Children’s Greenness Exposure and IQ-Associated DNA Methylation: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
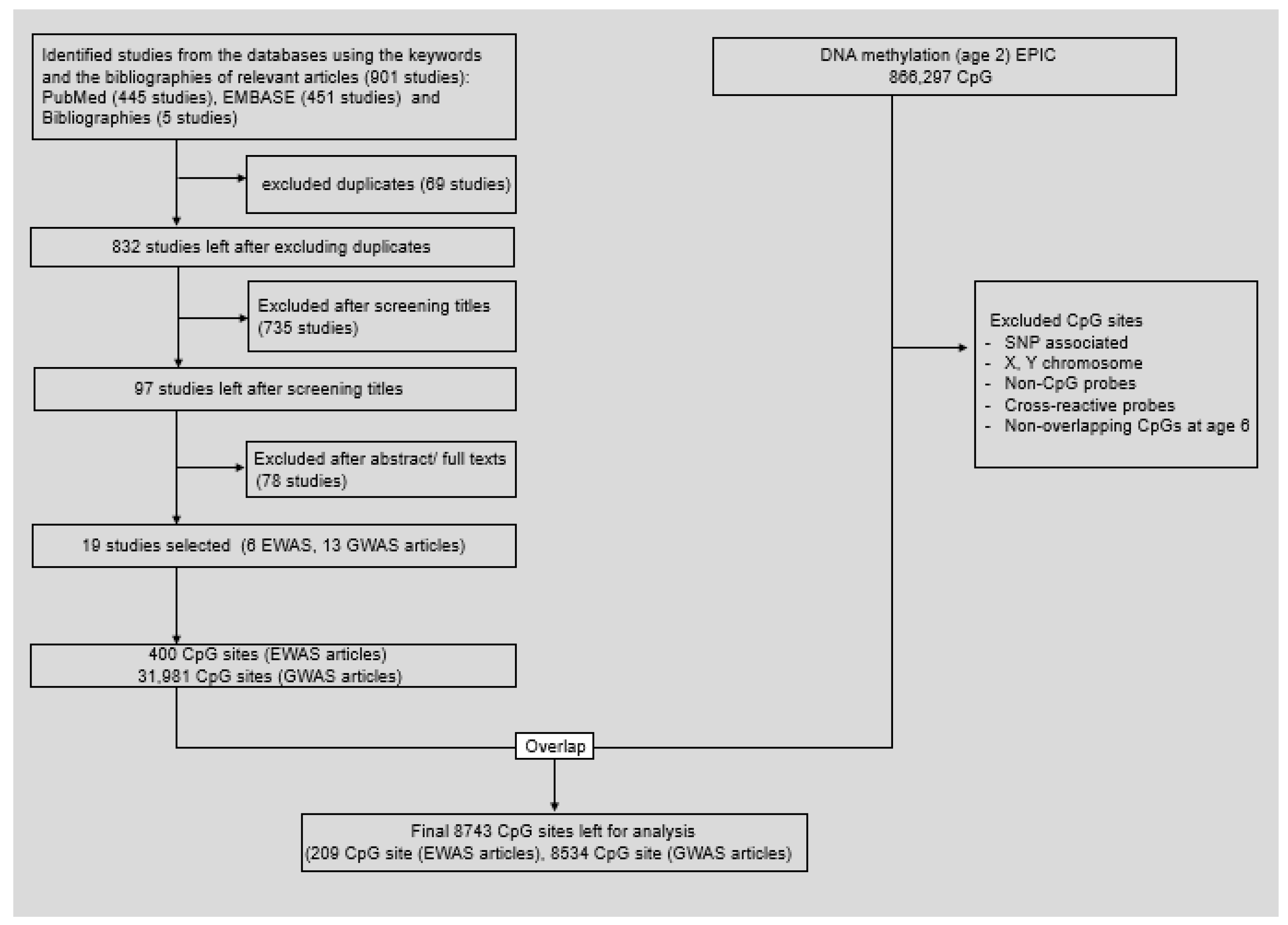
2.2. Systematic Review of Literature and Selection of Candidate Cytosine-Guanine Dinucleotide Sites
2.3. Measurement of Residential Surrounding Greenness
2.4. Measurement of DNA Methylation
2.4.1. Assessment of DNA Methylation at Age 2 in the Environment and the Development of Children Study Cohort
2.4.2. Quality Control of Methylation Data
2.5. Measurement of Intelligence Quotient in Children
2.6. Measurement of Other Exposure Variables
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Systematic Literature Review
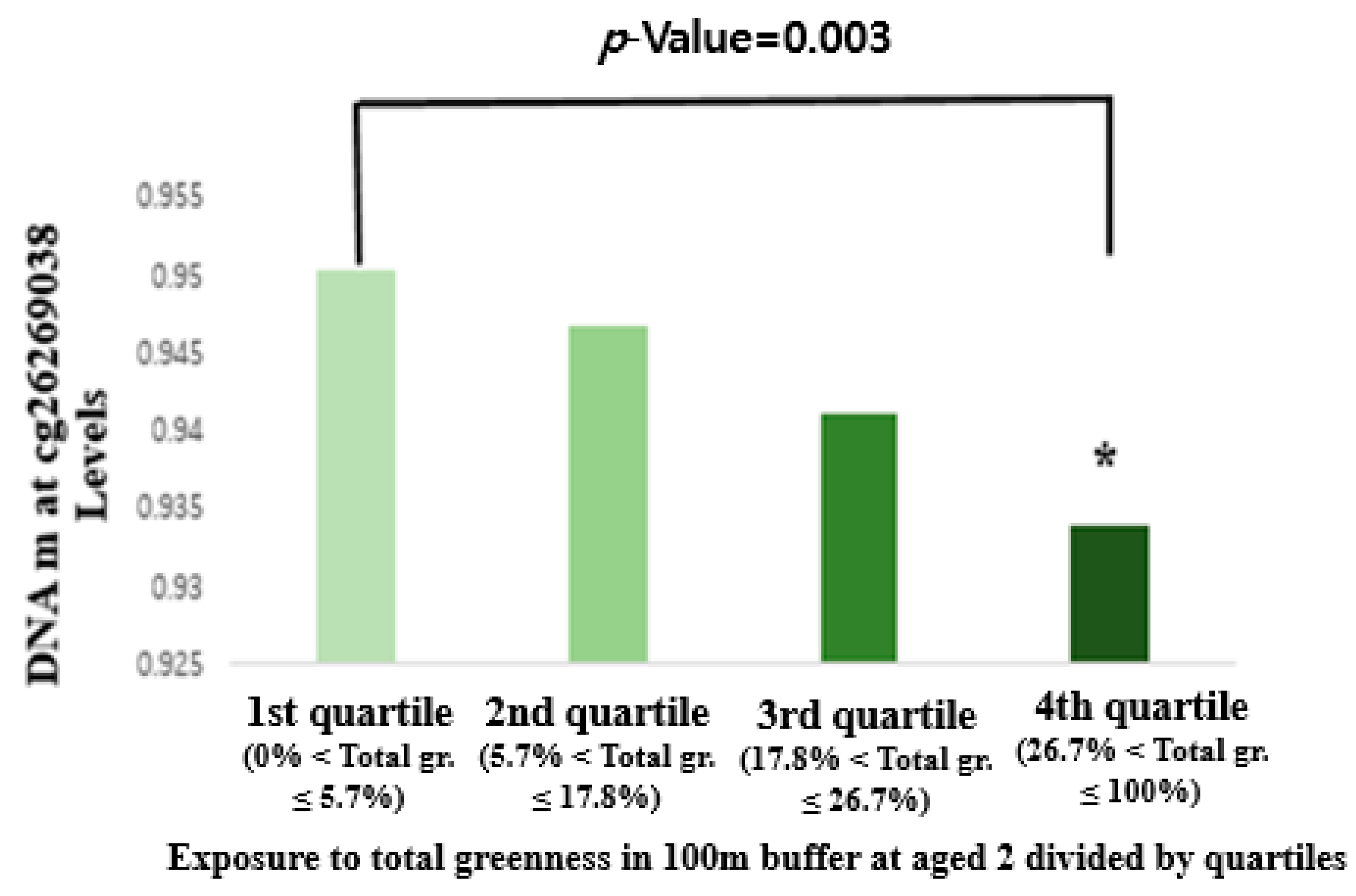
3.3. Association between Greenness Exposure and DNA Methylation
3.4. Pathway Enrichment Analysis
3.5. Association between DNA Methylation and Children’s Intelligence Quotient
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amoly, E.; Dadvand, P.; Forns, J.; López-Vicente, M.; Basagaña, X.; Julvez, J.; Alvarez-Pedrerol, M.; Mark, J.; Nieuwenhuijsen, M.J.; Sunyer, J. Green and blue spaces and behavioral development in Barcelona schoolchildren: The BREATHE project. Environ. Health Perspect. 2014, 122, 1351–1358. [Google Scholar] [CrossRef]
- Casey, J.A.; James, P.; Rudolph, K.E.; Wu, C.-D.; Schwartz, B.S. Greenness and birth outcomes in a range of Pennsylvania communities. Int. J. Environ. Res. Public Health 2016, 13, 311. [Google Scholar] [CrossRef]
- Dadvand, P.; de Nazelle, A.; Figueras, F.; Basagaña, X.; Su, J.; Amoly, E.; Jerrett, M.; Vrijheid, M.; Sunyer, J.; Nieuwenhuijsen, M.J. Green space, health inequality and pregnancy. Environ. Int. 2012, 40, 110–115. [Google Scholar] [CrossRef]
- Markevych, I.; Thiering, E.; Fuertes, E.; Sugiri, D.; Berdel, D.; Koletzko, S.; Von Berg, A.; Bauer, C.-P.; Heinrich, J. A cross-sectional analysis of the effects of residential greenness on blood pressure in 10-year old children: Results from the GINIplus and LISAplus studies. BMC Public Health 2014, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, E.; Markevych, I.; von Berg, A.; Bauer, C.-P.; Berdel, D.; Koletzko, S.; Sugiri, D.; Heinrich, J. Greenness and allergies: Evidence of differential associations in two areas in Germany. J. Epidemiol. Community Health 2014, 68, 787–790. [Google Scholar] [CrossRef]
- Kabisch, N.; van den Bosch, M.; Lafortezza, R. The health benefits of nature-based solutions to urbanization challenges for children and the elderly–A systematic review. Environ. Res. 2017, 159, 362–373. [Google Scholar] [CrossRef]
- Cilluffo, G.; Ferrante, G.; Fasola, S.; Montalbano, L.; Malizia, V.; Piscini, A.; Romaniello, V.; Silvestri, M.; Stramondo, S.; Stafoggia, M.; et al. Associations of greenness, greyness and air pollution exposure with children’s health: A cross-sectional study in Southern Italy. Environ. Health 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dzhambov, A.M.; Markevych, I.; Lercher, P. Associations of residential greenness, traffic noise, and air pollution with birth outcomes across Alpine areas. Sci. Total Environ. 2019, 678, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Grigsby-Toussaint, D.S.; Chi, S.-H.; Fiese, B.H. Where they live, how they play: Neighborhood greenness and outdoor physical activity among preschoolers. Int. J. Health Geogr. 2011, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.S.; Duncan, J.S.; Jarden, A.; Stewart, T. The impact of children’s exposure to greenspace on physical activity, cognitive development, emotional wellbeing, and ability to appraise risk. Health Place 2016, 40, 44–50. [Google Scholar] [CrossRef]
- Wan, C.; Shen, G.Q.; Choi, S.J. Underlying relationships between public urban green spaces and social cohesion: A systematic literature review. City Cult. Soc. 2021, 24, 100383. [Google Scholar] [CrossRef]
- Joubert, B.; Håberg, S.E.; Nilsen, R.M.; Wang, X.; Vollset, S.E.; Murphy, S.; Huang, Z.; Hoyo, C.; Midttun, Ø.; Uicab, L.C.; et al. 450K Epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ. Health Perspect. 2012, 120, 1425–1431. [Google Scholar] [CrossRef]
- Lee, K.W.K.; Richmond, R.; Hu, P.; French, L.; Shin, J.; Bourdon, C.; Reischl, E.; Waldenberger, M.; Zeilinger, S.; Gaunt, T.; et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: Epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of Age. Environ. Health Perspect. 2015, 123, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Markunas, C.; Xu, Z.; Harlid, S.; Wade, P.; Lie, R.T.; Taylor, J.; Wilcox, A.J. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ. Health Perspect. 2014, 122, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Broberg, K.; Ahmed, S.; Engström, K.; Hossain, M.; Mlakar, S.J.; Bottai, M.; Grander, M.; Raqib, R.; Vahter, M. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J. Dev. Orig. Health Dis. 2014, 5, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Koestler, D.C.; Houseman, E.A.; Jackson, B.P.; Kile, M.L.; Karagas, M.R.; Marsit, C.J. Differential DNA methylation in umbilical cord blood of infants exposed to mercury and arsenic in utero. Epigenetics 2015, 10, 508–515. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Tang, D.; Zhu, D.; Qu, L.; Sjödin, A.; Li, Z.; Camann, D.; Perera, F.P. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene–DNA adducts, and genomic DNA methylation in cord blood. Environ. Health Perspect. 2012, 120, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Dean, W.; Brown, D.; Reik, W.; Feil, R. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol. Reprod. 2001, 64, 918–926. [Google Scholar] [CrossRef]
- Kingsley, S.L.; Eliot, M.N.; Whitsel, E.A.; Huang, Y.-T.; Kelsey, K.T.; Marsit, C.; Wellenius, G.A. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ. Int. 2016, 92–93, 43–49. [Google Scholar] [CrossRef]
- Gruzieva, O.; Xu, C.; Breton, C.V.; Annesi-Maesano, I.; Antó, J.M.; Auffray, C.; Ballereau, S.; Bellander, T.; Bousquet, J.; Bustamante, M.; et al. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ. Health Perspect. 2017, 125, 104–110. [Google Scholar] [CrossRef]
- Cao-Lei, L.; Very, F.; Elgbeili, G.; Szyf, M.; Laplante, D.P.; King, S. DNA methylation mediates the effect of exposure to prenatal maternal stress on cytokine production in children at age 13½ years: Project Ice Storm. Clin. Epigenet. 2016, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.C.; Neelon, S.E.B.; Liu, Y.; Tuli, A.M.; Fuemmeler, B.; Hoyo, C.; Murtha, A.P.; Huang, Z.; Schildkraut, J.; Overcash, F.; et al. Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genet. Epigenet. 2014, 6. [Google Scholar] [CrossRef]
- Goodrich, J.; Dolinoy, D.C.; Sánchez, B.N.; Zhang, Z.; Meeker, J.D.; Mercado-Garcia, A.; Solano-González, M.; Hu, H.; Rojo, M.T.; Peterson, K.E. Adolescent epigenetic profiles and environmental exposures from early life through peri-adolescence. Environ. Epigenet. 2016, 2, dvw018. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; Van Dijk, S.J.; Ozanne, S. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Peng, C.; Dekker, M.D.; Cardenas, A.; Rifas-Shiman, S.L.; Gibson, H.; Agha, G.; Harris, M.H.; Coull, B.A.; Schwartz, J.; Litonjua, A.A.; et al. Residential proximity to major roadways at birth, DNA methylation at birth and midchildhood, and childhood cognitive test scores: Project Viva(Massachusetts, USA). Environ. Health Perspect. 2018, 126, 097006. [Google Scholar] [CrossRef]
- Marioni, R.E.; McRae, A.F.; Bressler, J.; Colicino, E.; Hannon, E.; Li, S.; Prada, D.; Smith, J.A.; Trevisi, L.; Tsai, P.-C.; et al. Meta-analysis of epigenome-wide association studies of cognitive abilities. Mol. Psychiatry 2018, 23, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, S.; Li, S.; Wong, E.M.; Southey, M.C.; Hopper, J.L.; Abramson, M.J.; Guo, Y. Residential surrounding greenness and DNA methylation: An epigenome-wide association study. Environ. Int. 2021, 154, 106556. [Google Scholar] [CrossRef] [PubMed]
- Peirce, T.R.; Bray, N.J.; Williams, N.M.; Norton, N.; Moskvina, V.; Preece, A.; Haroutunian, V.; Buxbaum, J.D.; Owen, M.J.; O’Donovan, M.C. Convergent evidence for 2′,3′-cyclic nucleotide 3′-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch. Gen. Psychiatry 2006, 63, 18–24. [Google Scholar] [CrossRef]
- Rajkowska, G.; Mahajan, G.; Maciag, D.; Sathyanesan, M.; Iyo, A.H.; Moulana, M.; Kyle, P.B.; Woolverton, W.L.; Miguel-Hidalgo, J.J.; Stockmeier, C.A.; et al. Oligodendrocyte morphometry and expression of myelin–Related mRNA in ventral prefrontal white matter in major depressive disorder. J. Psychiatr. Res. 2015, 65, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Kim, B.-N.; Cho, J.; Jang, Y.-Y.; Choi, Y.-J.; Lee, W.-S.; Han, C.; Bae, H.-J.; Lim, Y.-H.; Kim, J.I.; et al. Associations between surrounding residential greenness and intelligence quotient in 6-year-old children. Sci. Total Environ. 2021, 759, 143561. [Google Scholar] [CrossRef] [PubMed]
- Schoon, I.; Nasim, B.; Sehmi, R.; Cook, R. The Impact of Early Life Skills on Later Outcomes; OECD (Early Childhood Education and Care): Paris, France, 2015. [Google Scholar]
- Kim, K.-N.; Lim, Y.-H.; Shin, C.H.; Lee, Y.A.; Kim, B.-N.; Kim, J.I.; Hwang, I.G.; Hwang, M.S.; Suh, J.-H.; Hong, Y.-C. Cohort Profile: The Environment and Development of Children (EDC) study: A prospective children’s cohort. Int. J. Epidemiol. 2018, 47, 1049–1050f. [Google Scholar] [CrossRef]
- Dial, G.; Bowen, H.; Gerlach, F.; Grodecki, J.; Oleszczuk, R. IKONOS satellite, imagery, and products. Remote Sens. Environ. 2003, 88, 23–36. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Lee, Y.A.; Hong, Y.-C.; Cho, J.; Lee, K.-S.; Shin, C.H.; Kim, B.-N.; Kim, J.I.; Park, S.J.; Bisgaard, H.; et al. Effect of prenatal bisphenol A exposure on early childhood body mass index through epigenetic influence on the insulin-like growth factor 2 receptor (IGF2R) gene. Environ. Int. 2020, 143, 105929. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Marabita, F.; Lechner, M.; Bartlett, T.; Tegner, J.; Gomez-Cabrero, D.; Beck, S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2012, 29, 189–196. [Google Scholar] [CrossRef]
- Maechler, M. R Package Version 0.75-5. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing#citation (accessed on 30 May 2021).
- Park, K.; Yoon, J.; Park, H.; Park, H.; Kwon, K. Development of KEDI-WISC, Individual Intelligence Test for Korean Children; Korea Educational Development Institute: Seoul, Korea, 1996. [Google Scholar]
- Kim, J.I.; Hong, Y.-C.; Shin, C.H.; Lee, Y.A.; Lim, Y.-H.; Kim, B.-N. The effects of maternal and children phthalate exposure on the neurocognitive function of 6-year-old children. Environ. Res. 2017, 156, 519–525. [Google Scholar] [CrossRef]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-R.; Lee, W.-K.; Lee, W.-H.; Park, J.-W. The study on the accuracy and validity of Korean Wechsler Intelligence Scale short forms: A comparison of the WARD7 subtest vs Doppelt subtest. Korean J. Clin. Psychol. 2000, 19, 563–574. [Google Scholar]
- Johnson, W.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2006, 8, 118–127. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Yu, G.; He, Q.-Y.J.M.B. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef]
- Caramaschi, D.; Neumann, A.; Cardenas, A.; Tindula, G.; Alemany, S.; Zilich, L.; Pesce, G.; Lahti, J.M.T.; Havdahl, A.; Mulder, R.; et al. Meta-analysis of epigenome-wide associations between DNA methylation at birth and childhood cognitive skills. Biorxiv 2020. [Google Scholar] [CrossRef]
- Caramaschi, D.; Sharp, G.C.; Nohr, E.A.; Berryman, K.; Lewis, S.J.; Smith, G.D.; Relton, C.L. Exploring a causal role of DNA methylation in the relationship between maternal vitamin B12 during pregnancy and child’s IQ at age 8, cognitive performance and educational attainment: A two-step Mendelian randomization study. Hum. Mol. Genet. 2017, 26, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Krushkal, J.; Murphy, L.E.; Palmer, F.B.; Graff, J.C.; Sutter, T.R.; Mozhui, K.; Hovinga, C.A.; Thomas, F.; Park, V.; Tylavsky, F.A.; et al. Epigenetic analysis of neurocognitive development at 1 year of age in a community-based pregnancy cohort. Behav. Genet. 2014, 44, 113–125. [Google Scholar] [CrossRef][Green Version]
- Linnér, R.K.; Marioni, R.E.; Rietveld, C.A.; Simpkin, A.J.; Davies, N.; Watanabe, K.; Armstrong, N.J.; Auro, K.; Baumbach, C.; Bonder, M.J.; et al. An epigenome-wide association study meta-analysis of educational attainment. Mol. Psychiatry 2017, 22, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.G.; Houseman, E.A.; Green, B.B.; Lesseur, C.; Armstrong, D.A.; Lester, B.; Marsit, C.J. Regions of variable DNA methylation in human placenta associated with newborn neurobehavior. Epigenetics 2016, 11, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; Pourcain, B.S.; Davis, O.; Davies, G.; Hansell, N.; Brion, M.-J.; Kirkpatrick, R.M.; Cents, R.A.M.; Franic, S.; Miller, M.B.; et al. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol. Psychiatry 2014, 19, 253–258. [Google Scholar] [CrossRef]
- Coleman, J.R.; Bryois, J.; Gaspar, H.A.; Jansen, P.R.; Savage, J.E.; Skene, N.; Plomin, R.; Muñoz-Manchado, A.B.; Linnarsson, S.; Crawford, G.; et al. Biological annotation of genetic loci associated with intelligence in a meta-analysis of 87,740 individuals. Mol. Psychiatry 2019, 24, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.D.; Davies, G.; Van De Lagemaat, L.N.; Christoforou, A.; Marioni, R.; Fernandes, C.; Liewald, D.C.; Croning, M.D.R.; Payton, A.; Craig, L.C.A.; et al. Human cognitive ability is influenced by genetic variation in components of postsynaptic signalling complexes assembled by NMDA receptors and MAGUK proteins. Transl. Psychiatry 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.R.; Nagel, M.; Watanabe, K.; Wei, Y.; Savage, J.E.; De Leeuw, C.A.; Heuvel, M.P.V.D.; Van Der Sluis, S.; Posthuma, D. Genome-wide meta-analysis of brain volume identifies genomic loci and genes shared with intelligence. Nat. Commun. 2020, 11, 5606. [Google Scholar] [CrossRef]
- Kong, L.; Cheng, L.; Fan, L.-Y.; Zhao, M.; Qu, H. IQdb: An intelligence quotient score-associated gene resource for human intelligence. Database 2013, 2013, bat063. [Google Scholar] [CrossRef]
- Kornilov, S.; Tan, M.; Aljughaiman, A.; Naumova, O.; Grigorenko, E.L. Genome-wide homozygosity mapping reveals genes associated with cognitive ability in children from Saudi Arabia. Front. Genet. 2019, 10, 888. [Google Scholar] [CrossRef]
- Savage, J.E.; Jansen, P.R.; Stringer, S.; Watanabe, K.; Bryois, J.; De Leeuw, C.A.; Nagel, M.; Awasthi, S.; Barr, P.B.; Coleman, J.R.I.; et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 2018, 50, 912–919. [Google Scholar] [CrossRef]
- Luciano, M.; Wright, M.J.; Duffy, D.L.; Wainwright, M.A.; Zhu, G.; Evans, D.M.; Geffen, G.M.; Montgomery, G.W.; Martin, N.G. Genome-wide scan of IQ finds significant linkage to a quantitative trait locus on 2q. Behav. Genet. 2005, 36, 45–55. [Google Scholar] [CrossRef][Green Version]
- Smajlagić, D.; Jacobsen, K.K.; Myrum, C.; Haavik, J.; Johansson, S.; Zayats, T. Moderating effect of mode of delivery on the genetics of intelligence: Explorative genome-wide analyses in ALSPAC. Brain Behav. 2018, 8, e01144. [Google Scholar] [CrossRef]
- Sniekers, S.; Stringer, S.; Watanabe, K.; Jansen, P.R.; Coleman, J.R.; Krapohl, E.; Taskesen, E.; Hammerschlag, A.R.; Okbay, A.; Zabaneh, D.; et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat. Genet. 2017, 49, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Zabaneh, D.; Krapohl, E.; Gaspar, H.; Curtis, C.; Lee, S.H.; Patel, H.; Newhouse, S.; Wu, H.M.; Simpson, M.A.; Putallaz, M.; et al. A genome-wide association study for extremely high intelligence. Mol. Psychiatry 2018, 23, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Kong, L.; Qu, H. A systems biology approach to identify intelligence quotient score-related genomic regions, and pathways relevant to potential therapeutic treatments. Sci. Rep. 2014, 4, 4176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, B.; Yan, H.; Fang, W.; Zhou, Q.; Zhou, S.; Lei, H.; Huang, A.; Chen, T.; Gao, T.; et al. Multi-level genomic analyses suggest new genetic variants involved in human memory. Eur. J. Hum. Genet. 2018, 26, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Fraga, M.F. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2012, 13, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Reik, W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nat. Cell Biol. 2007, 447, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Young, L.E.; Fernandes, K.; McEvoy, T.G.; Butterwith, S.C.; Gutierrez, C.G.; Carolan, C.; Broadbent, P.J.; Robinson, J.J.; Wilmut, I.; Sinclair, K.D. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 2001, 27, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-H.; Reith, M.E.A.; Quick, M.W. Synaptic uptake and beyond: The sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflügers Arch. 2004, 447, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Fuke, S.; Suo, S.; Takahashi, N.; Koike, H.; Sasagawa, N.; Ishiura, S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenom. J. 2001, 1, 152–156. [Google Scholar] [CrossRef]
- Vandenbergh, D.; Thompson, M.; Cook, E.; Bendahhou, E.; Nguyen, T.; Krasowski, M.; Zarrabian, D.; Comings, D.; Sellers, E.M.; Tyndale, R.F.; et al. Human dopamine transporter gene: Coding region conservation among normal, Tourette’s disorder, alcohol dependence and attention-deficit hyperactivity disorder populations. Mol. Psychiatry 2000, 5, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Cómbita, L.M.; Voelker, P.; Abundis-Gutiérrez, A.; Pozuelos, J.P.; Rueda, M.R. Influence of the SLC6A3-DAT1 gene on multifaceted measures of self-regulation in preschool children. Front. Psychol. 2017, 8, 26. [Google Scholar] [CrossRef]
- Kaminski, J.A.; Schlagenhauf, F.; Rapp, M.; Awasthi, S.; Ruggeri, B.; Deserno, L.; Banaschewski, T.; Bokde, A.L.W.; Bromberg, U.; Büchel, C.; et al. Epigenetic variance in dopamine D2 receptor: A marker of IQ malleability? Transl. Psychiatry 2018, 8, 169. [Google Scholar] [CrossRef]
- Montague, P.R.; Hyman, S.E.; Cohen, J.D. Computational roles for dopamine in behavioural control. Nat. Cell Biol. 2004, 431, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, F.; Rapp, M.A.; Huys, Q.; Beck, A.; Wüstenberg, T.; Deserno, L.; Buchholz, H.; Kalbitzer, J.; Buchert, R.; Bauer, M.; et al. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum. Brain Map. 2013, 34, 1490–1499. [Google Scholar] [CrossRef]
- Figlewicz, D.P. Endocrine regulation of neurotransmitter transporters. Epilepsy Res. 1999, 37, 203–210. [Google Scholar] [CrossRef]
- Yeager, R.; Riggs, D.W.; DeJarnett, N.; Tollerud, D.J.; Wilson, J.; Conklin, D.J.; O’Toole, T.E.; McCracken, J.; Lorkiewicz, P.; Xie, Z.; et al. Association between residential greenness and cardiovascular disease risk. J. Am. Heart Assoc. 2018, 7, e009117. [Google Scholar] [CrossRef] [PubMed]
- De Petris, S.; Squillacioti, G.; Bono, R.; Borgogno-Mondino, E. Geomatics and epidemiology: Associating oxidative stress and greenness in urban areas. Environ. Res. 2021, 197, 110999. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, G.; Carsin, A.E.; Borgogno-Mondino, E.; Bono, R.; Aymerich, J.G. Greenness and physical activity as possible oxidative stress modulators in children. Eur. J. Public Health 2020, 30, ckaa165.090. [Google Scholar] [CrossRef]
- Squillacioti, G.; Bellisario, V.; Ghelli, F.; Piccioni, P.; Bono, R. Greenness effect on oxidative stress and respiratory flows in children. Environ. Epidemiol 2019, 3, 35–36. [Google Scholar]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2016, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Herman, F.; Westfall, S.; Brathwaite, J.; Pasinetti, G.M. Suppression of presymptomatic oxidative stress and inflammation in neurodegeneration by grape-derived polyphenols. Front. Pharmacol. 2018, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Volf, N.V.; Sinyakova, N.A.; Osipova, L.P.; Kulikov, A.V.; Belousova, L.V. Association between intelligence quotient and the 5HTTLPR polymorphism of human serotonin transporter coding gene. J. Psychiatry Brain Funct. 2015, 2, 8. [Google Scholar] [CrossRef]
- Kunugi, H.; Hattori, M.; Kato, T.; Tatsumi, M.; Sakai, T.; Sasaki, T.; Hirose, T.; Nanko, S. Serotonin transporter gene polymorphisms: Ethnic difference and possible association with bipolar affective disorder. Mol. Psychiatry 1997, 2, 457–462. [Google Scholar] [CrossRef][Green Version]
- Gelernter, J.; Kranzler, H.; Cubells, J.F. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African-and European-American and Japanese populations and in alcohol-dependent subjects. Hum. Genet. 1997, 101, 243–246. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueno, S.; Sano, A.; Tanabe, H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol. Psychiatry 2000, 5, 32–38. [Google Scholar] [CrossRef]
- Breton, C.V.; Marsit, C.J.; Faustman, E.; Nadeau, K.; Goodrich, J.M.; Dolinoy, D.C.; Herbstman, J.; Holland, N.; LaSalle, J.M.; Schmidt, R.; et al. Small-magnitude effect sizes in epigenetic end points are important in children’s environmental health studies: The children’s environmental health and disease prevention research center’s epigenetics working group. Environ. Health Perspect. 2017, 125, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.K.; Adigun, A.; Huang, Z.; Overcash, F.; Wang, F.; Jirtle, R.L.; Schildkraut, J.M.; Murtha, A.P.; Iversen, E.S.; Hoyo, C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene 2012, 494, 36–43. [Google Scholar] [CrossRef] [PubMed]

| Variables | Study Population (n = 59) | EDC Population Excluded from the Study (n = 366) | p-Value | |
|---|---|---|---|---|
| n (%) or Mean ± SD | n (%) or Mean ± SD | |||
| Maternal age at pregnancy (years) | 31.10 ± 3.79 | 31.68 ± 3.60 | 0.256 | |
| Children’s age (months) | 23.32 ± 0.77 | 23.31 ± 0.76 | 0.922 | |
| Children’s BMI at age 2 | 16.57 ± 1.20 | 16.48 ± 1.44 | 0.666 | |
| Maternal IQ | 117.8 ± 11.5 | 115.8 ± 11.1 | 0.248 | |
| Maternal education level | High school graduate | 9 (15.25) | 70 (19.13) | 0.669 |
| College graduate | 42 (71.19) | 257 (70.22) | ||
| Graduate school | 8 (13.56) | 39 (10.66) | ||
| Prenatal exposure to ETS | Yes | 14 (23.73) | 89 (24.32) | 0.922 |
| No | 45 (76.27) | 277 (75.68) | ||
| Children’s sex | Girl | 30 (50.85) | 172 (46.99) | 0.582 |
| Boy | 29 (49.15) | 194 (53.01) | ||
| Percentage of total greenness at the home address at age 2 | 100 m | 17.67 ± 12.8 | 19.82 ± 14.0 | 0.276 |
| 500 m | 18.71 ± 11.1 | 21.36 ± 13.9 | 0.108 | |
| 1000 m | 19.95 ± 11.2 | 23.61 ± 13.6 | 0.028 | |
| 1500 m | 23.63 ± 12.8 | 24.77 ± 12.9 | 0.529 | |
| 2000 m | 25.29 ± 13.4 | 26.32 ± 12.7 | 0.569 | |
| IQ at age 6 | Total IQ | 107.4 ± 13.7 | 110.6 ± 12.5 | 0.088 |
| Verbal IQ | 21.08 ± 5.03 | 20.81 ± 7.06 | 0.732 | |
| Performance IQ | 23.47 ± 5.10 | 22.91 ± 7.26 | 0.487 | |
| Origin Study | Greenness Type | Buffer | CpG | Gene | Difference (95% CI) § | p-Value |
|---|---|---|---|---|---|---|
| EWAS Study | Total | 100 m | cg13092901 | TYMP | 0.021(0.012, 0.029) | 2.0 × 10−5 |
| 500 m | cg04789403 | NA | 0.031(0.015, 0.047) | 1.1 × 10−4 | ||
| cg07266431 | CDK6 | 0.028(0.015, 0.040) | 1.9 × 10−4 | |||
| cg13599020 | SAMD3 | 0.026(0.014, 0.039) | 1.9 × 10−4 | |||
| cg27492942 | CISD3 | 0.029(0.013, 0.045) | 1.7 × 10−4 | |||
| 1000 m | cg00252813 | GAPDH | 0.013(0.006, 0.020) | 6.1 × 10−5 | ||
| Natural | 1000 m | cg00252813 | GAPDH | 0.013(0.007, 0.019) | 5.8 × 10−5 | |
| cg04789403 | NA | 0.020(0.010, 0.030) | 7.7 × 10−5 | |||
| Built | 1000 m | cg16594502 | NA | 0.015(0.008, 0.022) | 9.4 × 10−5 | |
| 1500 m | cg25189904 | GNG12 | 0.028(0.013, 0.044) | 2.3 × 10−4 | ||
| GWAS Study | Total | 100 m | cg26269038 | SLC6A3 | −0.011(−0.015, −0.007) | 3.2 × 10−8 |
| cg14464361 | AGAP1 | −0.023(−0.032, −0.016) | 2.2 × 10−6 | |||
| cg21175642 | CELSR3 | 0.013(0.007, 0.016) | 3.4 × 10−6 | |||
| 1000 m | cg23651585 | AUTS2 | −0.039(−0.056, −0.024) | 9.9 × 10−7 | ||
| cg27636559 | EFTUD1 | 0.007(0.004, 0.009) | 1.2 × 10−6 | |||
| cg27609819 | PLCL1 | −0.027(−0.038, −0.015) | 2.3 × 10−6 | |||
| cg16296679 | WBP2NL | 0.015(0.009, 0.022) | 2.9 × 10−6 | |||
| 1500 m | cg17146029 | AUTS2 | 0.010(0.007, 0.013) | 1.0 × 10−7 | ||
| cg00809988 | ELAVL2 | −0.006(−0.009, −0.003) | 1.5 × 10−7 | |||
| 2000 m | cg17146029 | AUTS2 | 0.009(0.006, 0.012) | 3.9 × 10−8 | ||
| cg00809988 | ELAVL2 | −0.004(−0.008, −0.002) | 3.2 × 10−7 | |||
| cg03367519 | PDE4D | −0.005(−0.008, −0.002) | 3.3 × 10−6 | |||
| Natural | 1000 m | cg27609819 | PLCL1 | −0.029(0.039, −0.020) | 3.7 × 10−8 | |
| cg23651585 | AUTS2 | −0.043(−0.059, −0.027) | 7.4 × 10−8 | |||
| cg27636559 | EFTUD1 | 0.007(0.005, 0.009) | 2.2 × 10−7 | |||
| 1500 m | cg23651585 | AUTS2 | −0.041(−0.055, −0.025) | 2.6 × 10−7 | ||
| cg23159678 | NOVA1 | 0.009(0.004, 0.014) | 1.9 × 10−6 | |||
| cg05016953 | SLC6A4 | −0.004(−0.006, −0.001) | 2.2 × 10−6 | |||
| cg27609819 | PLCL1 | −0.025(−0.036, −0.016) | 2.2 × 10−6 | |||
| cg03367519 | PDE4D | −0.005(−0.007, −0.002) | 2.9 × 10−6 | |||
| cg00809988 | ELAVL2 | −0.005(−0.007, −0.002) | 5.3 × 10−6 | |||
| 2000 m | cg17146029 | AUTS2 | 0.010(0.006, 0.012) | 1.8 × 10−6 | ||
| cg23651585 | AUTS2 | −0.046(−0.064, −0.026) | 1.9 × 10−6 | |||
| cg11176256 | BAIAP2 | 0.016(0.010, 0.023) | 3.5 × 10−6 | |||
| cg05897638 | PROS1 | −0.007(−0.010, −0.003) | 5.1 × 10−6 | |||
| cg00809988 | ELAVL2 | −0.005(−0.009, −0.002) | 5.5 × 10−6 | |||
| cg12414502 | BTN2A1 | 0.010(0.006, 0.012) | 5.6 × 10−6 | |||
| Built | 1500 m | cg19258882 | ERBB3 | 0.024(0.015, 0.032) | 4.6 × 10−6 | |
| cg18311871 | PTPRN2 | 0.081(0.047, 0.115) | 3.2 × 10−6 |
| IQ | CpG Sites | Chr | Gene | Gene Group | Difference (95% CI) § | p-Value * |
|---|---|---|---|---|---|---|
| Total IQ | cg26269038 | 5 | SLC6A3 | Body | 3.68(−0.90, 8.26) | 0.115 |
| Verbal IQ | −0.66(−2.47, 1.15) | 0.475 | ||||
| Performance IQ | 2.89(1.27, 4.51) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-S.; Choi, Y.-J.; Cho, J.-W.; Moon, S.-J.; Lim, Y.-H.; Kim, J.-I.; Lee, Y.-A.; Shin, C.-H.; Kim, B.-N.; Hong, Y.-C. Children’s Greenness Exposure and IQ-Associated DNA Methylation: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 7429. https://doi.org/10.3390/ijerph18147429
Lee K-S, Choi Y-J, Cho J-W, Moon S-J, Lim Y-H, Kim J-I, Lee Y-A, Shin C-H, Kim B-N, Hong Y-C. Children’s Greenness Exposure and IQ-Associated DNA Methylation: A Prospective Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(14):7429. https://doi.org/10.3390/ijerph18147429
Chicago/Turabian StyleLee, Kyung-Shin, Yoon-Jung Choi, Jin-Woo Cho, Sung-Ji Moon, Youn-Hee Lim, Johanna-Inhyang Kim, Young-Ah Lee, Choong-Ho Shin, Bung-Nyun Kim, and Yun-Chul Hong. 2021. "Children’s Greenness Exposure and IQ-Associated DNA Methylation: A Prospective Cohort Study" International Journal of Environmental Research and Public Health 18, no. 14: 7429. https://doi.org/10.3390/ijerph18147429
APA StyleLee, K.-S., Choi, Y.-J., Cho, J.-W., Moon, S.-J., Lim, Y.-H., Kim, J.-I., Lee, Y.-A., Shin, C.-H., Kim, B.-N., & Hong, Y.-C. (2021). Children’s Greenness Exposure and IQ-Associated DNA Methylation: A Prospective Cohort Study. International Journal of Environmental Research and Public Health, 18(14), 7429. https://doi.org/10.3390/ijerph18147429






