteins, new functions. Horm. Res. Paediatr. 1999, 51, 53–67. [Google Scholar] [CrossRef]
- Al Dakheel, M.; Haridi, H.; Al Bashir, B.; Al Shingiti, A.; Al Shehri, S.; Gassem, M.; Hussein, I. Prevalence of iodine deficiency disorders among school children in Saudi Arabia: Results of a national iodine nutrition study. EMHJ-East. Mediterr. Health J. 2016, 22, 301–308. [Google Scholar] [CrossRef]
- Bagchi, K. Nutrition in the eastern Mediterranean region of the World Health Organization. EMHJ-East. Mediterr. Health J. 2008, 14, S107–S113. [Google Scholar]
- Lemeshow, S.; Hosmer, D.W.; Klar, J.; Lwanga, S.K.; World Health Organization. Adequacy of Sample Size in Health Studies; Wiley: Chichester, UK, 1990. [Google Scholar]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Sullivan, K.M.; May, S.; Maberly, G. Urinary Iodine Assessment: A Manual on Survey and Laboratory Methods; Department of International Health, Rollins School of Public Health: Atlanta, GA, USA, 2000. [Google Scholar]
- World Health Organization. Elimination of Iodine Deficiency Disorders: A Manual for Health Workers; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Neufeld, L.; Osendarp, S. Global, regional and country trends in underweight and stunting as indicators of nutrition and health of populations. In International Nutrition: Achieving Millennium Goals and Beyond; Karger Publishers: Basel, Switzerland, 2014; Volume 78, pp. 11–19. [Google Scholar]
- Blössner, M.; Siyam, A.; Borghi, E.; Onyango, A.; De Onis, M. WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World’s Children and Adolescents; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Sellen, D. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series No. 854. Pp. 452.(WHO, Geneva, 1995.) Swiss Fr 71.00. J. Biosoc. Sci. 1998, 30, 135–144. [Google Scholar] [CrossRef]
- Tabi, E.S.B.; Cumber, S.N.; Juma, K.O.; Ngoh, E.A.; Akum, E.A.; Eyong, E.M. A cross-sectional survey on the prevalence of anaemia and malnutrition in primary school children in the Tiko Health District, Cameroon. Pan Afr. Med. J. 2019, 32, 111. [Google Scholar] [CrossRef]
- Emamian, M.H.; Hashemi, H.; Fotouhi, A. Obesity and underweight: Serious health problems in Iranian primary school children. Pediatrics Int. 2019, 61, 1030–1035. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Machado, A.; Embalo, A.R.; Bordalo, A.A. Endemic goiter and iodine deficiency status among Guinea-Bissau school-age children. Eur. J. Clin. Nutr. 2018, 72, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Egal, A.; Oldewage-Theron, W. Association of micronutrients and child growth in children aged 7-15 years from Qwa-Qwa, South Africa. S. Afr. J. Clin. Nutr. 2018, 31, 62–66. [Google Scholar] [CrossRef] [Green Version]
- von Oettingen, J.E.; Brathwaite, T.D.; Carpenter, C.; Bonnell, R.; He, X.; Braverman, L.E.; Pearce, E.N.; Larco, P.; Larco, N.C.; Jean-Baptiste, E. Population survey of iodine deficiency and environmental disruptors of thyroid function in young children in Haiti. J. Clin. Endocrinol. Metab. 2017, 102, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Ugo, J.; Chinwe, E. A pilot study of iodine and anthropometric status of primary school children in Obukpa, a rural Nigerian community. J. Public Health Epidemiol. 2012, 4, 246–252. [Google Scholar]
- Getaneh, Z.; Melku, M.; Geta, M.; Melak, T.; Hunegnaw, M.T. Prevalence and determinants of stunting and wasting among public primary school children in Gondar town, northwest, Ethiopia. BMC Pediatrics 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Brahmbhatt, S.R.; Brahmbhatt, R.M.; Boyages, S.C. Impact of protein energy malnutrition on thyroid size in an iodine deficient population of Gujarat (India): Is it an aetiological factor for goiter? Eur. J. Endocrinol. 2001, 145, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Alshammari, E.; Suneetha, E.; Adnan, M.; Khan, S.; Alazzeh, A. Growth profile and its association with nutrient intake and dietary patterns among children and adolescents in Hail region of Saudi Arabia. BioMed Res. Int. 2017, 2017, 5740851. [Google Scholar] [CrossRef] [Green Version]
- Akombi, B.J.; Chitekwe, S.; Sahle, B.W.; Renzaho, A. Estimating the double burden of malnutrition among 595,975 children in 65 low-and middle-income countries: A meta-analysis of demographic and health surveys. Int. J. Environ. Res. Public Health 2019, 16, 2886. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Alsanosy, R.M.A.; Gaffar, A.M.; Khalafalla, H.E.E.; Mahfouz, M.S.; Zaid, A.N.S.; Bani, I.A. Current iodine nutrition status and progress toward elimination of iodine deficiency disorders in Jazan, Saudi Arabia. BMC Public Health 2012, 12, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Robinson, H.; Betton, H.; Jackson, A. Clinical nutrition case. Nutr. Rev. 1986, 44, 270–273. [Google Scholar]
- Gaitan, G.; Mayoral, L.G.; Gaitan, E. Defective thyroidal iodine concentration in protein-calorie malnutrition. J. Clin. Endocrinol. Metab. 1983, 57, 327–333. [Google Scholar] [CrossRef]
- Polge, A.; Bancel, E.; Strubel, H.B.D.; Peray, S.P.P.; Carlet, C.; De Bornier, B.M. Plasma amino acid concentrations in elderly patients with protein energy malnutrition. Age Ageing 1997, 26, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Farebrother, J.; Naude, C.E.; Nicol, L.; Sang, Z.; Yang, Z.; Jooste, P.L.; Andersson, M.; Zimmermann, M.B. Effects of iodized salt and iodine supplements on prenatal and postnatal growth: A systematic review. Adv. Nutr. 2018, 9, 219–237. [Google Scholar] [CrossRef] [Green Version]
- Branca, F.; Ferrari, M. Impact of micronutrient deficiencies on growth: The stunting syndrome. Ann. Nutr. Metab. 2002, 46 (Suppl. 1), 8–17. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E.; The Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Patel, A.A. Iodine in Table Salt in the Aseer Region, Southwestern Saudi Arabia. World Fam. Med. 2021, 19, 20–24. [Google Scholar]
- Roberts, J.L.; Stein, A.D. The impact of nutritional interventions beyond the first 2 years of life on linear growth: A systematic review and meta-analysis. Adv. Nutr. 2017, 8, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffler, C.; Hermanussen, M.; Bogin, B.; Liana, D.; Taolin, F.; Cempaka, P.; Irawan, M.; Ibbibah, L.; Mappapa, N.; Payong, M. Stunting is not a synonym of malnutrition. Eur. J. Clin. Nutr. 2020, 74, 377–386. [Google Scholar] [CrossRef]
- Bogin, B. Social-Economic-Political-Emotional (SEPE) factors regulate human growth. Hum. Biol. Public Health 2021, 1. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).