Abstract
Liver fibrosis might be linked to the prevalence of chronic kidney disease (CKD). However, there is little information about the association between liver fibrosis and decreased kidney function in middle-aged and older subjects. We aimed to evaluate the influence of liver fibrosis on the incidence or prevalence of CKD stage 3–5 in a retrospective cross-sectional study (Study 1, n = 806) and a 6-year longitudinal study (Study 2, n = 380) of middle-aged and older subjects. We evaluated liver fibrosis using the Fibrosis-4 (FIB-4) index and kidney function using the estimated glomerular filtration rate (eGFR) of all subjects. All subjects were divided into four groups on the basis of their FIB-4 score quartiles (low to high). In the Jonckheere–Terpstra trend test of Study 1, the eGFR decreased significantly from the lowest group to the highest group (p < 0.001). The Kaplan–Meier survival curve in Study 2 showed that the cumulative prevalence of CKD stage 3–5 was higher in the third quartile than the other quartiles. Our results suggest that liver fibrosis could be a useful indicator for the prevalence of CKD, even within a relatively healthy population, although liver fibrosis was not an independent risk factor.
1. Introduction
Chronic liver disease includes the progressive destruction and regeneration of liver parenchyma, which results in fibrosis and cirrhosis that is caused by inflammation and abnormal metabolism [1]. Chronic kidney disease (CKD) has multiple traditional and nontraditional risk factors that are similar to those of chronic liver disease, such as insulin resistance and chronic inflammation [2]. Previous studies have shown that non-alcoholic fatty liver disease (NAFLD) is associated with CKD complication [3,4,5,6], suggesting that liver fibrosis might predict the early stage of CKD development. A previous study showed that NAFLD was independently associated with a high prevalence of microalbuminuria and an increased risk of CKD; if the disease is identified early, it can be appropriately managed using standard medical therapies [6].
The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines indicate that the kidneys are the target organ of many diseases, and significant aggravation or systemic pathophysiological processes can begin through their complex functions and effects on body homeostasis [7]. The causes of CKD have been reported to be complex and include common diseases such as hypertension, metabolic syndrome, diabetes, etc., that mainly affect the kidney [7,8,9]. As mentioned earlier, an increasing amount of evidence suggests that chronic liver disease is tightly linked to metabolic syndrome, which may be associated with many comorbidities such as insulin resistance, cardiovascular disease (CVD), and acute kidney injury [1].
It is known that kidney and liver dysfunction progress through common risk factors such as obesity, type 2 diabetes, hypertension, and dyslipidemia [5]. Although it is known that kidney and liver fibrosis are promoted by these common risk factors, the relationship between the two remains unclear. This study focused on relatively healthy middle-aged and older subjects. We believe that clarifying the relationship between kidney dysfunction and liver fibrosis, even in relatively healthy subjects, can contribute to the onset of CKD and the progression to NAFLD and NASH prevention in the future. A previous study showed that the Fibrosis-4 index (FIB4-index; a simple index for assessing liver fibrosis) was superior to other tested noninvasive markers of fibrosis in Japanese patients with NAFLD, with a high negative predictive value for excluding advanced fibrosis [10].
The aim of this study was to determine the association between the progression of liver fibrosis, which was evaluated using the FIB-4 index and the estimated glomerular filtration rate (eGFR), on the incidence of CKD stage 3–5 in a retrospective cross-sectional study and a 6-year longitudinal study in middle-aged and older subjects.
2. Materials and Methods
2.1. Setting
This study was based on the previous cohort study, and all data were collected as described for our previous study in 2017 [11].
2.2. Study Design
2.2.1. Study 1
A retrospective cross-sectional study identified the baseline clinical characteristics that were associated with CKD stage 3–5 in middle-aged and older subjects.
2.2.2. Study 2
A retrospective longitudinal cohort study identified baseline clinical characteristics that predict the progression of CKD stage 3–5 in middle-aged and older subjects.
2.3. Study Population
There were 4919 adults who received a periodic health checkup at a health center at Fukuoka University from 2008 to 2014. All subjects aged 29 to 72 years were included in the study. All subjects provided informed consent to participate after agreeing with the purpose, methods, and significance of the study. The study conformed to the Declaration of Helsinki guidelines and was approved by the Ethics Committee of Fukuoka University (No. 11-08-01). All subjects in these studies were divided into four groups on the basis of their FIB-4 score quartiles, as follows: Study 1—Group A was 0.78 or less (<0.78), Group B was between 0.78 and 1.01 (≥0.78, <1.01), Group C was between 1.01 and 1.29 (≥1.01, <1.29), and Group D was 1.29 or greater (≥1.29); Study 2—Group A was 0.80 or less (<0.80), Group B was between 0.80 and 1.02 (≥0.80, <1.02), Group C was between 1.02 and 1.32 (≥1.02, <1.32), and Group D was 1.32 or greater (≥1.32).
2.3.1. Study 1
A flowchart for participant inclusion in Study 1 is shown in Figure 1. Subjects undergoing dialysis for kidney failure and with a previous history of liver disease were excluded from the analysis. Eight hundred six people were eligible for Study 1.
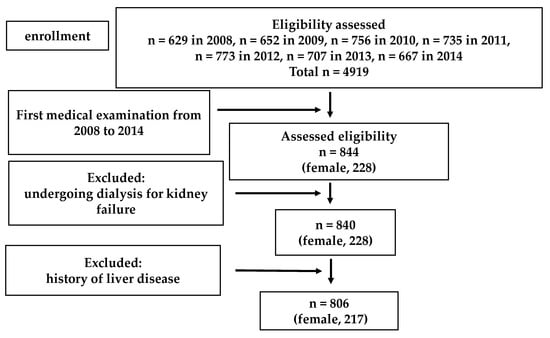
Figure 1.
Flow chart of the Study 1 population selection.
2.3.2. Study 2
A flowchart for participant inclusion in Study 2 is shown in Figure 2. Subjects with a previous history of liver disease and decreased kidney function (eGFR estimated by the Japanese GFR inference formula < 60 mL/min/1.73 m2) were excluded from the analysis. Three hundred eighty subjects with no missing information over the previous 6 years were eligible for Study 2.
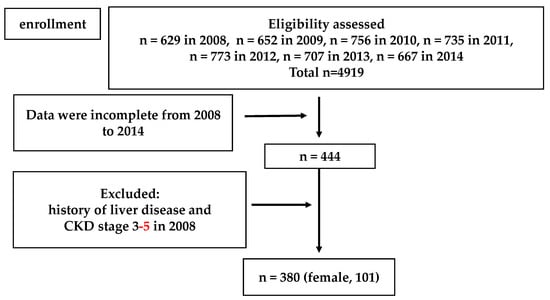
Figure 2.
Flow chart of the Study 2 population selection.
2.4. Blood Sampling, Blood Pressure, and Anthropometry Measurements
Physical characteristics were examined for each subject. The physical factors included age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), and body mass index (BMI). Plasma hemoglobin, platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γ-GTP), blood urea nitrogen (BUN), uric acid, fasting glucose, hemoglobin A1c (HbA1c), serum creatinine, triglyceride levels, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured for all subjects. The AST-to-ALT ratio was calculated as AST/ALT.
2.5. Definitions
Kidney function: The CKD grade was classified in accordance with the eGFR. The eGFR level was calculated using the following Japanese GFR inference formula: eGFR (mL/min/1.73 m2) = 194 × serum creatinine (mg/dL)−1.094 × age (years)−0.287 (×0.739, if women) [12]. In this study, CKD stage 3–5 was defined in accordance with the following Japanese Society Nephrology definition: eGFR < 60 mL/min/1.73 m2.
Liver function: Pathological examination of a liver biopsy specimen is required to diagnose liver fibrosis. However, because liver biopsy is invasive, various scoring systems were used that are associated with liver fibrosis induction, and these included age, AST-to-ALT ratio, and platelet count. The FIB-4 index has been reported to be the liver fibrosis system with the most valid AUROC (area under the receiver operating characteristic) [3,10,13], and it was assessed for each subject. It was calculated using the following formula: [age × AST (IU/L)/platelet count (×109/L) × ALT (IU/L)] [10,13,14].
2.6. Assessment of Lifestyle Behavior
The subjects’ lifestyle behaviors regarding drinking and smoking habits were selected for the present study based on the standardized self-administered questionnaire from the National Health Promotion Program. Each measurement method was followed as described in our previous study [11].
2.7. Statistical Analysis
The data were expressed as the mean ± standard deviation (SD). We evaluated differences between the four FIB-4 index groups using a one-way analysis of variance (ANOVA). Gender, drinking and smoking habits, taking antihypertensive drugs, lipid-lowering drugs, or anti-hyperglycemic drugs were evaluated using the chi-square test.
2.7.1. Study 1
Differences in the incidence of decreased kidney function among the four FIB-4 index groups were visualized using a Jonckheere–Terpstra trend test. To explore the risk factors that are associated with CKD stage 3–5, univariable and multivariable logistic regression models were used. Adjusted variables were chosen on the basis of previous findings [15] and the outcome of the one-way ANOVA. Age, BMI, HDL-C, triglycerides, fasting glucose, and FIB-4 index group were reported to be associated with the incidence of decreased kidney function [15].
2.7.2. Study 2
Differences in the incidence of decreased kidney function among the four FIB-4 index groups were visualized using Kaplan–Meier curves and the log-rank test. A Cox proportional hazards regression model was used to predict the incidence of CKD stage 3–5 for middle-aged and older subjects using the parameters as categorical variables. Hazard ratios were initially adjusted for age, BMI, HDL-C, triglycerides, fasting glucose, and FIB-4 index group at baseline. A probability value < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS software v. 24 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Study 1
3.1.1. Study Population Characteristics
The subjects’ clinical and biochemical features are presented in Table 1. Eight hundred six subjects were included in this analysis. The overall mean age of the cohort was 49.9 ± 8.8 years. Among these subjects, 217 (26.9%) were women, 88 (10.9%) were taking antihypertensive drugs, 61 (7.6%) were taking lipid-lowering drugs, and 25 (3.1%) were taking anti-hyperglycemic drugs. The mean eGFR was 76.7 ± 13.2 mL/min/1.73 m2, and the mean FIB-4 index score was 1.10 ± 0.48 (Table 1). There were significant differences in gender, age, eGFR, FIB-4 index, AST/ALT ratio, BMI, SBP, DBP, platelet count, HDL-C, BUN, creatinine, fasting glucose, and HbA1c among the four groups.

Table 1.
The characteristics of subjects in Study 1.
In the Jonckheere–Terpstra trend test, the eGFR decreased significantly from Group A to Group D.
3.1.2. Association between Liver Fibrosis and the Prevalence of CKD Stage 3–5
A stratified analysis of FIB-4 index groups as an independent variable was conducted (Table 2, Model 1). Group C showed 4.136 times higher and Group D showed 3.775 times higher rates of CKD stage 3–5 than Group A. A stratified analysis using the FIB-4 index, age, gender, BMI, HDL-C, triglycerides, and fasting glucose as independent variables was also conducted (Table 2, Model 2). Age was thought to be an independent variable that affected CKD stage 3–5 onset.

Table 2.
Multivariable analysis of odds ratio for CKD stage 3–5 in Study 1.
3.2. Study 2
3.2.1. Study Population Characteristics Categorized by the FIB-4 Index
Subjects’ clinical and biochemical features are presented in Table 3. Three hundred eighty subjects were included in this analysis. The overall mean age for the cohort was 50.5 ± 7.0 years. Among these subjects, 101 (26.6%) were women, 38 (10.0%) were taking antihypertensive drugs, 8 (2.1%) were taking antihyperglycemic drugs, and 26 (6.8%) were taking lipid-lowering drugs. The mean eGFR was 77.8 ± 10.3 mL/min/1.73 m2, and the mean FIB-4 index score was 1.1 ± 0.5. There were significant differences in age, eGFR, FIB-4 index, AST/ALT ratio, BMI, platelet count, HDL-C, LDL-C, and triglycerides among the four groups (Table 3). The AST/ALT ratio was significantly different between Groups A, C, and D. The BMI was significantly different between Groups A and D. Additionally, HDL and LDL cholesterol were significantly different between Group D and Groups A, B, and C. Triglycerides were significantly different between Groups C and D. Moreover, fasting glucose levels in Groups A and C were significantly different.

Table 3.
The characteristics of subjects in Study 2 (Baseline).
3.2.2. Clinical Utility of the FIB-4 Index to Predict the Prevalence of CKD Stage 3–5
Figure 3 shows the cumulative incidence and relative risk of the prevalence of CKD stage 3–5 over a six-year follow up period in the study participants. On categorization by FIB-4 index group, the Kaplan–Meier survival curve showed that the cumulative prevalence of CKD stage 3–5 was higher in FIB-4 index Group C than in Groups A, B, and D. The log-rank test result was 0.06.
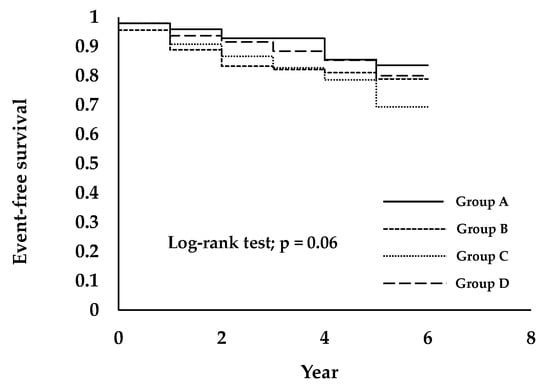
Figure 3.
Prevalence of CKD stage 3–5 is associated with a higher FIB-4 index group in healthy people. Subjects were divided into four groups, as follows: Group A was 0.80 or less (<0.80), Group B was between 0.80 and 1.02 (≥0.80, <1.02), Group C was between 1.02 and 1.32 (≥1.02, <1.32), and Group D was 1.32 or greater (≥1.32).
Table 4 shows the prevalence of CKD stage 3–5. Because only a stratified group of the FIB-4 index was input as an independent factor, the prevalence of CKD stage 3–5 in Group C was 2.237 times higher than in Group A (Table 4, model 1). In Study 2, a stratified group including the FIB-4 index, gender, age, BMI, triglycerides, HDL-C, and fasting glucose as independent factors (Table 4, model 2) was used. In this analysis, age was an independent variable for the prevalence of CKD stage 3–5.

Table 4.
Multivariable analysis of the hazard ratios for CKD stage 3–5 in Study 2.
4. Discussion
We researched whether the progression of liver fibrosis was a factor for kidney function deterioration in a retrospective cross-sectional study and a 6-year longitudinal study in middle-aged and older subjects. We found that liver fibrosis was not an independent risk factor of the prevalence of CKD stage 3–5 in middle-aged and older subjects. However, the FIB-4 index and eGFR showed a liner relationship in Study 1. Additionally, when the FIB-4 index was greater than 1.01 (Groups C and D), the prevalence of CKD stage 3–5 increased more than it did for groups with a FIB-4 index that was less than 1.01. These results suggested that the FIB-4 index could be a useful indicator to predict for the prevalence of CKD stage 3–5.
4.1. Association between Liver Fibrosis and Decreased Kidney Function
Liver fibrosis is the proliferation of connective tissue that occurs in response to chronic, recurring hepatocellular injury, and it is thought to progress when chronic inflammation is present [1]. Because inflammation is also a factor in the development of CKD [2], it is possible that liver fibrosis caused by inflammation also affects the kidneys and causes CKD. The relationship between liver and kidney pathogenesis is also considered to be a clinical issue, e.g., hepatorenal syndrome [16]. Previous studies indicate that as liver fibrosis progresses, it becomes diseases such as NASH or NAFLD [3,4,5,6,17]; although it is difficult to determine if NAFLD directly causes CKD, it is indirectly related to the onset. In the present study, as liver fibrosis progressed, the prevalence of CKD stage 3–5 morbidity increased, and it is possible that such results were related to each other.
The progress of liver fibrosis is considered to be the progress of chronic inflammation in hemodynamics; chronic inflammation also has an adverse effect on the kidneys [2]. Although the complex interactions among NAFLD, inflammation, oxidative stress, impaired glucose tolerance, atherogenic, and dyslipidemia make it difficult to unravel the specific role of the liver and the underlying mechanisms responsible for the association between NAFLD and the risk of developing CKD, it is clear that chronic inflammation caused by insulin resistance, such as obesity, diabetes, and NAFLD or NASH, induces glomerulosclerosis [3,18]. A previous study also demonstrated that a significant, positive association between NAFLD and CKD was prevalent in the univariate analysis; however, this relationship was attenuated by adjusting for features of metabolic syndrome [19]. A previous review also suggested that it was uncertain whether NAFLD was associated with a specific type of kidney disease, although it is reasonable to assume that NAFLD might promote kidney damage, mostly thorough accelerated atherothrombosis [20].
It is clear that patients with NAFLD or NASH exhibit the typical features of metabolic syndrome and have a myriad of other emerging risk factors and risk markers for CKD [2]. Insulin resistance increases free fatty acids and causes systemic vasculitis. The inflammatory cytokines caused by this chronic inflammation not only cause liver fibrosis through the bloodstream, but also act on the kidney (which is a distant tissue), so that the renal blood vessels also cause chronic inflammation. Specifically, chronic inflammation, which causes liver fibrosis, also affects the kidneys at the same time [21,22,23].
The previous study suggested that it was of great importance to implore public health efforts and strategies to curb the epidemics of obesity and metabolic syndrome. Otherwise, the prevalence of NAFLD and CKD will continue to rise [19]. Kidney function in patients with liver fibrosis, such as NAFLD, should be evaluated and followed-up. Additionally, in the present study, when the FIB-4 index exceeded 1.01, the prevalence of CKD stage 3–5 morbidity doubled. Thus, it might be better to follow liver and kidney function using a FIB-4 index of 1.01. In a previous study, discussions on setting the cutoff point for the FIB-4 index suggested that 1.105 should be regarded as a measure of liver fibrosis progression and an initial decrease in kidney function [3]. No previous studies focused on the FIB-4 index and clarified the relationship between decreased kidney function and liver fibrosis. This is the first study of the relationship between them. As long as the FIB-4 index is expected to be useful for evaluating liver fibrosis [10], it could be a useful indicator for evaluating the prevalence of CKD stage 3–5 morbidity. Following-up on liver fibrosis and decreased kidney function progression at the same time would be beneficial. This could be used during specific health checkups and in certain health guidelines because both the FIB-4 index and eGFR are widely used.
4.2. Metabolic Disorder and Arteriosclerosis Induced Deterioration of Kidney and Liver Structure and Function
Liver fibrosis and kidney dysfunction are related and develop together. The common causes of liver fibrosis and kidney dysfunction need to be clarified. Previous studies showed that many factors cause liver fibrosis and kidney disfunction, such as glycolipid metabolism [24,25]. Specifically, not only NAFLD but also CKD are strongly associated with obesity, insulin resistance, and type 2 diabetes [2,24,25]. In the present study, a higher fasting glucose induced a higher FIB-4 index and lower eGFR. This suggests that a metabolic disorder, such as metabolic syndrome, would be a progression factor for liver fibrosis and the prevalence of CKD stage 3–5. Additionally, we determined that the highest prevalence of CKD stage 3–5 was in FIB-4 index Group C, which showed the highest fasting glucose and a lower HDL-C. This finding also suggests that the major predictor must be glucose metabolism and lipid metabolism. Our results suggest that the evaluation of liver fibrosis by means of non-invasive markers is especially important in patients with diabetes and metabolic syndrome for preventing further deterioration.
HDL-C is associated with a lower risk of coronary heart disease and arteriosclerosis [26,27]. Moreover, decreased eGFR was independently associated with decreased HDL-C levels [28,29,30]. HDL-C has anti-inflammatory, anti-oxidative, and anti-apoptotic functions and improves endothelial function; these functions are considered to be anti-atherogenic [26,27]. In the present study, a higher HDL-C effect decreased the risk of CKD stage 3–5 in older age groups. These results suggest that HDL-C could be a prevention factor for liver fibrosis and decreased kidney function.
4.3. Age-Related Deterioration of Kidney and Liver Structure and Function
Age and age-related changes to kidney and liver structure and function are the main risk factors for major debilitating and life-threatening conditions, including CVD [31,32,33]. Notably, old age is a risk factor for NAFLD, CKD, and type 2 diabetes, and NAFLD and CKD are major factors for CVD [20]. The present study also shows that eGFR decreases and liver fibrosis increases with aging. Kidney and liver function are the main predictors of longevity; thus, preventing deteriorating kidney and liver function might slow liver fibrosis and CKD stage 3–5 prevalence during aging. In the present study, the group with a higher FIB-4 index score had a greater risk for CKD stage 3–5 prevalence than lower FIB-4 index groups. As discussed above, metabolic disorder and arteriosclerosis might affect kidney and liver function. This result suggests that decreased kidney function and liver fibrosis might be more strongly affected by metabolic disorder and arteriosclerosis than aging.
Most importantly, not only are liver fibrosis and decreased kidney function induced by aging, but they are also accelerated by chronic inflammation such as metabolic disorders and insulin resistance. The early detection of these declines in kidney function, via signs of advanced liver fibrosis, may prevent further complications such as CVD. The FIB4-index was positively correlated with cardiovascular risk scores in patients with chronic liver disease [34]. NAFLD, CKD, and type 2 diabetes are all major risk factors for CVD; therefore, the FIB4-index can be a useful non-invasive indicator for the early detection of these diseases.
4.4. Study Limitations and Clinical Implications
Our study had several limitations. First, this was an observational study in middle-aged and older subjects in just one cohort. Therefore, the results may not be generalizable to all people. Second, insulin resistance was not assessed, and thus, it is not clear if there is another association between CKD and the FIB-4 index. Third, although the FIB-4 index is useful for diagnosing liver fibrosis, the gold standard is liver biopsy, which was not evaluated. More studies are needed to determine these relationships. Finally, the subjects of this study were relatively healthy middle-aged and older people, and most of the eGFR and Fib4 values were within the normal range.
However, this was the first study to evaluate FIB-4 index and CKD stage 3–5 prevalence in middle-aged and older subjects. In Japan, not only the number of patients with the introduction of dialysis but also cirrhotic NASH associated with liver fibrosis are steadily increasing [35,36]. Especially, survival in NASH is lower than the expected survival of the matched general population, due to the higher prevalence of cardiovascular and liver-related deaths [34]. We consider that capturing a decrease in eGFR and an increase in FIB-4 index in a relatively healthy population, even within the normal range, is important from the perspective of introducing dialysis and cirrhotic NASH prevention in the future. The present study suggests that liver fibrosis and decreased kidney function are associated with aging. However, it is possible for aging people to reduce CKD stage 3–5 prevalence through a healthy lifestyle, such as preventing increasing insulin resistance and increasing HDL-C levels.
5. Conclusions
Our retrospective study evaluated the association of liver fibrosis and other risk factors with the prevalence of CKD stage 3–5 during a 6-year follow-up in middle-aged and older subjects. We found that liver fibrosis was not an independent risk factor of the prevalence of CKD stage 3–5 in middle-aged and older subjects. Although it was not significant, higher FIB-4 index groups (FIB-4 index > 1.01) tended to show more CKD stage 3–5. These results suggested that liver fibrosis could be a useful indicator for the prevalence of CKD, even within a relatively healthy population.
Author Contributions
Conceptualization, K.K., R.M. and Y.H.; Methodology, K.K., R.M., T.M., N.M., Y.U. and Y.H.; Software, K.K., R.M., T.M. and N.M.; Validation, K.K., R.M., T.M., S.K., N.M., Y.U. and Y.H.; Formal Analysis, K.K. and R.M.; Investigation, R.M., T.M., N.M. and Y.H.; Resources, K.K., R.M., N.M. and Y.H.; Data Curation, K.K., R.M. and Y.H.; Writing—Original Draft Preparation, K.K.; Writing—Review and Editing, K.K., R.M., T.M., S.K., N.M., Y.U. and Y.H.; Visualization, K.K., R.M., T.M., S.K. and Y.H.; Supervision, K.K., R.M. and Y.H.; Project Administration, K.K., R.M. and Y.H.; Funding Acquisition, R.M., S.K., Y.U. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed with the support of Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 15H03082, No. 19H04012) and the Fukuoka University Institute for Physical Activity.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Fukuoka University (No. 11-08-01).
Informed Consent Statement
All of the subjects gave their informed consent to participate after agreeing with the purpose, methods, and significance of the study.
Data Availability Statement
The contents of the ethics committee’s approval resolution as well as the wording of participants’ written consent do not render open public data access possible. Access to the study’s data set may be requested by contacting the Ethics Committee of Fukuoka University and inform and consent to each participant.
Acknowledgments
We would like to thank the members of the Laboratory of Exercise Physiology and Health Care Center of Fukuoka University for their assistance with the data evaluation. We are grateful to the participants of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chancharoenthana, W.; Leelahavanichkul, A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J. Gastroenterol. 2019, 25, 3684–3703. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.B.; Byrne, C.D. CKD and Nonalcoholic Fatty Liver Disease. Am. J. Kidney Dis. 2014, 64, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-W.; Hsu, Y.-C.; Chang, C.-H.; Wei, K.-L.; Lin, C.-L. High FIB-4 index as an independent risk factor of prevalent chronic kidney disease in patients with nonalcoholic fatty liver disease. Hepatol. Int. 2015, 10, 340–346. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Chonchol, M.; Zoppini, G.; Abaterusso, C.; Bonora, E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: Is there a link? J. Hepatol. 2011, 54, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.; Adams, L.A.; Canbay, A.; Syn, W.-K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef]
- EEckardt, K.-U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Levin, A.; Kellum, J.A. Definition and Classification of Kidney Diseases. Am. J. Kidney Dis. 2013, 61, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD); Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michishita, R.; Matsuda, T.; Kawakami, S.; Tanaka, S.; Kiyonaga, A.; Tanaka, H.; Morito, N.; Higaki, Y. Hypertension and hyperglycemia and the combination thereof enhances the incidence of chronic kidney disease (CKD) in middle-aged and older males. Clin. Exp. Hypertens. 2017, 39, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Sumida, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francoz, C.; Durand, F.; Kahn, J.A.; Genyk, Y.S.; Nadim, M.K. Hepatorenal Syndrome. Clin. J. Am. Soc. Nephrol. 2019, 14, 774–781. [Google Scholar] [CrossRef]
- Okamura, K.; Okuda, T.; Takamiya, Y.; Shirai, K.; Urata, H. High Fib4 index in patients with suspected NASH is associated with elevation of chymase-dependent angiotensin II-forming activity in circulating mononuclear leucocytes. Heart Vessel. 2019, 34, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Camici, M. Podocyte dysfunction in aging-related glomerulosclerosis. Front. Biosci. 2011, S3, 995–1006. [Google Scholar] [CrossRef] [Green Version]
- Sirota, J.C.; McFann, K.; Targher, G.; Chonchol, M.; Jalal, D.I. Association between Nonalcoholic Liver Disease and Chronic Kidney Disease: An Ultrasound Analysis from NHANES 1988–1994. Am. J. Nephrol. 2012, 36, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: Revisited after a decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Ren. Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D. Nonalcoholic Fatty Liver Disease: A Novel Cardiometabolic Risk Factor for Type 2 Diabetes and Its Complications. J. Clin. Endocrinol. Metab. 2013, 98, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehara, Y. High-density lipoprotein and atherosclerosis: Roles of lipid transporters. World J. Cardiol. 2014, 6, 1049–1059. [Google Scholar] [CrossRef]
- Ikenaga, M.; Higaki, Y.; Saku, K.; Uehara, Y. High-Density Lipoprotein Mimetics: A Therapeutic Tool for Atherosclerotic Diseases. J. Atheroscler. Thromb. 2016, 23, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.; Ray, U.; Yu, R.; Hudspeth, A.; Smillie, M.; Jordan, N.; Bartle, J. Kidney Function as a Determinant of HDL and Triglyceride Concentrations in the Australian Population. J. Clin. Med. 2016, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Muntner, P.; He, J.; Astor, B.C.; Folsom, A.R.; Coresh, J. Traditional and Nontraditional Risk Factors Predict Coronary Heart Disease in Chronic Kidney Disease: Results from the Atherosclerosis Risk in Communities Study. J. Am. Soc. Nephrol. 2004, 16, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Kwan, B.C.; Kronenberg, F.; Beddhu, S.; Cheung, A.K. Lipoprotein Metabolism and Lipid Management in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2007, 18, 1246–1261. [Google Scholar] [CrossRef]
- Martin, J.E.; Sheaff, M.T. Renal ageing. J. Pathol. 2007, 211, 198–205. [Google Scholar] [CrossRef]
- Jin, C.J.; Baumann, A.; Brandt, A.; Engstler, A.J.; Nier, A.; Hege, M.; Schmeer, C.; Kehm, R.; Höhn, A.; Grune, T.; et al. Aging-related liver degeneration is associated with increased bacterial endotoxin and lipopolysaccharide binding protein levels. Am. J. Physiol. Liver Physiol. 2020, 318, G736–G747. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballestri, S.; Mantovani, A.; Baldelli, E.; Lugari, S.; Maurantonio, M.; Nascimbeni, F.; Marrazzo, A.; Romagnoli, D.; Targher, G.; Lonardo, A. Liver Fibrosis Biomarkers Accurately Exclude Advanced Fibrosis and Are Associated with Higher Cardiovascular Risk Scores in Patients with NAFLD or Viral Chronic Liver Disease. Diagnostics 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society for Dialysis Therapy. 2019. Available online: https://docs.jsdt.or.jp/overview/index.html (accessed on 1 June 2021).
- Hashimoto, E.; Tokushige, K. Prevalence, gender, ethnic variations, and prognosis of NASH. J. Gastroenterol. 2010, 46, 63–69. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).