Performance of the Cognitive Performance Scale of the Resident Assessment Instrument (interRAI) for Detecting Dementia amongst Older Adults in the Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
- (i)
- an interRAI Version 9.1 Home Care assessment was completed within 90 days of discharge from psychogeriatric inpatient care or memory clinic assessment;
- (ii)
- participants gave permission, at the time of their interRAI assessment, for the interRAI data to be used for research purposes.
2.2. Measures
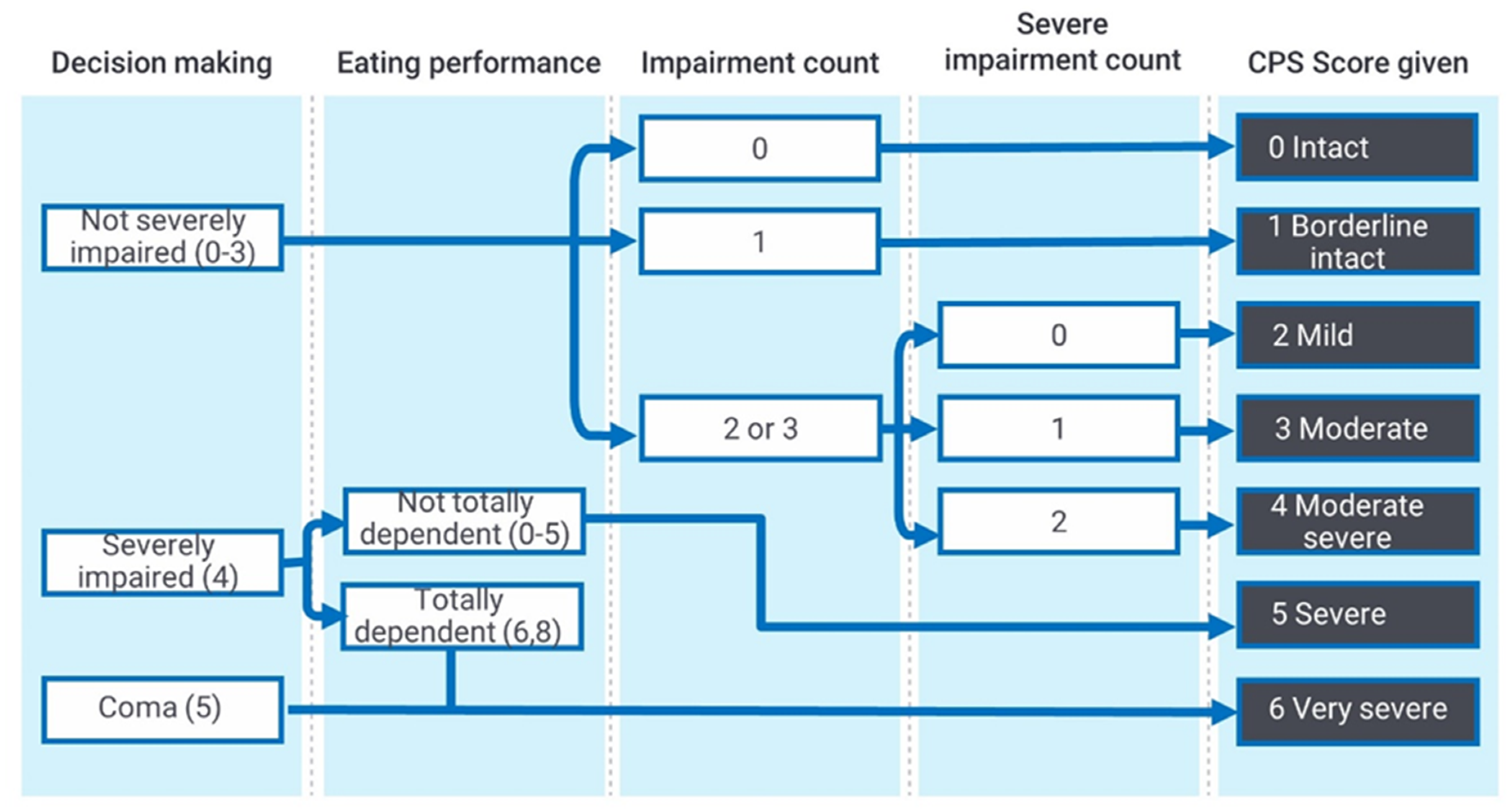
2.2.1. Index Test: Interrai Cognitive Performance Scale (2005 Revision)
2.2.2. Reference Standard: ‘Clinical Dementia Diagnosis’ or ‘No Clinical Dementia Diagnosis’
2.3. Data Collection
2.4. Data Analysis
- Sensitivity: probability that the CPS score will be ‘positive’ (over cut-off) when the diagnosis is present.
- Specificity: probability that the CPS score will be ‘negative’ (under cut-off) when the diagnosis is not present.
- Positive likelihood ratio: ratio between the probability of a positive (over cut-off) CPS score given the presence of the diagnosis and the probability of a positive (over cut-off) CPS score given the absence of the diagnosis, i.e., Sensitivity/(1-Specificity).
- Negative likelihood ratio: ratio between the probability of a negative (under cut-off) CPS score given the presence of the diagnosis and the probability of a negative (under cut-off) InterRAI scale score given the absence of the diagnosis, i.e., (1-Sensitivity)/Specificity.
- Positive predictive value: probability that the diagnosis is present when the CPS is ‘positive’ (over cut-off).
- Negative predictive value: probability that the diagnosis is not present when the CPS score is negative (below cut-off).
3. Results
3.1. Sample
3.2. Accuracy for Diagnosis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia: A Public Health Priority; World Health Organisation: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Mittelman, M.S.; Halley, W.E.; Clay, O.J.; Roth, D.L. Improving caregiver wellbeing delays nursing home placement of patients with Alzheimer’s disease. Neurology 2006, 67, 1592–1599. [Google Scholar] [CrossRef]
- Olivari, B.S.; French, M.E.; McGuire, L.C. The Public Health Road Map to Respond to the Growing Dementia Crisis. Innov. Aging 2020, 4, igz043. [Google Scholar] [CrossRef]
- Dubois, B.; Padovani, A.; Scheltens, P.; Rossie, A.; Dell’Agnello, G. Timely diagnosis for Alzheimer’s Disease: A literature review on benefits and challenges. J. Alzheimers Dis. 2016, 49, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Boustani, M.; Peterson, B.; Hanson, L.; Harris, R.; Lohr, K.N. Screening for Dementia in Primary Care: A Summary of the Evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2003, 138, 927–937. [Google Scholar] [CrossRef]
- InterRai, N.Z. For the Public. Available online: interrai.co.nz/for-the-public/ (accessed on 22 June 2021).
- Hirdes, J.P.; Ljunggren, G.; Morris, J.N.; Frijters, D.H.M.; Soveri, H.F.; Gray, L.; Björkgren, M.; Gilgen, R. Reliability of the interRAI suite of assessment instruments: A 12-country study of an integrated health information system. BMC Health Serv. Res. 2008, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.N.; Fries, B.E.; Mehr, D.R.; Hawes, C.; Phillips, C.; Mor, V.; Lipsitz, L.A. MDS Cognitive Performance Scale(C). J. Gerontol. 1994, 49, M174–M182. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Albert, M.; Cohen, C. The Test for Severe Impairment: An Instrument for the Assessment of Patients with Severe Cognitive Dysfunction. J. Am. Geriatr. Soc. 1992, 40, 449–453. [Google Scholar] [CrossRef]
- Bula, C.J.; Wietlisbach, V. Use of the Cognitive Performance Scale (CPS) to detect cognitive impairment in the acute care setting: Concurrent and predictive validity. Brain Res. Bull. 2009, 80, 173–178. [Google Scholar] [CrossRef]
- Wellens, N.; Flamaing, J.; Tournoy, J.; Hanon, T.; Moons, P.; Verbeke, G.; Boonen, S.; Milisen, K. Convergent Validity of the Cognitive Performance Scale of the interRAI Acute Care and the Mini-Mental State Examination. Am. J. Geriatr. Psychiatry 2013, 21, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Smart, K.A.; Herrmann, N.; Lanctôt, K.L. Validity and Responsiveness to Change of Clinically Derived MDS Scales in Alzheimer Disease Outcomes Research. J. Geriatr. Psychiatry Neurol. 2011, 24, 67–72. [Google Scholar] [CrossRef]
- Frederiksen, K.; Tariot, P.; De Jonghe, E. Minimum Data Set Plus (MDS+) Scores Compared with Scores from Five Rating Scales. J. Am. Geriatr. Soc. 1996, 44, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Baldini, A.; Zimmerman, S.; Mortimore, E.; Jay, M. The Validity of the Minimum data set in measuring the cognitive impairment of persons admitted to nursing homes. J. Am. Geriatr. Soc. 2000, 48, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Hartmaier, S.L.; Sloane, P.D.; Guess, H.A.; Koch, G.G.; Mitchell, C.M.; Phillips, C.D. Validation of the Minimum Data Set Cognitive Performance Scale: Agreement with the Mini-Mental State Examination. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, M128–M133. [Google Scholar] [CrossRef]
- Snowden, M.; McCormick, W.; Russo, J.; Srebnik, D.; Comtois, K.; Bowen, J.; Teri, L.; Larson, E.B. Validity and responsiveness of the Minimum Data Set. J. Am. Geriatr. Soc. 1999, 47, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Travers, C.; Byrne, G.J.; Pachana, N.A.; Klein, K.; Gray, L. Validation of the interRAI cognitive performance scale against independent clinical diagnosis and the mini-mental state examination in older hospitalized patients. J. Nutr. Health Aging 2013, 17, 435–439. [Google Scholar] [CrossRef]
- Chan, C.L.F.; Lai, C.K.Y.; Chi, I. Initial validation of the Chinese interRAI Mental Health in people with psychiatric illness. Int. J. Psychiatry Clin. Pract. 2014, 18, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Perlman, C.M.; Hirdes, J.P.; Scott, T. Screening Cognitive Performance with the Resident Assessment Instrument for Mental Health Cognitive Performance Scale. Can. J. Psychiatry 2010, 55, 736–740. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Tua, E.; Onder, G.; Carrara, B.; Sgadari, A.; Rinaldi, C.; Gambassi, G.; Lattanzio, F.; Bernabei, R. Minimum data set for home care: A valid instrument to assess frail older people living in the community. Med. Care 2000, 38, 1184–1190. [Google Scholar] [CrossRef]
- Morris, J.N.; Howard, E.P.; Steel, K.; Perlman, C.; Fries, B.E.; Garms-Homolová, V.; Henrard, J.-C.; Hirdes, J.P.; Ljunggren, G.; Gray, L.; et al. Updating the Cognitive Performance Scale. J. Geriatr. Psychiatry Neurol. 2015, 29, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Paquay, L.; De Lepeleire, J.; Schoenmakers, B.; Ylieff, M.; Fontaine, O.; Buntinx, F. Comparison of the diagnostic accuracy of the cognitive performance scale (minimum data set) and the mini-mental state exam for the detection of cognitive impairment in nursing home residents. Int. J. Geriatr. Psychiatry 2007, 22, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Zeng, N.; Wang, N. Sensitivity, Accuracy, Associated Confidence Interval and ROC Analysis with Practical SAS Implementations; NESUG Proceedings: Health and Life Care Sciences; NESUG: Baltimore, MD, USA, 2010; pp. 1–9. [Google Scholar]
- Furukawa, T.A.; Strauss, S.; Buscher, H.C.; Guyatt, G. Diagnostic tests. In Users’ Guides to the Medical Literature: Essentials of Evidence-Based Clinical Practice, 2nd ed.; Guyatt, G., Rennie, D., Eds.; AMA Press: Chicago, IL, USA, 2008; pp. 195–222. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; The QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. Dementia: US recommends against screening over 65s because of insufficient evidence. BMJ 2020, 368, m750. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.; Edwards, S.; Sundram, F. Death wishes among older people assessed for home support and long-term aged residential care. Int. J. Geriatr. Psychiatry 2017, 32, 1371–1380. [Google Scholar] [CrossRef]
- Central Region’s Technical Advisory Services Limited. interRAI NZ Annual Report 2019/2020; TAS: Wellington, New Zealand, 2020. [Google Scholar]
- Martinez-Ruiz, A.; Huang, Y.; Gee, S.; Jamieson, H.; Cheung, G. Individual risk factors for possible undetected dementia amongst community-dwelling older people in New Zealand. Dementia 2020, 19, 750–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Central Region’s Technical Advisory Services Limited. interRAI NZ Annual Report 2016/2017; TAS: Wellington, New Zealand, 2017. [Google Scholar]
- National Dementia Cooperative, Alzheimers New Zealand, Dementia New Zealand. Improving Dementia Services in New Zealand: Dementia Action Plan 2020–2025; National Dementia Cooperative, Alzheimers New Zealand, Dementia New Zealand: Wellington, New Zealand, 2020; Available online: https://www.nzdementia.org/Portals/0/Uploads/New-Zealand-Dementia-Action-Plan.pdf?ver=2020-05-27-151033-190 (accessed on 22 June 2021).
- Central Region’s Technical Advisory Services Limited. interRAI NZ Annual Report 2018/2019; TAS: Wellington, New Zealand, 2019. [Google Scholar]
| CPS Score | Clinical Diagnosis | |
|---|---|---|
| Description | No Dementia (N = 62) | Dementia (N = 72) |
| 21 (91%) | 2 (9%) |
| 16 (76%) | 5 (24%) |
| 21 (39%) | 33 (61%) |
| 3 (12%) | 22 (88%) |
| 1 (9%) | 10 (91%) |
| Cut Point | Sensitivity (95% CI) | Specificity (95% CI) | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|---|---|---|
| 1/2 | 0.90 (0.81–0.96) | 0.60 (0.46–0.72) | 2.24 (1.64–3.06) | 0.16 (0.08–0.34) | 0.72 (0.66–0.78) | 0.84 (0.72–0.92) | 0.76 (0.68–0.83) |
| 2/3 | 0.44 (0.33–0.57) | 0.94 (0.84–0.98) | 6.89 (2.58–18.40) | 0.59 (0.48–0.74) | 0.89 (0.75–0.96) | 0.59 (0.54–0.64) | 0.67 (0.59–0.75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gee, S.; Croucher, M.; Cheung, G. Performance of the Cognitive Performance Scale of the Resident Assessment Instrument (interRAI) for Detecting Dementia amongst Older Adults in the Community. Int. J. Environ. Res. Public Health 2021, 18, 6708. https://doi.org/10.3390/ijerph18136708
Gee S, Croucher M, Cheung G. Performance of the Cognitive Performance Scale of the Resident Assessment Instrument (interRAI) for Detecting Dementia amongst Older Adults in the Community. International Journal of Environmental Research and Public Health. 2021; 18(13):6708. https://doi.org/10.3390/ijerph18136708
Chicago/Turabian StyleGee, Susan, Matthew Croucher, and Gary Cheung. 2021. "Performance of the Cognitive Performance Scale of the Resident Assessment Instrument (interRAI) for Detecting Dementia amongst Older Adults in the Community" International Journal of Environmental Research and Public Health 18, no. 13: 6708. https://doi.org/10.3390/ijerph18136708
APA StyleGee, S., Croucher, M., & Cheung, G. (2021). Performance of the Cognitive Performance Scale of the Resident Assessment Instrument (interRAI) for Detecting Dementia amongst Older Adults in the Community. International Journal of Environmental Research and Public Health, 18(13), 6708. https://doi.org/10.3390/ijerph18136708






