Association between Physical Activity and Sport Participation on Hemoglobin A1c Among Children and Adolescents with Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedures
2.2. Demographic Characteristics, Diabetes Monitoring, Treatment Plans and Outcomes
2.3. Physical Activity and Sport Participation
2.4. Data Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Associations between Frequency of Physical Activity, Sport Participation and Diabetes Health Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J., Liguori, G., Magal, M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- American College of Sports Medicine. Exercise is Medicine 2018. Available online: http://www.exerciseismedicine.org/ (accessed on 2 August 2020).
- MacMillan, F.; Kirk, A.; Mutrie, N.; Matthews, L.; Robertson, K.; Saunders, D.H. A systematic review of physical activity and sedentary behavior intervention studies in youth with type 1 diabetes: Study characteristics, intervention design, and efficacy. Pediatric Diabetes 2014, 15, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, L.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colberg, S.R. The Athlete’s Guide to Diabetes; Human Kinetics: Champaign, IL, USA, 2020. [Google Scholar]
- United States Department of Health and Sport Sciences (USDHHS). Physical Activity Guidelines for Americans, 2nd ed.; Services USDHHS: Washington, DC, USA, 2018. [Google Scholar]
- Jaggers, J.R.; Casto Hynes, K.; Wintergerst, K.A. Exercise and Sport Participation for Individuals with Type 1 Diabetes. ACSM’s Health Fit. J. 2016, 20, 40–44. [Google Scholar] [CrossRef]
- Jaggers, J.R.; King, K.M.; Watson, S.E.; Wintergerst, K.A. Predicting nocturnal hypoglycemia with measures of physical activity intensity in adolescent athletes with type 1 diabetes. Diabetes Technolol. Ther. 2019, 21, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Åman, J.; Skinner, T.; de Beaufort, C.; Swift, P.; Aanstoot, H.; Cameron, F. Associations between physical activity, sedentary behavior, and glycemic control in a large cohort of adolescents with type 1 diabetes: The Hvidoere Study Group on Childhood Diabetes. Pediatric Diabetes 2009, 10, 234–239. [Google Scholar] [CrossRef]
- Aljawarneh, Y.M.; Wardell, D.W.; Wood, G.L.; Rozmus, C.L. A Systematic Review of Physical Activity and Exercise on Physiological and Biochemical Outcomes in Children and Adolescents With Type 1 Diabetes. J. Nurs. Scholarsh. 2019, 51, 337–345. [Google Scholar] [CrossRef]
- Elmesmari, R.; Reilly, J.J.; Martin, A.; Paton, J.Y. Accelerometer measured levels of moderate-to-vigorous intensity physical activity and sedentary time in children and adolescents with chronic disease: A systematic review and meta-analysis. PLoS ONE 2017, 12, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galler, A.; Lindau, M.; Ernert, A.; Thalemann, R.; Raile, K. Associations between media consumption habits, physical activity, socioeconomic status, and glycemic control in children, adolescents, and young adults with type 1 diabetes. Diabetes Care 2011, 34, 2356–2359. [Google Scholar] [CrossRef] [Green Version]
- Mandic, S.; Bengoechea, E.G.; Stevens, E.; de la Barra, S.L.; Skidmore, P. Getting kids active by participating in sport and doing it more often: Focusing on what matters. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beraki, A.; Magnuson, A.; Särnblad, S.; Aman, J.; Samuelsson, U. Increase in physical activity is associated with lower HbA1c levels in children and adolescents with type 1 diabetes: Results from a cross-sectional study based on the Swedish pediatric diabetes quality registry (SWEDIABKIDS). Diabetes Res. Clin. Pract. 2014, 105, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, G.; Spagnuolo, M.I.; Lombardi, F.; Spadaro, R.; Siano, M.; Franzese, A. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 376–382. [Google Scholar] [CrossRef]
- American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S163–S182.
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.M.; Su, H.; Thomas, D.M.; Heo, M.; Golnabi, A.H.; Pietrobelli, A.; Heymsfield, S.B. Tri-Ponderal Mass Index vs Body Mass Index in Estimating Body Fat During Adolescence. JAMA Pediatrics 2017, 171, 629–636. [Google Scholar] [CrossRef]
- Park, H.K.; Shim, Y.S. Distribution of Tri-Ponderal Mass Index and its Relation to Body Mass Index in Children and Adolescents Aged 10 to 20 Years. J. Clin. Endocrinol. Metab. 2020, 105, e826–e834. [Google Scholar] [CrossRef] [PubMed]
- Kann, L.; McManus, T.; Harris, W.A.; Shanklin, S.L.; Flint, K.H.; Queen, B.; Lowry, R.; Chyen, D.; Whittle, L.; Thornton, J.; et al. Youth Risk Behavior Surveillance—United States, 2017. MMWR Surveill. Summ. 2018, 67, 1–114. [Google Scholar] [CrossRef]
- Booth, M.L.; Okely, A.D.; Chey, T.; Bauman, A. The reliability and validity of the adolescent physical activity recall questionnaire. /Fiabilite du questionnaire de rappel sur l’ activite physique des adolescents. Med. Sci. Sports Exerc. 2002, 34, 1986–1995. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S. Exercise and Diabetes: A Clinician’s Guide to Prescribing Physical Activity, 1st ed.; American Diabetes Association: Arlington, VA, USA, 2013; pp. 28–29. [Google Scholar]
- Quirk, H.; Blake, H.; Tennyson, R.; Randell, T.L.; Glazebrook, C. Physical activity interventions in children and young people with Type 1 diabetes mellitus: A systematic review with meta-analysis. Diabet. Med. 2014, 31, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.; Noyes, J.; Lowes, L.; Haf Spencer, L.; Gregory, J.W. An ongoing struggle: A mixed-method systematic review of interventions, barriers and facilitators to achieving optimal self-care by children and young people with type 1 diabetes in educational settings. BMC Pediatr. 2014, 14, 228. [Google Scholar] [CrossRef] [Green Version]
- Ryninks, K.; Sutton, E.; Thomas, E.; Jago, R.; Shield, J.P.; Burren, C.P. Attitudes to Exercise and Diabetes in Young People with Type 1 Diabetes Mellitus: A Qualitative Analysis. PLoS ONE 2015, 10, e0137562. [Google Scholar] [CrossRef] [PubMed]
- Belcher, B.R.; Berrigan, D.; Dodd, K.W.; Emken, B.A.; Chou, C.P.; Spruijt-Metz, D. Physical activity in US youth: Effect of race/ethnicity, age, gender, and weight status. Med. Sci. Sports Exerc. 2010, 42, 2211–2221. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.A.; Moore, L.V.; Moore, K.; Zagorski, M.; Brines, S.J.; Roux, A.V.D.; Evenson, K.R. Disparities in physical activity resource availability in six US regions. Prev. Med. 2015, 78, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzmarzyk, P.T.; Denstel, K.D.; Beals, K.; Carlson, J.; Crouter, S.E.; McKenzie, T.L.; Pate, R.R.; Sisson, S.B.; Staiano, A.E.; Stanish, H.; et al. Results from the United States 2018 Report Card on Physical Activity for Children and Youth. J. Phys. Act. Health 2018, 15, S422–S424. [Google Scholar] [CrossRef] [Green Version]
- Kahkoska, A.R.; Watts, M.E.; Driscoll, K.A.; Bishop, F.K.; Mihas, P.; Thomas, J.; Law, J.R.; Jain, N.; Mayer-Davis, E.J. Understanding antagonism and synergism: A qualitative assessment of weight management in youth with Type 1 diabetes mellitus. Obes. Med. 2018, 9, 21–31. [Google Scholar] [CrossRef] [PubMed]
| Variable | Category | All | Inactive | Active | Most Active | p-Value |
|---|---|---|---|---|---|---|
| Number | 152 | 51 (33.55%) | 50 (32.9%) | 51 (33.55%) | ||
| Age (avg. yrs) | 13 ± 3 | 14 ± 3 | 13 ± 3 | 13 ± 3 | 0.12 | |
| Gender | 0.25 | |||||
| Female | 71 (46.71%) | 26 (50.98%) | 26 (52%) | 19 (37.25%) | ||
| Male | 81 (53.29%) | 25 (49.02%) | 24 (48%) | 32 (62.75%) | ||
| Race | 0.05 | |||||
| Black | 13 (8.55%) | 8 (15.69%) | 1 (2%) | 4 (7.84%) | ||
| White | 134 (88.16%) | 41 (80.39%) | 46 (92%) | 47 (92.16%) | ||
| Other | 5 (3.29%) | 2 (3.92%) | 3 (6%) | 0 | ||
| Ethnicity | 0.07 | |||||
| Hispanic or Latino | 5 (3.29%) | 4 (7.84%) | 1 (2%) | 0 | ||
| Not Hispanic or Latino | 147 (96.71%) | 47 (92.16%) | 49 (98%) | 51 (100%) | ||
| CGM Use | 0.73 | |||||
| Yes | 77 (50.66%) | 24 (47.06%) | 25 (50%) | 28 (54.9%) | ||
| No | 75 (49.34%) | 27 (52.94%) | 25 (50%) | 23 (45.1%) | ||
| Insulin Pump | 0.85 | |||||
| Yes | 115 (75.66%) | 40 (78.43%) | 37 (74%) | 38 (74.51%) | ||
| No | 37 (24.34%) | 11 (21.57%) | 13 (26%) | 13 (25.49%) | ||
| Years diagnosed with T1D | 4.78 ± 3.91 | 5.67 ± 4.06 | 3.84 ± 3.59 | 4.82 ± 3.91 | 0.06 | |
| Height (m) | 156.54 ± 15.60 | 158.43 ± 14.83 | 154.32 ± 15.28 | 157.12 ± 16.67 | 0.40 | |
| Weight (kg) | 54.36 ± 16.97 | 57.30 ± 18.92 | 53.32 ± 14.99 | 52.45 ± 16.67 | 0.31 | |
| TMI (kg/m3) | 13.91 ± 2.85 | 14.09 ± 3.14 | 14.34 ± 2.75 | 13.31 ± 2.59 | 0.16 | |
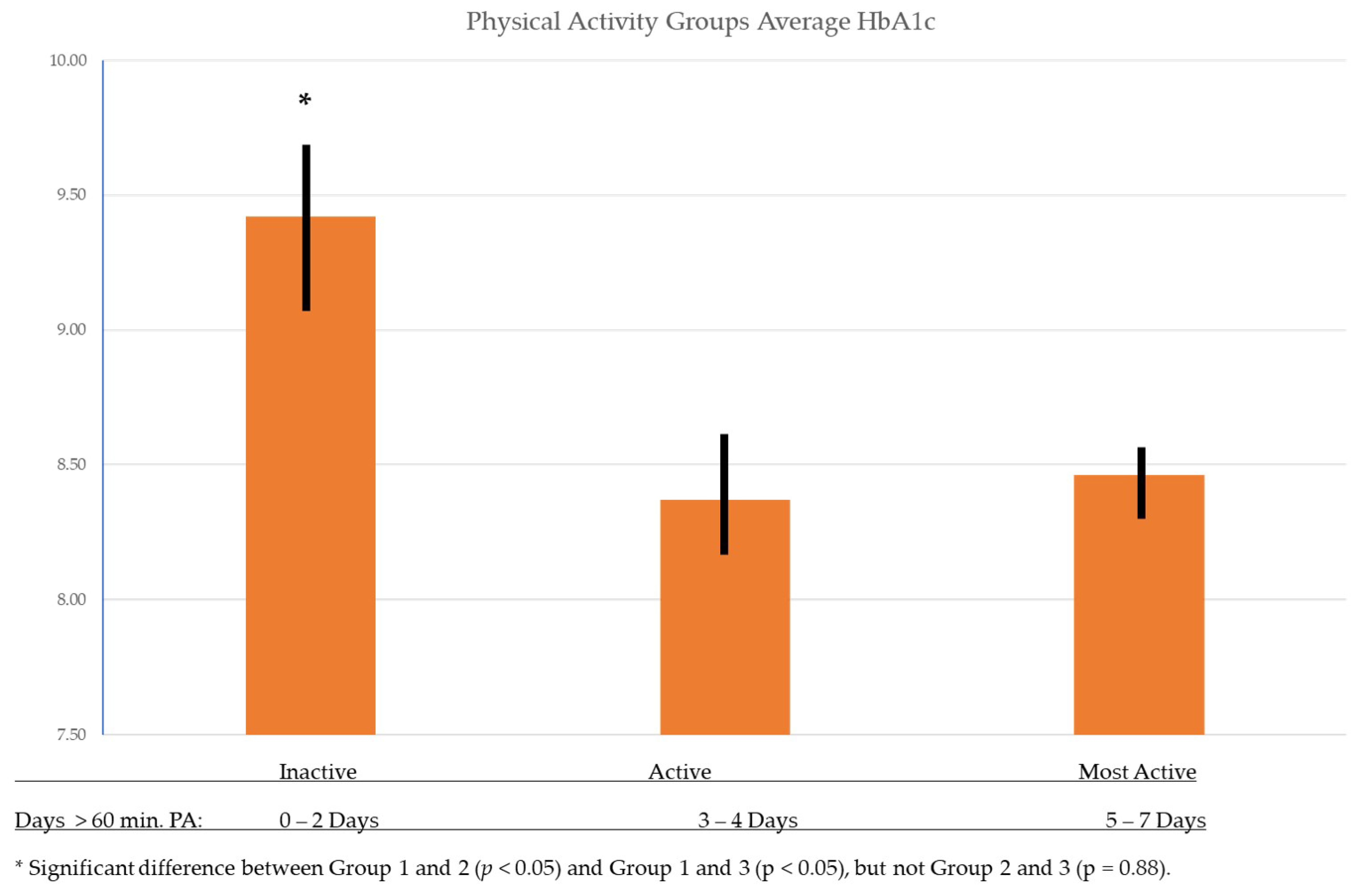
| HbA1c | 8.75 ± 1.81 | 9.42 ± 2.18 | 8.37 ± 1.70 | 8.46 ± 1.29 | 0.007 | |
| Sport Participation | <0.001 | |||||
| Yes | 97 (63.82%) | 22 (43.14%) | 35 (70%) | 40 (78.43%) | ||
| No | 55 (36.18%) | 29 56.86%) | 15 (30%) | 11 (21.57%) | ||
| Days of PA | 3.49 ± 1.95 | 1.29 ± 0.83 | 3.48 ± 0.50 | 5.69 ± 0.84 | <0.001 | |
| Insurance Type | ||||||
| Private Company | 87 (57.24%) | 29 (56.86%) | 30 (60%) | 28 (54.9%) | 0.88 | |
| Medicare/Medicaid | 122 (80.26%) | 46 (90.2%) | 39 (78%) | 37 (72.55%) | 0.07 | |
| None | 2 (1.32%) | 1 (1.96%) | 0 | 1 (1.96%) | 0.61 |
| Independent Variable HbA1c | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 a | Model 2 b | |||||||
| Variable | β (SE) | Standardized Beta | t | p-Value | β (SE) | Standardized Beta | t | p-Value |
| Intercept | 11.14 (1.35) | 8.23 | <0.01 | 13.2 (2.32) | 5.69 | <0.001 | ||
| Days of PA per week | −0.15 (0.07) | −0.16 | −2.08 | 0.03 | −0.17 (0.07) | −0.18 | −2.26 | 0.02 |
| Age | 0.06 (0.05) | 0.09 | 1.06 | 0.29 | 0.04 (0.05) | 0.06 | 0.72 | 0.47 |
| Race | −0.66 (0.29) | −0.17 | −2.25 | 0.03 | −0.73 (0.34) | −0.19 | −2.12 | 0.04 |
| CGM use | −0.66 (0.28) | −0.18 | −2.40 | 0.02 | −0.74 (0.28) | −0.21 | −2.69 | 0.01 |
| Diagnosis Duration | 0.08 (0.04) | 0.17 | 2.15 | 0.03 | 0.09 (0.04) | 0.19 | 2.30 | 0.02 |
| Gender | −0.50 (0.29) | −0.14 | −1.73 | 0.09 | ||||
| Ethnicity | −0.27 (0.92) | −0.03 | −0.30 | 0.77 | ||||
| TMI (kg/mt2) | −0.02 (0.05) | −0.03 | −0.30 | 0.76 | ||||
| Insulin Pump use | 0.04 (0.32) | 0.01 | 0.13 | 0.89 | ||||
| Insurance Type | −0.70 (0.29) | −0.15 | −1.94 | 0.06 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, K.M.; Jaggers, J.R.; Della, L.J.; McKay, T.; Watson, S.; Kozerski, A.E.; Hartson, K.R.; Wintergerst, K.A. Association between Physical Activity and Sport Participation on Hemoglobin A1c Among Children and Adolescents with Type 1 Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 7490. https://doi.org/10.3390/ijerph18147490
King KM, Jaggers JR, Della LJ, McKay T, Watson S, Kozerski AE, Hartson KR, Wintergerst KA. Association between Physical Activity and Sport Participation on Hemoglobin A1c Among Children and Adolescents with Type 1 Diabetes. International Journal of Environmental Research and Public Health. 2021; 18(14):7490. https://doi.org/10.3390/ijerph18147490
Chicago/Turabian StyleKing, Kristi M., Jason R. Jaggers, Lindsay J. Della, Timothy McKay, Sara Watson, Amy E. Kozerski, Kimberly R. Hartson, and Kupper A. Wintergerst. 2021. "Association between Physical Activity and Sport Participation on Hemoglobin A1c Among Children and Adolescents with Type 1 Diabetes" International Journal of Environmental Research and Public Health 18, no. 14: 7490. https://doi.org/10.3390/ijerph18147490
APA StyleKing, K. M., Jaggers, J. R., Della, L. J., McKay, T., Watson, S., Kozerski, A. E., Hartson, K. R., & Wintergerst, K. A. (2021). Association between Physical Activity and Sport Participation on Hemoglobin A1c Among Children and Adolescents with Type 1 Diabetes. International Journal of Environmental Research and Public Health, 18(14), 7490. https://doi.org/10.3390/ijerph18147490







