Salivary Pro-Inflammatory Markers and Smoking Status Influences the Treatment Effectiveness of Periodontal Disease Patients with Hypertension
Abstract
:1. Introduction
2. Materials and Methods
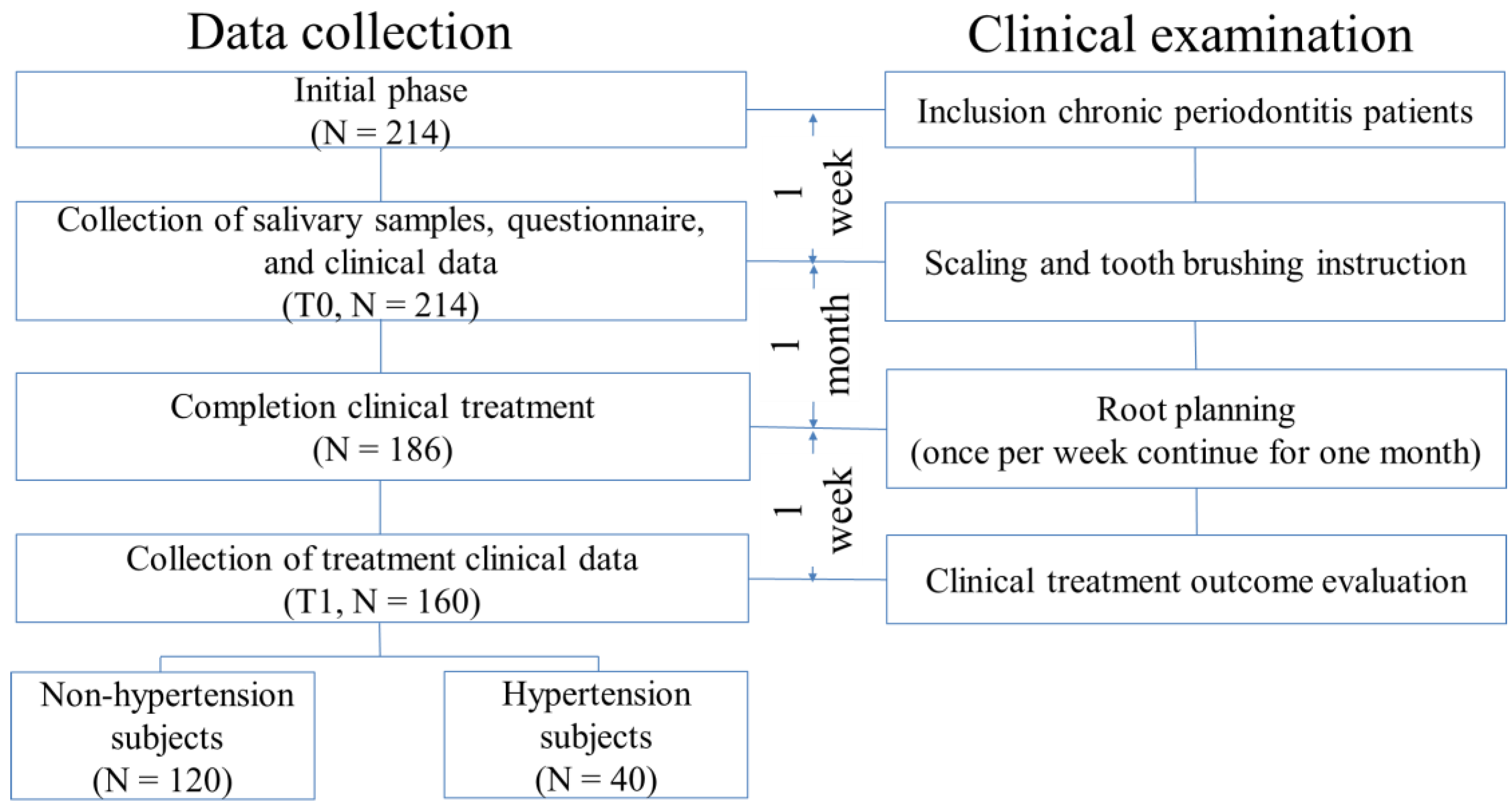
2.1. Subject Recruitment
2.2. Clinical Parameters and Treatment Evaluation
2.3. Specimen Collection and Inflammatory Biomarker Detection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44, S5–S11. [Google Scholar] [CrossRef]
- Barros, S.P.; Offenbacher, S. Modifiable risk factors in periodontal disease: Epigenetic regulation of gene expression in the inflammatory response. Periodontology 2000 2014, 64, 95–110. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Joffres, M.; Falaschetti, E.; Gillespie, C.; Robitaille, C.; Loustalot, F.; Poulter, N.; McAlister, F.A.; Johansen, H.; Baclic, O.; Campbell, N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: A cross-sectional study. BMJ Open 2013, 3, e003423. [Google Scholar] [CrossRef] [Green Version]
- Beck, J.D.; Papapanou, P.N.; Philips, K.H.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef]
- DeStefano, F.; Anda, R.F.; Kahn, H.S.; Williamson, D.F.; Russell, C.M. Dental disease and risk of coronary heart disease and mortality. BMJ 1993, 306, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Beck, J.D.; Elter, J.R.; Heiss, G.; Couper, D.; Mauriello, S.M.; Offenbacher, S. Relationship of periodontal disease to carotid artery intima-media wall thickness: The atherosclerosis risk in communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1816–1822. [Google Scholar] [CrossRef] [Green Version]
- Morita, T.; Yamazaki, Y.; Mita, A.; Takada, K.; Seto, M.; Nishinoue, N.; Sasaki, Y.; Motohashi, M.; Maeno, M. A Cohort Study on the Association Between Periodontal Disease and the Development of Metabolic Syndrome. J. Periodontol. 2010, 81, 512–519. [Google Scholar] [CrossRef]
- Carrizales-Sepulveda, E.F.; Ordaz-Farias, A.; Vera-Pineda, R.; Flores-Ramirez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Links between atherosclerotic and periodontal disease. Exp. Mol. Pathol. 2016, 100, 220–235. [Google Scholar] [CrossRef]
- Higashi, Y.; Goto, C.; Jitsuiki, D.; Umemura, T.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Soga, J.; Chayama, K.; et al. Periodontal Infection Is Associated With Endothelial Dysfunction in Healthy Subjects and Hypertensive Patients. Hypertension 2008, 51, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Clin. Periodontol. 2013, 40, S51–S69. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.W.M.; Quan, V.H.; Nguyen, B.H.; Tran, S.D. Periodontal associations in cardiovascular diseases: The latest evidence and understanding. J. Oral Biol. Craniofacial Res. 2015, 5, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Pouso, A.I.; Perez-Sayans, M.; Bravo, S.B.; Lopez-Jornet, P.; Garcia-Vence, M.; Alonso-Sampedro, M.; Carballo, J.; Garcia-Garcia, A. Protein—Based Salivary Profiles as Novel Biomarkers for Oral Diseases. Dis. Markers 2018, 2018, 6141845. [Google Scholar] [CrossRef]
- de Lima, C.L.; Acevedo, A.C.; Grisi, D.C.; Taba, M., Jr.; Guerra, E.; De Luca Canto, G. Host—Derived salivary biomarkers in diagnosing periodontal disease: Systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 492–502. [Google Scholar] [CrossRef]
- Jaedicke, K.M.; Preshaw, P.M.; Taylor, J.J. Salivary cytokines as biomarkers of periodontal diseases. Periodontology 2000 2016, 70, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, Y.W.; Tu, Y.K.; Wu, Y.C.; Chang, P.C. The potential of salivary biomarkers for predicting the sensitivity and monitoring the response to nonsurgical periodontal therapy: A preliminary assessment. J. Periodontal. Res. 2018, 53, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Hefti, A.F.; Preshaw, P.M. Examiner alignment and assessment in clinical periodontal research. Periodontology 2000 2012, 59, 41–60. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Han, M.L.; Teng, N.C.; Lee, C.Y.; Huang, W.T.; Lin, C.T.; Huang, Y.K. Cigarette Smoking Aggravates the Activity of Periodontal Disease by Disrupting Redox Homeostasis—An Observational Study. Sci Rep. 2018, 8, 11055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2019, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Chukkapalli, S.S.; Easwaran, M.; Rivera-Kweh, M.F.; Velsko, I.M.; Ambadapadi, S.; Dai, J.; Larjava, H.; Lucas, A.R.; Kesavalu, L. Sequential colonization of periodontal pathogens in induction of periodontal disease and atherosclerosis in LDLRnull mice. Pathog. Dis. 2017, 75, ftx003. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.A.; Hasturk, H.; Kantarci, A.; Serhan, C.N.; Van Dyke, T. Atherosclerosis, Periodontal Disease, and Treatment with Resolvins. Curr. Atheroscler. Rep. 2017, 19, 57. [Google Scholar] [CrossRef]
- Delaleu, N.; Bickel, M. Interleukin-1beta and interleukin-18: Regulation and activity in local inflammation. Periodontology 2000 2004, 35, 42–52. [Google Scholar] [CrossRef]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119. [Google Scholar] [CrossRef] [Green Version]
- Offenbacher, S.; Barros, S.; Mendoza, L.; Mauriello, S.; Preisser, J.; Moss, K.; De Jager, M.; Aspiras, M. Changes in gingival crevicular fluid inflammatory mediator levels during the induction and resolution of experimental gingivitis in humans. J. Clin. Periodontol. 2010, 37, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stashenko, P.; Fujiyoshi, P.; Obernesser, M.S.; Prostak, L.; Haffajee, A.D.; Socransky, S.S. Levels of interleukin 1β in tissue from sites of active periodontal disease. J. Clin. Periodontol. 1991, 18, 548–554. [Google Scholar] [CrossRef]
- Koide, M.; Suda, S.; Saitoh, S.; Ofuji, Y.; Suzuki, T.; Yoshie, H.; Takai, M.; Ono, Y.; Taniguchi, Y.; Hara, K. In vivo administration of IL-1β accelerates silk ligature-induced alveolar bone resorption in rats. J. Oral Pathol. Med. 1995, 24, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontology 2000 1997, 14, 33–53. [Google Scholar] [CrossRef]
- Passoja, A.; Puijola, I.; Knuuttila, M.; Niemela, O.; Karttunen, R.; Raunio, T.; Tervonen, T. Serum levels of interleukin-10 and tumour necrosis factor-alpha in chronic periodontitis. J. Clin. Periodontol. 2010, 37, 881–887. [Google Scholar] [CrossRef]
- Ertugrul, A.S.; Sahin, H.; Dikilitas, A.; Alpaslan, N.; Bozoglan, A. Comparison of CCL28, interleukin-8, interleukin-1beta and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J. Periodontal Res. 2013, 48, 44–51. [Google Scholar] [CrossRef]
- Graves, D.T.; Oskoui, M.; Volejnikova, S.; Naguib, G.; Cai, S.; Desta, T.; Kakouras, A.; Jiang, Y. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J. Dent. Res. 2001, 80, 1875–1879. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Heulsmann, A.; Tondravi, M.M.; Mukherjee, A.; Abu-Amer, Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J. Biol. Chem. 2001, 276, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Milward, M.R.; Chapple, I.L.C.; Wright, H.J.; Millard, J.L.; Matthews, J.B.; Cooper, P.R. Differential activation of NF-kappa B and gene expression in oral epithelial cells by periodontal pathogens. Clin. Exp. Immunol. 2007, 148, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Jagdev, D.; Coward, P.Y.; Crook, M.; Barclay, G.R.; Wilson, R.F. The Short-Term Effects of Treatment of Chronic Periodontitis on Circulating Levels of Endotoxin, C-Reactive Protein, Tumor Necrosis Factor-α, and Interleukin-6. J. Periodontol. 2004, 75, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Khocht, A.; Rogers, T.; Janal, M.N.; Brown, M. Gingival Fluid Inflammatory Biomarkers and Hypertension in African Americans. JDR Clin. Trans. Res. 2017, 2, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenstein, G. Contemporary Interpretation of Probing Depth Assessments: Diagnostic and Therapeutic Implications. A Literature Review. J. Periodontol. 1997, 68, 1194–1205. [Google Scholar] [CrossRef]
- Santarelli, A.; Mascitti, M.; Rubini, C.; Bambini, F.; Zizzi, A.; Offidani, A.; Ganzetti, G.; Laino, L.; Cicciù, M.; Muzio, L.L. Active inflammatory biomarkers in oral lichen planus. Int. J. Immunopathol. Pharmacol. 2015, 28, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary Biomarkers: Toward Future Clinical and Diagnostic Utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, R. Sex hormones and hypertension. Cardiovasc. Res. 2002, 53, 688–708. [Google Scholar] [CrossRef] [Green Version]
- Stimpson, J.P.; Wilson, F.A. Cholesterol screening by marital status and sex in the United States. Prev. Chronic Dis. 2009, 6, A55. [Google Scholar]
- Cornwell, E.Y.; Waite, L.J. Social Network Resources and Management of Hypertension. J. Health Soc. Behav. 2012, 53, 215–231. [Google Scholar] [CrossRef] [Green Version]
| Non-Hypertension (N = 120) | Hypertension (N = 40) | χ2 p Value | |
|---|---|---|---|
| Age, n (%) | <0.001 | ||
| <50 years | 38 (31.67) | 4 (10.00) | |
| 50–55 years | 26 (21.67) | 11 (27.50) | |
| 55–50 years | 33 (27.50) | 4 (10.00) | |
| >60 | 23 (19.17) | 21 (52.50) | |
| Gender, n (%) | <0.01 | ||
| Female | 82 (68.33) | 16 (40.00) | |
| Male | 38 (31.67) | 24 (60.00) | |
| Marital status, N (%) | 0.01 | ||
| Single | 28 (23.33) | 2 (5.00) | |
| Married/separated or divorced | 92 (76.67) | 38 (95.00) | |
| Years of schooling, N (%) | 0.26 | ||
| ≤9 years | 10 (8.33) | 5 (12.50) | |
| 10~12 years | 27 (22.50) | 13 (32.50) | |
| ≥13 years | 83 (69.17) | 22 (55.00) | |
| Annual income (NTD), N (%) | 0.37 | ||
| <200,000 | 31 (25.83) | 11 (27.50) | |
| 200,000~500,000 | 16 (13.33) | 3 (7.50) | |
| 500,000~1,000,000 | 25 (20.83) | 4 (10.00) | |
| >1,000,000 | 19 (15.83) | 9 (22.50) | |
| Missing | 29 (24.17) | 13 (32.50) | |
| Smoking, n (%) | <0.01 | ||
| Non-smokers | 101 (84.17) | 25 (52.50) | |
| Former smokers | 9 (7.50) | 11 (27.50) | |
| Smokers | 10 (8.33) | 4 (10.00) | |
| Clinical Parameters | Non-HT (N = 120) | Hypertension (N = 40) | ||
|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | |
| Plaque index (%) | ||||
| At the baseline | 56.7 | 44.1–67.97 | 60.91 | 44.46–77.58 |
| After completing treatment | 33.95 | 25.6–44.1 | 37.5 | 24.66–49.15 |
| p-value a | <0.001 | <0.001 | ||
| Bleeding on probing (%) | ||||
| At the baseline | 41.51 | 30.65–54.91 | 45.1 | 27.24–67.15 |
| After completing treatment | 18.47 | 13.51–30.4 | 23.47 | 15.29–31.07 |
| p-value a | <0.001 | <0.001 | ||
| PD mean (mm) | ||||
| At the baseline | 3.36 | 3.09–3.83 | 3.35 | 3.17–3.83 |
| After completing treatment | 2.73 | 2.56–2.99 | 2.86 | 2.69–3.11 |
| p-value a | <0.001 | <0.001 | ||
| PD 4–6 mm (%) | ||||
| At the baseline | 23.86 | 16.04–31.65 | 22.88 | 17.77–32.46 |
| After completing treatment | 9.70 | 6.25–14.40 | 12.6 | 6.72–16.98 |
| p-value a | <0.001 | <0.001 | ||
| PD 7–9 mm (%) | ||||
| At the baseline | 3.35 | 1.75–9.06 | 3.57 | 2.08–8.43 |
| After completing treatment | 0.64 | 0–2.04 | 1.23 | 0.29–2.08 |
| p-value a | <0.001 | <0.001 | ||
| PD 4–9 mm (%) | ||||
| At the baseline | 27.97 | 19.09–38.96 | 24.68 | 22.56–40.43 |
| After completing treatment | 10.60 | 6.79–16.67 | 13.39 | 7.14–20.03 |
| p-value a | <0.001 | <0.001 | ||
| Non-HT (N = 120) | Hypertension (N = 40) | p-Value b | |||
|---|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | ||
| Recovery rate of clinical parameters (%) | |||||
| Plaque index (%) | 39.77 | 13.11–54.81 | 36.79 | 13.81–50.19 | 0.84 |
| Bleeding on probing (%) | 53.70 | 27.02–69.01 | 52.76 | 27.66–66.23 | 0.78 |
| Mean of probing depth (mm) | 0.64 | 0.45–0.92 | 0.63 | 0.39–0.77 | 0.39 |
| PD 4–6 mm percentage (%) | 55.44 | 40.63–68.03 | 45.96 | 29.09–68.39 | 0.15 |
| PD 7–9 mm percentage (%) | 77.78 | 51.32–100.00 | 66.67 | 25.00–81.53 | 0.03 |
| PD 4–9 mm percentage (%) | 60.47 | 49.04–70.43 | 52.60 | 40.59–69.05 | 0.04 |
| Salivary inflammatory biomarker levels | |||||
| IL1-β(ng/mL) | 16.65 | 4.3–47.8 | 22.27 | 6.28–124.11 | 0.05 |
| IL-6 (ng/mL) | 5.45 | 2.27–9.75 | 5.16 | 3.02–12.74 | 0.46 |
| IL-8 (ng/mL) | 415.79 | 228.23–651.11 | 544.16 | 281.75–864.11 | 0.05 |
| TNF-α (ng/mL) | 4.11 | 1.02–8.49 | 5.51 | 1.03–10.77 | 0.50 |
| Non-HT (N = 120) | HT (N = 40) | |||||||
|---|---|---|---|---|---|---|---|---|
| IL1-β | IL-6 | IL-8 | TNF-α | IL1-β | IL-6 | IL-8 | TNF-α | |
| Periodontal clinical parameters at baseline. | ||||||||
| PCR (%) | 0.09 | 0.03 | 0.12 | −0.17 | −0.08 | 0.04 | 0.22 | −0.15 |
| BOP (%) | 0.42 *** | 0.20 * | 0.26 ** | 0.006 | 0.55 *** | 0.18 | 0.44 ** | 0.33 * |
| PD mean (mm) | 0.42 *** | 0.11 | 0.25 ** | −0.05 | 0.57 *** | 0.57 *** | 0.37 * | 0.45 ** |
| PD 4–6mm (%) | 0.27 ** | 0.10 | 0.18 | −0.02 | 0.43 ** | 0.37 * | 0.43 ** | 0.35 * |
| PD 7–9mm (%) | 0.46 *** | 0.07 | 0.21 * | −0.08 | 0.64 *** | 0.58 *** | 0.26 | 0.48 ** |
| PD 4–9mm (%) | 0.37 *** | 0.11 | 0.22 * | −0.05 | 0.52 *** | 0.48 ** | 0.38 * | 0.45 ** |
| Periodontal clinical parameters after completing treatment. | ||||||||
| PCR (%) | 0.19 * | 0.07 | 0.04 | −0.02 | 0.25 | 0.22 | 0.22 | 0.23 |
| BOP (%) | 0.22 * | 0.11 | 0.06 | −0.03 | 0.59 *** | 0.36 * | 0.48 ** | 0.49 ** |
| PD mean (mm) | 0.33 ** | 0.02 | 0.12 | −0.01 | 0.61 *** | 0.47 ** | 0.37 * | 0.46 ** |
| PD 4–6mm (%) | 0.27 ** | 0.02 | 0.14 | −0.03 | 0.52 *** | 0.47 ** | 0.32 * | 0.42 ** |
| PD 7–9mm (%) | 0.33 *** | 0.02 | 0.20 * | −0.06 | 0.56 *** | 0.47 ** | 0.05 | 0.49 ** |
| PD 4–9mm (%) | 0.30 *** | 0.01 | 0.17 | −0.05 | 0.57 *** | 0.47 ** | 0.30 | 0.45 ** |
| Dependent Variable | Recovery Rate of PI (%) | Recovery Rate of PD 4–6 mm (%) | |||
|---|---|---|---|---|---|
| Non HT | HT | Non HT | HT | ||
| Independent Variable | β (SE) | β (SE) | β (SE) | β (SE) | |
| Model I | Model II | Model III | Model IV | ||
| Salivary IL-1β level | −7.40 (8.37) | −27.65 (13.58) * | −0.19 (3.84) | −17.05 (7.15) * | |
| Smoking status | −3.54 (13.10) | 2.76 (14.87) | −4.75 (6.01) | −26.8 (7.83) ** | |
| Age | 4.97 (3.88) | 9.37 (6.26) | 4.89 (1.78) * | 1.20 (3.30) | |
| Gender | 3.72 (10.29) | −21.17 (14.67) | −0.75 (4.73) | 7.15 (7.73) | |
| Marital status | −6.55 (10.22) | −24.08 (31.11) | −2.98 (4.69) | 19.23 (16.39) | |
| Model V | Model VI | Model VII | Model VIII | ||
| Salivary IL-6 level | 0.81 (8.58) | −27.55 (12.90) * | 2.30 (3.92) | −6.58 (7.29) | |
| Smoking status | −4.32 (13.14) | 7.08 (14.69) | −4.99 (6.01) | −24.44 (8.31) * | |
| Age | 5.23 (3.88) | 9.83 (6.22) | 4.9 (1.77) * | 1.38 (3.52) | |
| Gender | 3.55 (10.45) | −11.31 (14.48) | −1.18 (4.78) | 11.6 (8.19) | |
| Marital status | −6.42 (10.27) | −28.95 (30.91) | −3.15 (4.69) | 16.82 (17.48) | |
| Model IX | Model X | Model XI | Model XII | ||
| Salivary IL-8 level | 10.67 (8.45) | −14.09 (15.20) | −5.1 (3.87) | −10.98 (8.05) | |
| Smoking status | −4.52 (13.03) | 9.63 (15.86) | −4.63 (5.96) | −22.01 (8.4) * | |
| Age | 4.64 (3.88) | 9.32 (6.55) | 5.18 (1.78) ** | 1.14 (3.47) | |
| Gender | 2.63 (10.29) | −20.37 (15.92) | −0.23 (4.71) | 6.88 (8.44) | |
| Marital status | −7.35 (10.21) | −23.51 (32.76) | −2.5 (4.67) | 20.21 (17.36) | |
| Model XIII | Model XIV | Model XV | Model XVI | ||
| Salivary TNF-α level | −5.00 (8.38) | −37.96 (13.04) ** | −3.99 (3.82) | −12.74 (7.52) | |
| Smoking status | −4.85 (13.14) | 8.25 (14.01) | −5.25 (5.99) | −23.96 (8.08) * | |
| Age | 5.09 (3.88) | 9.48 (5.93) | 4.79 (1.77) * | 1.29 (3.42) | |
| Gender | 3.94 (10.32) | −27.22 (14.23) | −0.55 (4.71) | 6.68 (8.21) | |
| Marital status | −6.51 (10.24) | −42.81 (29.91) | −3.1 (4.67) | 12.01 (17.25) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-T.; Guo, Z.-L.; Teng, N.-C.; Hsu, K.-L.C.; Chen, I.-H.; Lee, C.-Y.; Chang, H.-M.; Huang, Y.-K. Salivary Pro-Inflammatory Markers and Smoking Status Influences the Treatment Effectiveness of Periodontal Disease Patients with Hypertension. Int. J. Environ. Res. Public Health 2021, 18, 7364. https://doi.org/10.3390/ijerph18147364
Lee K-T, Guo Z-L, Teng N-C, Hsu K-LC, Chen I-H, Lee C-Y, Chang H-M, Huang Y-K. Salivary Pro-Inflammatory Markers and Smoking Status Influences the Treatment Effectiveness of Periodontal Disease Patients with Hypertension. International Journal of Environmental Research and Public Health. 2021; 18(14):7364. https://doi.org/10.3390/ijerph18147364
Chicago/Turabian StyleLee, Kun-Tsung, Zhu-Ling Guo, Nai-Chia Teng, Kuei-Ling Christine Hsu, I-Hui Chen, Chang-Yu Lee, Hung-Ming Chang, and Yung-Kai Huang. 2021. "Salivary Pro-Inflammatory Markers and Smoking Status Influences the Treatment Effectiveness of Periodontal Disease Patients with Hypertension" International Journal of Environmental Research and Public Health 18, no. 14: 7364. https://doi.org/10.3390/ijerph18147364
APA StyleLee, K.-T., Guo, Z.-L., Teng, N.-C., Hsu, K.-L. C., Chen, I.-H., Lee, C.-Y., Chang, H.-M., & Huang, Y.-K. (2021). Salivary Pro-Inflammatory Markers and Smoking Status Influences the Treatment Effectiveness of Periodontal Disease Patients with Hypertension. International Journal of Environmental Research and Public Health, 18(14), 7364. https://doi.org/10.3390/ijerph18147364








