Comparison of Point-of-Care Testing and Hospital-Based Methods in Screening for Potential Type 2 Diabetes Mellitus and Abnormal Glucose Regulation in a Dental Setting
Abstract
1. Introduction
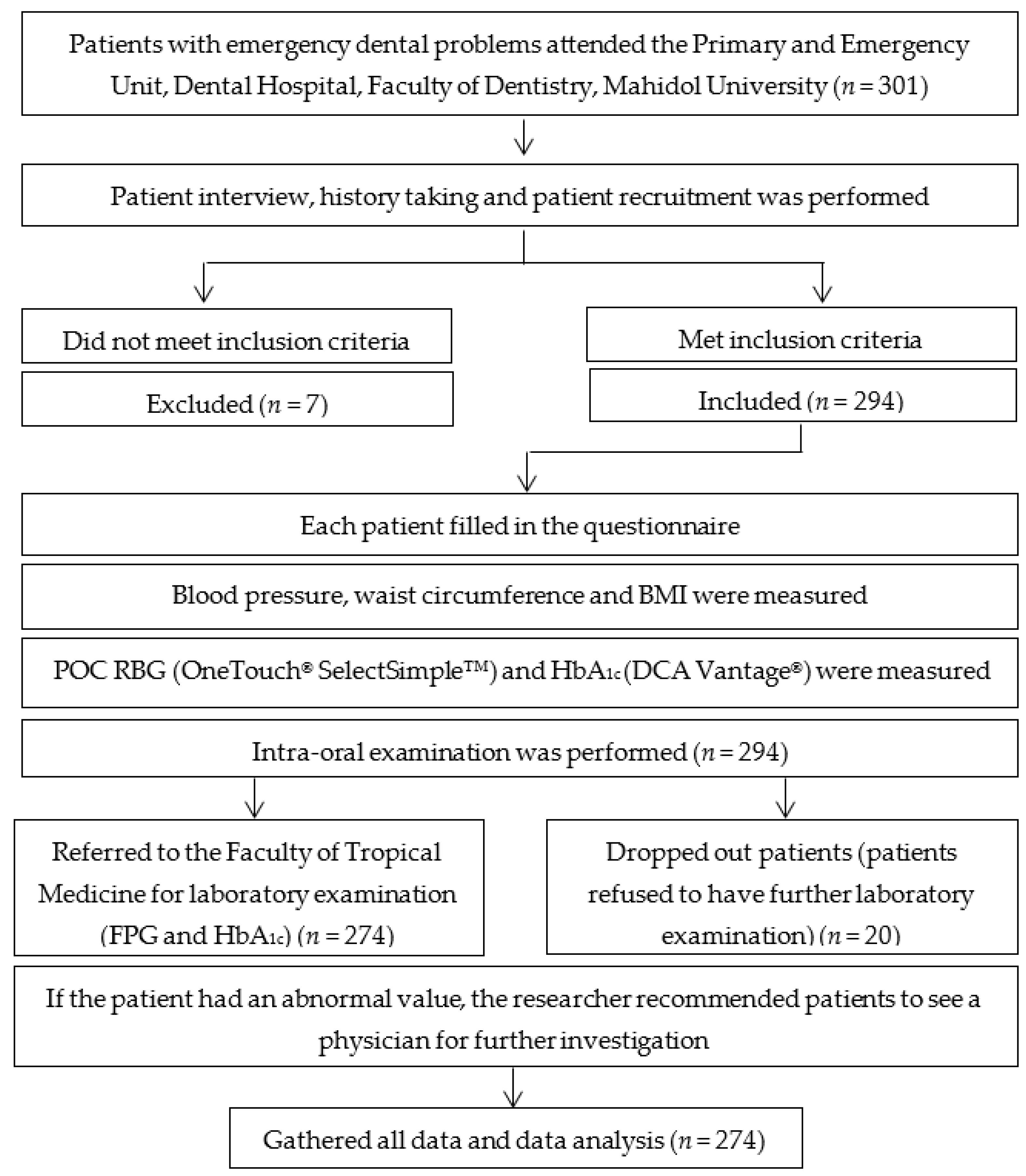
2. Materials and Methods
2.1. Study Population
2.2. Demographic Data Collection
2.3. Periodontal Examination
2.4. Glycemic Measurement
- -
- Hyperglycemia.
- -
- Potential type 2 DM.
- -
- AGR.
2.5. Statistical Analysis
3. Results
3.1. Subjects’ Characteristics
3.2. Prevalence of Hyperglycemia and Potential Type 2 DM
3.3. Agreement between Hospital-Based Laboratory Measurement and POC Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Saudek, C.D.; Herman, W.H.; Sacks, D.B.; Bergenstal, R.M.; Edelman, D.; Davidson, M.B. A new look at screening and diagnosing diabetes mellitus. J. Clin. Endocrinol. Metab. 2008, 93, 2447–2453. [Google Scholar] [CrossRef]
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Assanangkornchai, S.; Taneepanichskul, S.; Putwatana, P. Prevalence of Diabetes and Relationship with Socioeconomic Status in the Thai Population: National Health Examination Survey, 2004–2014. J. Diabetes Res. 2018, 2018, 1654530. [Google Scholar] [CrossRef]
- Rohlfing, C.L.; Wiedmeyer, H.M.; Little, R.R.; England, J.D.; Tennill, A.; Goldstein, D.E. Defining the relationship between plasma glucose and HbA(1c): Analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002, 25, 275–278. [Google Scholar] [CrossRef]
- Rahbar, S.; Blumenfeld, O.; Ranney, H.M. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem. Biophys. Res. Commun. 1969, 36, 838–843. [Google Scholar] [CrossRef]
- World Health Organization. Use of Glycated Haemoglobin (Hba1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of A WHO Consultation; World Health Organization: Geneva, Swizerland, 2011. [Google Scholar]
- Kennedy, L.; Herman, W.H.; Team, G.A.C.S. Glycated hemoglobin assessment in clinical practice: Comparison of the A1cNow point-of-care device with central laboratory testing (GOAL A1C Study). Diabetes Technol. Ther. 2005, 7, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, W.; Tang, J.; Dong, L.; Gu, C.; Zhang, X.; Zhou, J.; Jia, W. Verification of a novel point-of-care HbA1c device in real world clinical practice by comparison to three high performance liquid chromatography instruments. Biochem. Med. 2018, 28, 020705. [Google Scholar] [CrossRef] [PubMed]
- Mayega, R.W.; Guwatudde, D.; Makumbi, F.E.; Nakwagala, F.N.; Peterson, S.; Tomson, G.; Ostenson, C.G. Comparison of fasting plasma glucose and haemoglobin A1c point-of-care tests in screening for diabetes and abnormal glucose regulation in a rural low income setting. Diabetes Res. Clin. Pract. 2014, 104, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Albisser, A.M.; Sakkal, S.; Wright, C. Home blood glucose prediction: Validation, safety, and efficacy testing in clinical diabetes. Diabetes Technol. Ther. 2005, 7, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Glurich, I.; Bartkowiak, B.; Berg, R.L.; Acharya, A. Screening for dysglycaemia in dental primary care practice settings: Systematic review of the evidence. Int. Dent. J. 2018, 68, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Tantipoj, C.; Sakoolnamarka, S.S.; Supa-amornkul, S.; Lohsoonthorn, V.; Deerochanawong, C.; Khovidhunkit, S.P.; Hiransuthikul, N. Screening for Type 2 Diabetes Mellitus and Prediabetes Using Point-of-Care Testing for Hba1c among Thai Dental Patients. Southeast. Asian J. Trop. Med. Public Health 2017, 48, 455–465. [Google Scholar]
- Herman, W.H.; Taylor, G.W.; Jacobson, J.J.; Burke, R.; Brown, M.B. Screening for prediabetes and type 2 diabetes in dental offices. J. Public Health Dent. 2015, 75, 175–182. [Google Scholar] [CrossRef]
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Sangthong, R.; Inthawong, R.; Putwatana, P.; Taneepanichskul, S.; Thai National Health Examination Survey, I.V.S.G. Prevalence and management of diabetes and metabolic risk factors in Thai adults: The Thai National Health Examination Survey IV, 2009. Diabetes Care 2011, 34, 1980–1985. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Tytun, A.; Ury, H.K. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics 1980, 36, 343–346. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Engelgau, M.M.; Thompson, T.J.; Smith, P.J.; Herman, W.H.; Aubert, R.E.; Gunter, E.W.; Wetterhall, S.F.; Sous, E.S.; Ali, M.A. Screening for diabetes mellitus in adults. The utility of random capillary blood glucose measurements. Diabetes Care 1995, 18, 463–466. [Google Scholar] [CrossRef]
- Jadhav, A.N.; Tarte, P.R.; Puri, S.K. Dental clinic: Potential source of high-risk screening for prediabetes and type 2 diabetes. Indian J. Dent. Res. 2019, 30, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Leite, F.R.M.; Vestergaard, P.; Scheutz, F.; Lopez, R. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol 2018, 55, 653–667. [Google Scholar] [CrossRef]
- Nazir, M.A.; AlGhamdi, L.; AlKadi, M.; AlBeajan, N.; AlRashoudi, L.; AlHussan, M. The burden of Diabetes, Its Oral Complications and Their Prevention and Management. Open Access Maced. J. Med. Sci. 2018, 6, 1545–1553. [Google Scholar] [CrossRef]
- Genco, R.J.; Schifferle, R.E.; Dunford, R.G.; Falkner, K.L.; Hsu, W.C.; Balukjian, J. Screening for diabetes mellitus in dental practices: A field trial. J. Am. Dent. Assoc. 2014, 145, 57–64. [Google Scholar] [CrossRef]
- Evron, J.M.; Herman, W.H.; McEwen, L.N. Changes in Screening Practices for Prediabetes and Diabetes Since the Recommendation for Hemoglobin A1c Testing. Diabetes Care 2019, 42, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Maxim, L.D.; Niebo, R.; Utell, M.J. Screening tests: A review with examples. Inhal. Toxicol. 2014, 26, 811–828. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Laboratory HbA1c | FPG | ||
|---|---|---|---|---|---|
| n (%) | <5.7% n (%) | ≥5.7% n (%) | <100 mg/dl n (%) | ≥100 mg/dl n(%) | |
| Gender | |||||
| Male | 106 (38.7) | 80 (75.5) | 26 (24.5) | 71 (67.0) | 35 (33.0) |
| Female | 168 (61.3) | 147 (87.5) | 21 (12.5) | 135 (80.4) | 33 (19.6) |
| Age | |||||
| ≤40 y | 134 (48.9) | 115 (85.8) | 19 (14.2) | 113 (84.3) | 21 (15.7) |
| 41–60 y | 90 (32.9) | 74 (82.2) | 16 (17.8) | 63 (70.0) | 27 (30.0) |
| ≥61 y | 50 (18.3) | 38 (76.0) | 12 (24.0) | 30 (60.0) | 20 (40.0) |
| Marital status | |||||
| Single | 158 (57.7) | 133 (84.2) | 25 (15.8) | 114 (72.2) | 44 (27.8) |
| Married | 77 (28.1) | 63 (81.8) | 14 (18.2) | 60 (77.9) | 17 (22.1) |
| Divorced/widow | 39 (14.2) | 31 (79.5) | 8 (20.5) | 32 (82.1) | 7 (17.9) |
| Smoking status | |||||
| Non-smoking | 203 (74.1) | 175 (86.2) | 28 (13.8) | 158 (77.8) | 45 (22.2) |
| Former smoking | 61 (22.3) | 45 (73.8) | 16 (26.2) | 41 (67.2) | 20 (32.8) |
| Current smoking | 10 (3.7) | 7 (70.0) | 3 (30.0) | 7 (70.0) | 3 (30.0) |
| Alcohol consumption | |||||
| Never | 163 (59.5 | 139 (85.3) | 24 (14.7) | 124 (76.1) | 39 (23.9) |
| Sometimes | 56 (20.4) | 42 (75.0) | 14 (25.0) | 41 (73.2) | 15 (26.8) |
| Usually | 55 (20.1) | 46 (83.6) | 9 (16.4) | 41 (74.5) | 14 (25.5) |
| Underlying disease | |||||
| Yes | 193 (70.4) | 166 (86.0) | 27 (14.0) | 149 (72.2) | 44 (22.8) |
| No | 81 (29.6) | 61 (75.3) | 20 (24.7) | 57 (70.4) | 24 (29.6) |
| BMI | |||||
| ≥23 kg/m2 | 152 (55.5) | 118 (77.6) | 34 (22.4) | 117 (77.0) | 35 (23.0) |
| <23 kg/m2 | 122 (44.5) | 109 (89.3) | 13 (10.7) | 89 (73.0) | 33 (27.0) |
| Waist circumference | |||||
| Male | |||||
| ≥90 cm | 70 (66.0) | 51 (72.9) | 19 (27.1) | 46 (65.7) | 24 (34.3) |
| <90 cm | 36 (34.0) | 29 (80.6) | 7 (19.4) | 25 (69.4) | 11 (30.6) |
| Female | |||||
| ≥80 cm | 81 (48.2) | 64 (79.0) | 17 (21.0) | 61 (75.3) | 20 (24.7) |
| <80 cm | 87 (51.8) | 83 (95.4) | 4 (4.6) | 74 (85.1) | 13 (14.9) |
| Family history of DM | |||||
| Positive | 84 (30.7) | 63 (75.0) | 21 (25.0) | 63 (75.0) | 21 (25.0) |
| Negative | 190 (69.3) | 164 (86.3) | 26 (13.7) | 143 (75.3) | 47 (24.7) |
| Symptoms of DM | |||||
| With at least 1 symptom | 44 (16.1) | 33 (75.0) | 11 (25.0) | 31 (70.5) | 13 (29.5) |
| Without any symptoms | 230 (83.9) | 194 (84.3) | 36 (15.7) | 175 (76.1) | 55 (23.9) |
| Hypertension | |||||
| ≥140/90 mmHg | 66 (24.1) | 47 (71.2) | 19 (28.8) | 44 (66.7) | 22 (33.3) |
| <140/90 mmHg | 208 (75.9) | 180 (86.5) | 28 (13.5) | 162 (77.9) | 46 (22.1) |
| Periodontal status | |||||
| Mild/none | 173 (63.1) | 156 (90.2) | 17 (9.8) | 149 (86.1) | 24 (13.9) |
| Moderate | 48 (17.5) | 36 (75.0) | 12 (25.0) | 30 (62.5) | 18 (37.5) |
| Severe | 53 (19.3) | 35 (66.0) | 18 (34.0) | 27 (50.9) | 26 (49.1) |
| Method | Range | Frequency | Percent |
|---|---|---|---|
| POC HbA1c | <5.7% | 140 | 51 |
| 5.7–6.4% | 115 | 42 | |
| ≥6.5% | 19 | 7 | |
| ≥5.7% | 134 | 49 | |
| RBG | <110 mg/dl | 101 | 37 |
| 110–200 mg/dl | 154 | 56 | |
| >200 mg/dl | 19 | 7 | |
| ≥110 mg/dl | 173 | 63 | |
| RBG | <140 mg/dl | 186 | 68 |
| 140–200 mg/dl | 69 | 25 | |
| >200 mg/dl | 19 | 7 | |
| ≥140 mg/dl | 88 | 32 | |
| FPG | <100 mg/dl | 206 | 75 |
| 100–125 mg/dl | 56 | 20 | |
| ≥126 mg/dl | 12 | 4 | |
| ≥100 mg/dl | 68 | 25 | |
| Laboratory-HbA1c | <5.7% | 227 | 83 |
| 5.7–6.4% | 35 | 13 | |
| ≥6.5% | 12 | 4 | |
| ≥5.7% | 47 | 17 |
| Testing | Hospital-Based FPG Level | Sens ** | Spec ** | PPV | NPV | AUC | 95%CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal * n (%) | AGR * n (%) | Total n (%) | |||||||
| POC HbA1c | |||||||||
| <5.7% | 128 (46.72) | 12 (4.38) | 140 (51.09) | 82.35 | 62.14 | 41.79 | 91.43 | 0.72 | 0.67–0.78 |
| ≥5.7% | 78 (28.47) | 56 (20.44) | 134 (48.91) | ||||||
| Total | 206 (75.19) | 68 (24.82) | |||||||
| RBG cut-off = 110 mg/dl | |||||||||
| <110 mg/dl | 91 (33.21) | 10 (3.65) | 101 (36.86) | 85.29 | 44.17 | 33.53 | 90.10 | 0.65 | 0.59–0.70 |
| ≥110 mg/dl | 115 (41.97) | 58 (21.17) | 173 (63.14) | ||||||
| Total | 206 (75.19) | 68 (24.82) | |||||||
| RBG cut-off = 140 mg/dl | |||||||||
| <140 mg/dl | 159 (58.03) | 27 (9.85) | 186 (67.88) | 60.29 | 77.18 | 46.59 | 85.48 | 0.69 | 0.62–0.75 |
| ≥140 mg/dl | 47 (17.15) | 41 (14.96) | 88 (32.12) | ||||||
| Total | 206 (75.19) | 68 (24.82) | |||||||
| Non-DM * | Potential DM * | ||||||||
| POC HbA1c | |||||||||
| <6.5% | 255 (93.07) | 0 (0) | 255 (93.07) | 100.00 | 97.33 | 63.16 | 100 | 0.99 | 0.98–0.99 |
| ≥6.5% | 7 (2.55) | 12 (4.38) | 19 (6.93) | ||||||
| Total | 262 (95.62) | 12 (4.38) | |||||||
| RBG | |||||||||
| <200 mg/dl | 254 (92.70) | 1 (0.36) | 255 (93.07) | 91.67 | 96.95 | 57.89 | 99.61 | 0.94 | 0.86–1.00 |
| ≥200 mg/dl | 8 (2.92) | 11 (4.02) | 19 (6.93) | ||||||
| Total | 255 (93.07) | 12 (4.38) | |||||||
| Testing | Hospital-Based HbA1c Level | Sens ** | Spec ** | PPV | NPV | AUC | 95%CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal * n (%) | AGR * n (%) | Total n (%) | |||||||
| POC HbA1c | |||||||||
| <5.7% | 140 (51.09) | 0 (0) | 140 (51.09) | 100 | 61.67 | 35.07 | 100 | 0.81 | 0.78–0.84 |
| ≥5.7% | 87 (31.75) | 47 (17.15) | 134 (48.91) | ||||||
| Total | 227 (82.84) | 47 (17.15) | |||||||
| RBG cut-off = 110 mg/dl | |||||||||
| <110 mg/dl | 94 (34.31) | 7 (2.55) | 101 (36.86) | 85.11 | 41.41 | 23.12 | 93.07 | 0.63 | 0.57–0.69 |
| ≥110 mg/dl | 133 (48.54) | 40 (14.60) | 173 (63.14) | ||||||
| Total | 227 (82.84) | 47 (17.15) | |||||||
| RBG cut-off = 140 mg/dl | |||||||||
| <140 mg/dl | 169 (61.68) | 17 (6.20) | 186 (67.88) | 63.83 | 74.45 | 34.09 | 90.86 | 0.69 | 0.62–0.77 |
| ≥140 mg/dl | 58 (21.17) | 30 (10.95) | 88 (32.12) | ||||||
| Total | 227 (82.84) | 47 (17.15) | |||||||
| Non-DM * | Potential DM * | ||||||||
| POC HbA1c | |||||||||
| <6.5% | 255 (93.07) | 0 (0) | 255 (93.07) | 100 | 97.33 | 63.16 | 100 | 0.99 | 0.98–0.99 |
| ≥6.5% | 7 (2.55) | 12 (4.38) | 19 (6.93) | ||||||
| Total | 262 (95.62) | 12 (4.38) | |||||||
| RBG | |||||||||
| <200 mg/dl | 253 (92.34) | 2 (0.73) | 255 (93.07) | 83.33 | 96.56 | 52.63 | 99.22 | 0.90 | 0.79–1.00 |
| ≥200 mg/dl | 9 (3.28) | 10 (3.65) | 19 (6.93) | ||||||
| Total | 262 (95.62) | 12 (4.38) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwattipong, M.; Thuramonwong, T.; Tantipoj, C.; Fuangtharnthip, P.; Thanakun, S.; Khovidhunkit, W.; Khovidhunkit, S.-o.P. Comparison of Point-of-Care Testing and Hospital-Based Methods in Screening for Potential Type 2 Diabetes Mellitus and Abnormal Glucose Regulation in a Dental Setting. Int. J. Environ. Res. Public Health 2021, 18, 6459. https://doi.org/10.3390/ijerph18126459
Suwattipong M, Thuramonwong T, Tantipoj C, Fuangtharnthip P, Thanakun S, Khovidhunkit W, Khovidhunkit S-oP. Comparison of Point-of-Care Testing and Hospital-Based Methods in Screening for Potential Type 2 Diabetes Mellitus and Abnormal Glucose Regulation in a Dental Setting. International Journal of Environmental Research and Public Health. 2021; 18(12):6459. https://doi.org/10.3390/ijerph18126459
Chicago/Turabian StyleSuwattipong, Muneedej, Thitima Thuramonwong, Chanita Tantipoj, Pornpoj Fuangtharnthip, Supanee Thanakun, Weerapan Khovidhunkit, and Siribang-on Piboonniyom Khovidhunkit. 2021. "Comparison of Point-of-Care Testing and Hospital-Based Methods in Screening for Potential Type 2 Diabetes Mellitus and Abnormal Glucose Regulation in a Dental Setting" International Journal of Environmental Research and Public Health 18, no. 12: 6459. https://doi.org/10.3390/ijerph18126459
APA StyleSuwattipong, M., Thuramonwong, T., Tantipoj, C., Fuangtharnthip, P., Thanakun, S., Khovidhunkit, W., & Khovidhunkit, S.-o. P. (2021). Comparison of Point-of-Care Testing and Hospital-Based Methods in Screening for Potential Type 2 Diabetes Mellitus and Abnormal Glucose Regulation in a Dental Setting. International Journal of Environmental Research and Public Health, 18(12), 6459. https://doi.org/10.3390/ijerph18126459






