Walking Cadence during Moderate-Intensity Physical Activity in Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Data Analysis
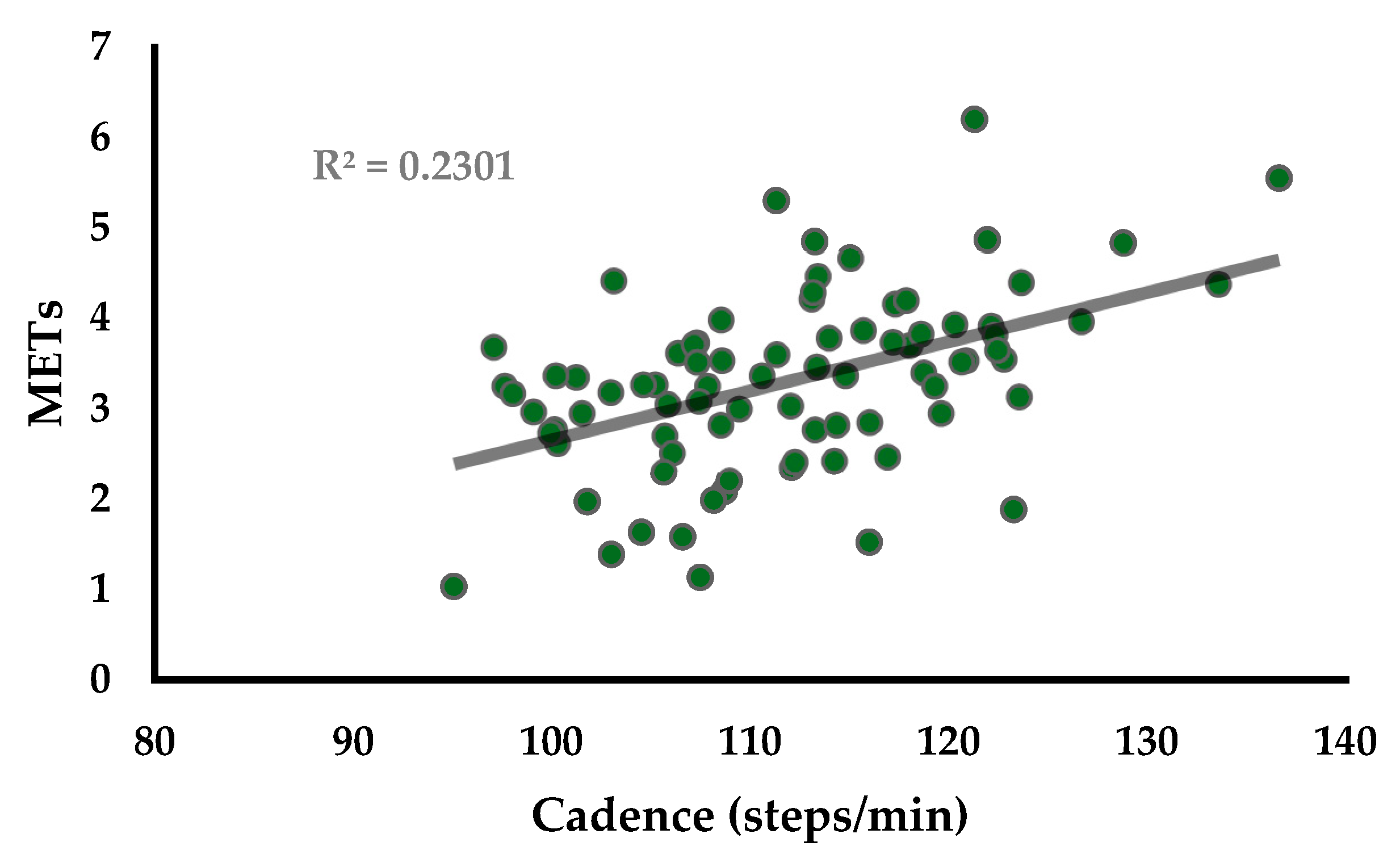
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tudor-Locke, C.; Aguiar, E.J.; Han, H.; Ducharme, S.W.; Schuna, J.M.; Barreira, T.V.; Moore, C.C.; Busa, M.A.; Lim, J.; Sirard, J.R. Walking cadence (steps/min) and intensity in 21–40 year olds: CADENCE-adults. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, E.J.; Gould, Z.R.; Ducharme, S.W.; Moore, C.C.; McCullough, A.K.; Tudor-Locke, C. Cadence-based classification of minimally moderate intensity during Overground walking in 21-to 40-year-old adults. J. Phys. Act. Health 2019, 16, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.; Hannon, J.; Mullineaux, D.; Beighle, A. Determination of step rate thresholds corresponding to physical activity intensity classifications in adults. J. Phys. Act Health 2011, 8, 45–51. [Google Scholar] [CrossRef]
- Marshall, S.J.; Levy, S.S.; Tudor-Locke, C.E.; Kolkhorst, F.W.; Wooten, K.M.; Ji, M.; Macera, C.A.; Ainsworth, B.E. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am. J. Prev. Med. 2009, 36, 410–415. [Google Scholar] [CrossRef]
- Rowe, D.A.; Welk, G.J.; Heil, D.P.; Mahar, M.T.; Kemble, C.D.; Calabro, M.A.; Camenisch, K. Stride rate recommendations for moderate-intensity walking. Med. Sci. Sports Exerc. 2011, 43, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Peacock, L.; Hewitt, A.; Rowe, D.A.; Sutherland, R. Stride rate and walking intensity in healthy older adults. J. Aging Phys. Act. 2014, 22, 276–283. [Google Scholar] [CrossRef]
- ACOG Committee Opinion. Number 267, January 2002: Exercise during pregnancy and the postpartum period. Obstet. Gynecol. 2002, 99, 171–173. [Google Scholar]
- Evenson, K.R.; Wen, F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev. Med. 2010, 50, 123–128. [Google Scholar] [CrossRef]
- Evenson, K.R.; Savitz, D.A.; Huston, S.L. Leisure-time physical activity among pregnant women in the US. Paediatr. Perinat. Epidemiol. 2004, 18, 400–407. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Henriksen, T.; Saugstad, O.D.; Tonstad, S. Physical activity and the risk of gestational diabetes mellitus: A systematic review and dose–response meta-analysis of epidemiological studies. Eur. J. Epidemiol. 2016, 31, 967–997. [Google Scholar] [CrossRef]
- Hayashi, A.; Matsuzaki, M.; Kusaka, M.; Shiraishi, M.; Haruna, M. Daily walking decreases casual glucose level among pregnant women in the second trimester. Drug Discov. Ther. 2016, 10, 218–222. [Google Scholar] [CrossRef]
- Aune, D.; Saugstad, O.D.; Henriksen, T.; Tonstad, S. Physical activity and the risk of preeclampsia: A systematic review and meta-analysis. Epidemiology 2014, 25, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Ruchat, S.M.; Davenport, M.H.; Giroux, I.; Hillier, M.; Batada, A.; Sopper, M.M.; Hammond, J.M.; Mottola, M.F. Nutrition and exercise reduce excessive weight gain in normal-weight pregnant women. Med. Sci. Sports Exerc. 2012, 44, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.L.; Campbell, C.G.; Foster, R.C.; Peterson, A.D.; Lanningham-Foster, L. A pilot walking program promotes moderate-intensity physical activity during pregnancy. Med. Sci. Sports Exerc. 2014, 46, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F.; Kim, H.; Burciu, B.; Schmidt, S.; Petry, K.; Lopez, B. Continuing regular exercise during pregnancy: Effect of exercise volume on fetoplacental growth. Am. J. Obstet. Gynecol. 2002, 186, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.R.; Pivarnik, J.M. Perceived Exertion of Physical Activity During Pregnancy. J. Phys. Act. Health 2015, 12, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Dobson, K.L.; da Silva, D.F.; Dervis, S.; Mohammad, S.; Nagpal, T.S.; Adamo, K.B. Physical activity and gestational weight gain predict physiological and perceptual responses to exercise during pregnancy. Birth Defects Res. 2021, 113, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Robson, S.C. Adaptation of the maternal heart in pregnancy. Br. Heart J. 1992, 68, 540. [Google Scholar] [CrossRef] [PubMed]
- Pivarnik, J.M. Cardiovascular responses to aerobic exercise during pregnancy and postpartum. In Seminars in Perinatology; WB Saunders: Philadelphia, PA, USA, 1996; Volume 20, pp. 242–249. [Google Scholar]
- Pivarnik, J.M.; Lee, W.; Miller, J.F.; Werch, J. Alterations in plasma volume and protein during cycle exercise throughout pregnancy. Med. Sci. Sports Exerc. 1990, 22, 751–755. [Google Scholar] [CrossRef]
- Capeless, E.L.; Clapp, J.F. Cardiovascular changes in early phase of pregnancy. Am. J. Obstet. Gynecol. 1989, 161, 1449–1453. [Google Scholar] [CrossRef]
- Forczek, W.; Staszkiewicz, R. Changes of kinematic gait parameters due to pregnancy. Acta Bioeng. Biomech. 2012, 14, 113–119. [Google Scholar]
- Gilleard, W.L. Trunk motion and gait characteristics of pregnant women when walking: Report of a longitudinal study with a control group. BMC Pregnancy Childbirth 2013, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Rowe, D.A. Using cadence to study free-living ambulatory behaviour. Sports Med. 2012, 42, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Ducharme, S.W.; Aguiar, E.J.; Schuna, J.M.; Barreira, T.V.; Moore, C.C.; Chase, C.J.; Gould, Z.R.; Amalbert-Birriel, M.A.; Mora-Gonzalez, J. Walking cadence (steps/min) and intensity in 41 to 60-year-old adults: The CADENCE-adults study. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pivarnik, J.M.; Stein, A.D.; Rivera, J.M. Effect of pregnancy on heart rate/oxygen consumption calibration curves. Med. Sci. Sports Exerc. 2002, 34, 750–755. [Google Scholar] [CrossRef]
- Lienhard, K.; Schneider, D.; Maffiuletti, N.A. Validity of the Optogait photoelectric system for the assessment of spatiotemporal gait parameters. Med. Eng. Phys. 2013, 35, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Song, C.H.; Lee, K.J.; Jung, S.W.; Shin, D.C.; Shin, S.H. Concurrent validity and test-retest reliability of the OPTOGait photoelectric cell system for the assessment of spatio-temporal parameters of the gait of young adults. J. Phys. Ther. Sci. 2014, 26, 81–85. [Google Scholar] [CrossRef] [PubMed]
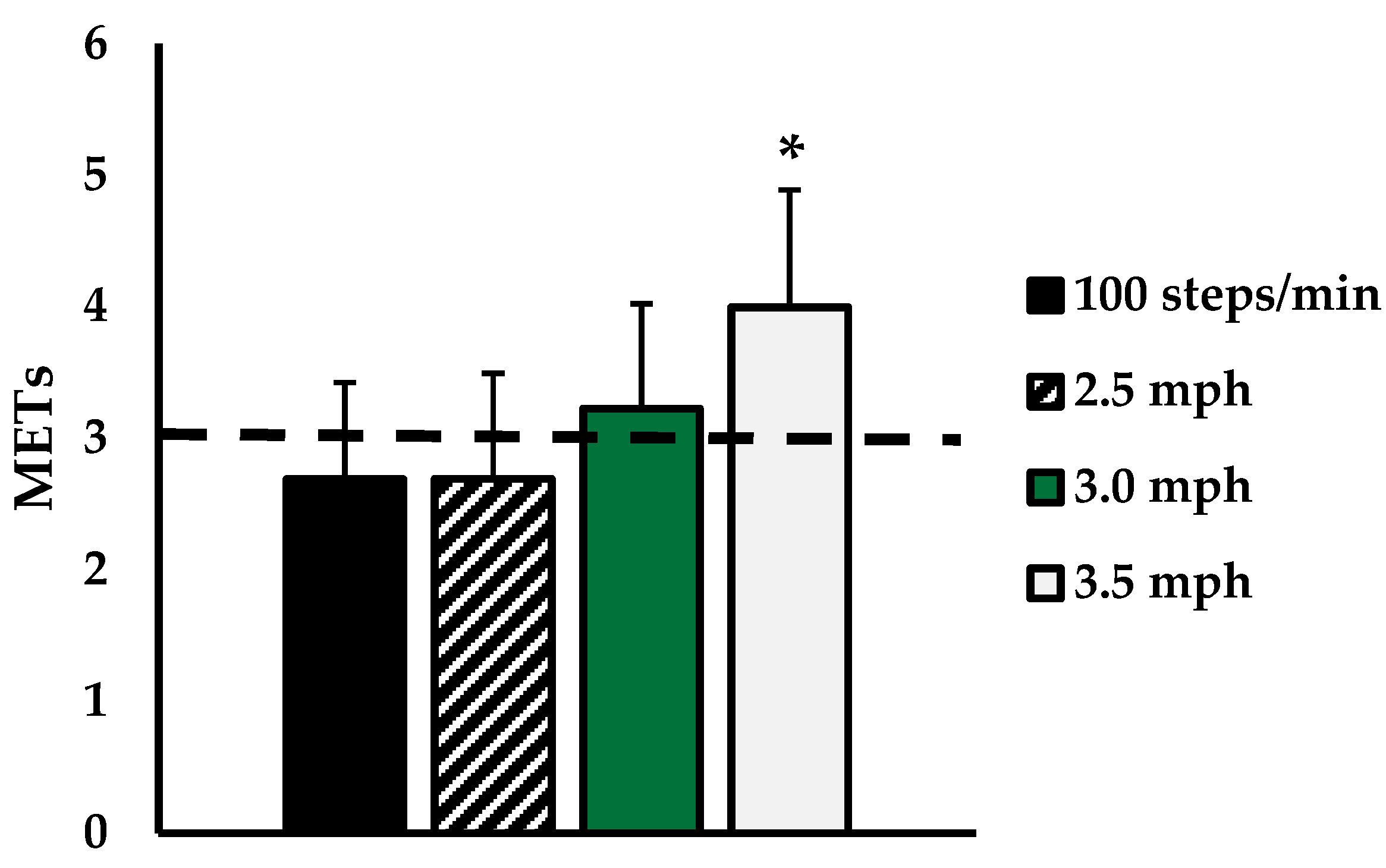
| 2.5 mph | 3.0 mph | 3.5 mph | ||
|---|---|---|---|---|
| 2nd Trimester | HR | 100.2 ± 13.7 * | 113.2 ± 2 | 123.8 ± 21.1 |
| VO2 | 9.1 ± 3.4 | 11.5 ± 3.2 | 13.8 ± 3.5 | |
| Steps/min | 103.5 ± 4.4 | 112.0 ± 5.3 | 121.0 ± 6.5 | |
| 3rd Trimester | HR | 116.1 ± 17.5 | 125.7 ± 22.0 | 137.9 ± 23.8 |
| VO2 | 9.0 ± 2.3 | 10.9 ± 2.1 | 14.1 ± 2.1 | |
| Steps/min | 104.1 ± 4.8 | 112.9 ± 5.7 | 120.1 ± 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, M.; Birchfield, B.; Rogers, R.; Senga, J.; Persch, M.; Currie, M.; Schmid, D.; Ballmann, C. Walking Cadence during Moderate-Intensity Physical Activity in Pregnant Women. Int. J. Environ. Res. Public Health 2021, 18, 6593. https://doi.org/10.3390/ijerph18126593
Marshall M, Birchfield B, Rogers R, Senga J, Persch M, Currie M, Schmid D, Ballmann C. Walking Cadence during Moderate-Intensity Physical Activity in Pregnant Women. International Journal of Environmental Research and Public Health. 2021; 18(12):6593. https://doi.org/10.3390/ijerph18126593
Chicago/Turabian StyleMarshall, Mallory, Beth Birchfield, Rebecca Rogers, Joyeuse Senga, McKenna Persch, Madison Currie, Daphne Schmid, and Christopher Ballmann. 2021. "Walking Cadence during Moderate-Intensity Physical Activity in Pregnant Women" International Journal of Environmental Research and Public Health 18, no. 12: 6593. https://doi.org/10.3390/ijerph18126593
APA StyleMarshall, M., Birchfield, B., Rogers, R., Senga, J., Persch, M., Currie, M., Schmid, D., & Ballmann, C. (2021). Walking Cadence during Moderate-Intensity Physical Activity in Pregnant Women. International Journal of Environmental Research and Public Health, 18(12), 6593. https://doi.org/10.3390/ijerph18126593







