Abstract
Reindeer husbandry is essential for the livelihood and culture of indigenous people in the Arctic. Parts of the herding areas are also used as pastures for farm animals, facilitating potential transmission of viruses between species. Following the Covid-19 pandemic, viruses circulating in the wild are receiving increased attention, since they might pose a potential threat to human health. Climate change will influence the prevalence of infectious diseases of both humans and animals. The aim of this study was to detect known and previously unknown viruses in Eurasian tundra reindeer. In total, 623 nasal and 477 rectal swab samples were collected from reindeer herds in Fennoscandia, Iceland, and Eastern Russia during 2016–2019. Next-generation sequencing analysis and BLAST-homology searches indicated the presence of viruses of domesticated and wild animals, such as bovine viral diarrhea virus, bovine papillomavirus, alcephaline herpesvirus 1 and 2, deer mastadenovirus B, bovine rotavirus, and roe deer picobirnavirus. Several viral species previously found in reindeer and some novel species were detected, although the clinical relevance of these viruses in reindeer is largely unknown. These results indicate that it should be possible to find emerging viruses of relevance for both human and animal health using reindeer as a sentinel species.
Keywords:
Rangifer tarandus; NGS; virus screening; orthobunyavirus; arenavirus; flavivirus; herpesvirus; picornavirus 1. Introduction
Climate change and anthropogenic activities (e.g., altered land use, agricultural practices, changes in human populations) are major drivers of the emergence and re-emergence of infectious diseases [1]. Climate change is predicted to have a greater impact in Arctic and sub-Arctic regions than in other parts of the world [2,3]. The threat from new and/or emerging infectious diseases may play a critical role for the survival of reindeer herding now and in the future. Free-ranging reindeer have numerous opportunities to exchange microorganisms with wildlife animals, but they also have regular contacts with humans. Thus, reindeer may be regarded as a sentinel species for potential pathogen microorganisms circulating in natural ecosystems, which may be relevant for livestock and human health. Better knowledge of circulating viruses is also important to avoid and understand emerging infectious diseases and pandemics, and also a central part of the One Health concept concerning zoonotic infectious diseases circulating in the wild.
Zoonoses are of special importance in the context of climate change. It has been estimated that more than 70% of current human infections are zoonoses [4]. Thus, both animal and human health will most likely be affected by changes in the distribution and virulence of zoonotic pathogens caused by climate change. It is likely that only a small proportion of the viruses circulating in nature have been detected and investigated. Improved knowledge within this research area is thus important for public health, as exemplified during the outbreak and course of the Covid-19 pandemic.
Reindeer husbandry is of great importance in northern Fennoscandia (Finland, Norway, Sweden) and in the Russian Federation, both for livelihoods and for cultural values. In wintertime, i.e., after slaughter and before calving, there are around 600,000 reindeer in Fennoscandia and 2.5 million in the Russian Federation. There are populations of wild Eurasian tundra reindeer in Iceland and Norway, and some smaller populations of wild forest reindeer (R. t. fennicus) in Finland and western Russia. The wild reindeer population in Iceland originates from 35 semi-domesticated reindeer imported from Finnmark, Norway, in 1787 [5]. At present, the Icelandic summer population consists of approximately 6500 animals, which are kept at low density by controlled hunting.
Under current reindeer herding regimes, the number of diseases and clinical cases detected are restricted under normal conditions. Semi-domesticated reindeer are free-ranging for most of the year, utilizing remote forest and mountain pastures, usually with little close contact and handling by people. Thus, reduced reproductive success or production, disease cases in individual animals and even small disease outbreaks may occur unobserved and veterinary attention and clinical investigations are not common, in contrast to the situation for livestock.
For cervids, including semi-domesticated reindeer, the most apparent impact of climate change may be increased frequency of difficult grazing conditions in wintertime [6]. The predicted more frequent rain-on-snow events will create multiple layers of hard ice, making lichen and other winter forage unavailable for reindeer, and causing starvation and emaciation. Future loss and fragmentation of pastures and habitats due to various human activities (e.g., exploitation in the form of increased wind power, forestry, and mining) and high predator pressure will make it difficult for animals and reindeer herders to mitigate the effects of climate change [7].
Infectious diseases directly or indirectly associated with climate change may become an increasing threat. When a new infectious disease is introduced to an immunologically naïve population, the effects may be serious. In the Fennoscandian countries and the Russian Federation, herding systems and levels of pastoralism vary, and the occurrence and epidemiology of certain diseases can also be expected to vary. When weather extremes hinder the ability of reindeer to smell forage under the ice and reach it through digging, to avoid starvation reindeer are fed supplementary fodder in the field or as full maintenance in enclosures. This mitigation strategy saves reindeer lives, but also leads to stress, increased animal density, challenging hygiene conditions, and sometimes lack of clean snow or water for drinking, all of which increase the risk of infectious disease transmission. Thus, opportunistic infections might become a more frequent threat. Infectious diseases of the mucosa of the eyes and mouth are increasingly being observed [8,9]. Arctic wildlife and indigenous peoples’ health are especially at risk due to their dependence on subsistence food resources and the fact that climate change will have a greater impact in the area [10]. Therefore, a transdisciplinary One Health approach in northern regions is a must, i.e., better management of human health, animal health and ecosystem health of this and other remote regions, combining traditional and scientific experience and knowledge.

Table 1.
Virus infections of known or potential clinical relevance identified to circulate in Eurasian tundra reindeer (Rangifer tarandus tarandus).
Table 1.
Virus infections of known or potential clinical relevance identified to circulate in Eurasian tundra reindeer (Rangifer tarandus tarandus).
| Virus | Information | References |
|---|---|---|
| Flaviviridae | Serological studies have reported pestivirus antibodies in reindeer from Finland, Norway, Sweden, and Iceland, as well as in caribou from Canada. The clinical relevance of pestivirus infections in reindeer is unknown. It may, however, be reasonable to assume that also reindeer may be persistently infected, with abortion, stillbirth, and the birth of persistently virus shedding offspring (i.e., persistently infected animals), as seen for many other host species. West Nile virus has also been demonstrated to infect reindeer, causing clinical disease. | [11,12,13,14,15] |
| Herpesviridae Alphaherpesvirinae | Cervid herpesvirus 2 (CvHV2) is enzootic in the Fennoscandian reindeer populations and antibodies against alphaherpesvirus have also been detected in caribou in Alaska (USA) and Canada. CvHV2 has been shown to act as the primary cause of infectious keratoconjunctivitis in reindeer during outbreaks and after experimental ocular inoculation, although many types of bacteria may contribute to the disease. CvHV2 may also cause respiratory infections in reindeer, and possibly abortion and weak-borne calves. | [16,17,18,19,20,21,22] |
| Herpesviridae Gammaherpesvirinae Genus Macavirus | The subfamily Gammaherpesvirinae contains several closely related virus species that are associated with malignant catarrhal fever (MCF). Sheep and goats are healthy carriers of ovine herpesvirus 2 and caprine herpesvirus 2, respectively, but may transmit the virus to susceptible domestic and wild ruminants. One clinical case of MCF in reindeer has been reported. The recorded symptoms were hair loss and thickening of the skin, with crusts in the axillary region, distal parts of the feet, and on the muzzle. Further, the animal had swollen eyelids, opaque cornea and fibrinopurulent eye discharge. | [23,24,25] |
| Papillomaviridae | Papillomaviruses cause mostly benign processes in the skin (papillomas, fibropapillomas or warts) or mucous membranes (condylomas) in many animal species, including reindeer. The clinical outcome may be serious for the individual. Papilloma viruses are considered species-specific, but several virus species may circulate in the same host species. The prevalence of papilloma viruses in reindeer is scarce. Generalized papillomatosis has been reported, affecting the skin in coalescing warts all over the body. | [25,26,27] |
| Poxviridae Genus Parapoxvirus | Orf virus (ORFV) and pseudocowpoxvirus (PCPV) have small ruminants and cattle as their main reservoirs. ORFV cause contagious ecthyma in and around the mouth in sheep and goats, and a similar disease has been reported in reindeer in Sweden, Finland, and Norway. Early outbreaks in Finland were caused by ORFV, whereas later outbreaks, from 1999–2000, have been associated with PCPV, with a milder clinical appearance as compared to ORFV. | [25,28,29,30,31] |
Another example of a new threat is expansion of the geographical distribution of arthropod vectors and host animals, such as roe deer and badgers, due to climate change [32,33].
Some virus infections of known or potential clinical relevance are known to circulate in reindeer (Table 1). Among other relevant viruses, exposure of reindeer or caribou (wild, semi-domesticated, or captive) has been indicated for rabies virus (Canada, Svalbard, Russia), parainfluenzavirus 3 (PIV3) (Sweden), polyomavirus (Alaska), West Nile virus (USA), bluetongue virus (Germany), Schmallenberg virus (Germany), and foot-and-mouth disease virus (Russia) [25,34,35,36].
Ongoing climate change and other drivers affecting ecosystems may influence the type and nature of virus infections directly, or by impacting herding strategies and management. For most virus infections of relevance for reindeer, the transmission potential between wildlife, domesticated animals, and reindeer is not known. The aim of the present study was thus to detect potential virus infections circulating in reindeer populations in northern Fennoscandia, Iceland, and Eastern Russia (Yakutia). Better knowledge of the viruses circulating among reindeer will make it possible to predict health and disease challenges in the vulnerable reindeer herding industry, and to track changes due to increased anthropogenic encroachment and climate change over time.
2. Materials and Methods
2.1. Ethical Statement
In Finland and Sweden, samples were obtained from slaughtered animals. In Norway, sampling was conducted in a general health surveillance of the herds when animals were gathered and handled for other herding purposes, and the study was not classified as an animal experiment. Animal handling procedures and sample collection were performed in accordance with regulations set by the Russian Authorization Board (FS/U.VN-03/163733/07.04.2016). In Iceland, opportunistic sampling from dead animals was perform during the hunting season, with appropriate permits from the Icelandic authorities.
2.2. Sample Collection
In total, 623 nasal and 477 rectal swab samples from Eurasian tundra reindeer (Rangifer t. tarandus) herds in Iceland, Finland, Norway, and Sweden, and the Republic of Sakha, Yakutia, Russia, were included in the study (Table 2 and Table 3, Figure 1). During the first year of sampling in the Nordic countries, eNAT swabs (Copan Italia, Brescia, Italy) were used, while for the remaining sampling UTM swabs (Copan Italia, Brescia, Italy) were used. The sampling performed in Yakutia was conducted with eNAT swabs (Copan Italia, Brescia, Italy) in 2017 and Amies Agar Gel with Charcoal Transport Swabs (JSHD Medical, Yancheng, China) in 2019. In Iceland, wild reindeer shot during the regular hunt were sampled. The reindeer sampled in the other countries were semi-domesticated. The samples from Finland and Sweden were obtained from slaughtered reindeer, whereas the samples from Norway were collected from live animals in corrals. Samples from Finland, Norway, and Sweden represented three geographical locations (Regions A, B, and C), reflecting different pasture and herding conditions. In Russia, sampling was performed during slaughter at two sampling locations (Regions A and B) in northern Yakutia (Figure 1b), while samples were obtained from live animals at one site (Region C) in southern Yakutia. Calves (≤1 year old) and adult animals (>1 year old) were both sampled, except in Iceland where only two calves were available due to a special permit in 2017. The samples were collected in two consecutive years at each site, during the period November 2016 to September 2018, in all countries except Russia, where sampling was performed at one site (Ust-Yansky, northern Yakutia) in December 2017 and at two sites (Eveno-Bytantay, north-central Yakutia, and Aldan, southern Yakutia) in November 2019. All animals sampled at slaughter were examined ante mortem by an official veterinarian and classified as healthy. The reindeer sampled in Iceland and Russia were all considered healthy by the hunters, slaughterers, or an official veterinarian, and were intended for human consumption.

Table 2.
Details of the 623 nasal swabs obtained from 484 Eurasian tundra reindeer (Rangifer t. tarandus), including calves (≤1 year) and adult animals (>1 year), in Finland, Norway, Sweden, Iceland, and Russia. Swabs were taken from three geographically separate herds in each country except for Iceland, where the wild reindeer population was sampled.

Table 3.
Details of the 477 rectal swabs Eurasian tundra reindeer (Rangifer t. tarandus), including calves (≤1 year) and adult animals (>1 year), in Finland, Norway, Sweden, Iceland, and Russia. Sampling was performed during two consecutive years. Swabs were taken from three geographically separate herds in each country except for Iceland, where the wild reindeer population was sampled.
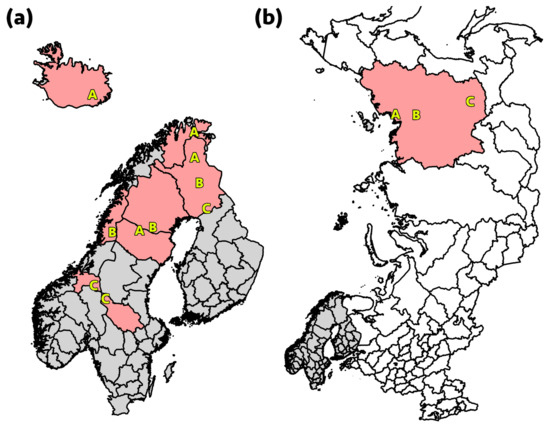
Figure 1.
(a) In Finland, Norway, and Sweden, samples were collected from three geographical locations, denoted A, B, and C, reflecting different pasture and herding conditions. The sampling region in Iceland is also displayed (A). (b) The sampling regions in Ust-Yansky, northern Yakutia (A), Eveno-Bytantay, north-central Yakutia (B), and Aldan, southern Yakutia (C), Russia.
2.3. Nucleic Acid Extraction
Before nucleic acid extraction, 1200 mL of swab collection buffer from each sample were initially filtered through a 0.45 µm filter to remove particles of bacterium size and larger. However, this filtration step was eventually excluded, since most samples were sufficiently clean and did not contain much debris. For swabs in eNAT buffer, five samples were pooled and 550 µL from the pool were used to extract nucleic acids with a magnetic bead-based kit (Viral NA Extraction Kit, Diasorin, Ireland) in an Arrow extraction robot (NorDiag, Oslo, Norway). For swabs in UTM buffer, buffer from each sample was mixed with 10xTURBO DNase Buffer (Kit TURBO DNase; Invitrogen, Carlsbad, CA, USA) to obtain a 1xTURBO DNase Buffer concentration, before pooling five samples per pool. Each UTM-buffer pool was treated with 2 U/µL TURBO DNase (Invitrogen, Carlsbad, CA, USA) to a concentration of 0.2 U/µL and 2.8 µL 40 U/µL of RNase One (Invitrogen, Carlsbad, CA, USA) at 37 °C for 30 min, to degrade unprotected nucleic acids. Then 250 µL was extracted from the pool with a magnetic bead-based kit (Viral NA Extraction Kit, Diasorin, Ireland) in an Arrow extraction robot (NorDiag, Oslo, Norway). Extracted RNA in the eluted total NA was converted to cDNA using random hexamers or the FR20RV-6N primer [37] with the SuperScript IV first-strand synthesis kit (Invitrogen). Double-stranded DNA was obtained by incubation of cDNA with Klenow Fragment DNA polymerase (New England Biolabs, Ipswich, MA, USA) at 37 °C for 1 h. The Klenow enzyme was then inactivated at 75 °C for 10 min. When the tagged primer was used for cDNA synthesis, random amplification of the tagged cDNA was performed using the FR20RV primer [37] under the following conditions: 10 min at 95 °C, followed by 40 cycles of 30 s at 95 °C, 30 s at 58 °C, and 90 s at 72 °C. The reaction was ended with an extra elongation step at 72 °C for 10 min. The PCR reaction contained 1x PCR buffer, 2.5 mM MgCl2, 2.5 mM dNTPs, 0.4 mM primer, and 1.25 U AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA). Some sample pools were run in triplicate with FR20RV primer, and then the products were pooled before purification by QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol [38]. The amplified DNA fragments were further treated with EcoRV (New England Biolabs) to remove the amplification primers and purified by QIAquick PCR purification kit (Qiagen, Hilden, Germany). Concentration was measured with a Qubit fluorometer using Qubit dsDNA HS (High Sensitivity) Assay Kit (Invitrogen, Carlsbad, CA, USA), and an 0.2 ng/µL aliquot was prepared for each sample.
2.4. NGS Library Preparation and Sequencing
Nextera XT DNA Library Preparation Kit (Illumina Inc., San Diego, CA, USA) was used to fragment the input DNA and tag the DNA from each sample with a pair of unique index primers by a 12-cycle PCR amplification. The libraries were purified with AMPure XP beads (Sigma-Aldrich, Milan, Italy), and Agilent High Sensitivity DNA Kit (Agilent Technologies, Waldbronn, Germany) was used to verify the length distribution of the fragments and for quantification of the libraries. Finally, an equimolar amount (preferably 4 nM, but when concentration was not high enough 2 nM was used) of each sample library DNA was pooled, denatured, and further diluted to a final concentration of 10 pM. Sequencing was performed on a MiSeq desktop sequencer using MiSeq 2 × 300 cycles reagent kit (v. 2) (Illumina, Inc.). Library preparation and sequencing was performed according to the manufacturer’s instructions.
2.5. Bioinformatics
The sequence reads were homology searched against the NCBI nt database using a Decypher server (TimeLogic®, Carlsbad, CA, USA). Before blasting, the sequence reads were quality checked and trimmed using HTStream [39]. Over 17 NGS runs the average read length after trimming was 216 nt and the average number of reads per run was 1,939,070. First, the trimmed reads were blasted against the VRL section of the NCBI nt database (i.e., the viral sequences) with a cut-off except value (e-value) of 10−5. A VRL blast database was created using the BLAST+ command line tools available from NCBI [40]. The reads that hit sequences in VRL within the limit of this e-value were collected with an in-house Python script and blasted against the whole nt database with the same e-value. The reads that again had the best hits (lowest e-value) to viral sequences were collected with an in-house Python script. This procedure reduced the computational burden on blasting against the large nt database by about 90%, since non-viral reads were filtered away against the much smaller VRL section.
3. Results
Extracted nucleic acids from 477 rectal swabs pools and 623 nasal swab pools were processed for next-generation sequencing (NGS). Most swab sample pools produced sequences classified as viruses, but there was a tendency for pools from Finland and Russia to contain fewer or no viral sequences. A summary of results for the study regions with positive nasal and/or rectal swab pools for viruses from selected viral families can be found in Table 4 and Figure 2, while a complete overview of the NGS sequence reads with positive pools, read counts, and e-values (min) is provided in the Supplementary Materials. In this results section, the main findings for selected viral families are described in more detail. Sequence reads referred to as ‘virus sequence reads’ were classified by the BLASTn algorithm as most similar to that particular virus sequence in the current version of the nt database of NCBI GenBank. These classifications are also referred to as ‘hits’, meaning the best sequence hits using the BLASTn algorithm.

Table 4.
Summary of regions with positive nasal and/or rectal swab pools (X) for viruses from selected viral families. Nasal and rectal swabs were collected from three different semi-domesticated reindeer herds in Finland, Norway, and Sweden (regions A, B, and C) and from wild reindeer in Iceland (region A) in in two consecutive sampling years (samplings 1 and 2). Nasal and rectal swabs were collected and pooled from one semi-domesticated reindeer herd in Yakutia, Russia, in 2016 (region A) and two different herds in 2019 (regions B and C).
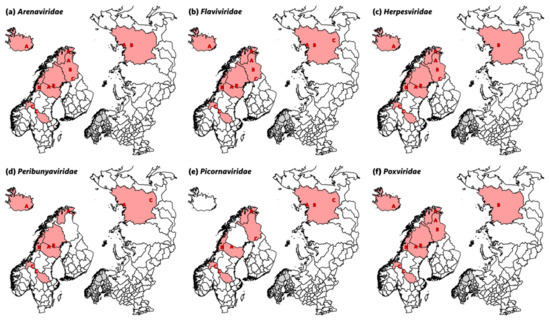
Figure 2.
Maps showing the regions in Finland (A, B, C), Norway (A, B, C), Sweden (A, B, C), Iceland (A), and Russia (A, B, C) in which sequence read hits were detected for viruses from (a) the family Arenaviridae, (b) the family Flaviviridae, (c) the family Herpesviridae, (d) the family Peribunyaviridae, (e) the family Picornaviridae, and (f) the family Poxviridae.
3.1. Arenaviridae
Overall, the most abundant sequence reads, both in number of pools and in geographical distribution (Figure 2a), were reads similar to various viruses from the family Arenaviridae, i.e., Lassa mammarenavirus (104 pools in the range 2–242), Guanarito mammarenavirus (85 pools in the range 2–866) and Luna mammarenavirus (three pools with one sequence read each). The range of sequence reads was 1–860 in the different pools. Sequence reads classified as Arenaviridae were detected in all countries and sampling years except for Finland (Region B) and Russia (Region C) in sampling 2.
3.2. Flaviviridae
Flaviviridae sequence reads were very common and fell in the range 2–47 per pool when present. Sequence reads mainly belonged to two species, dengue virus 1 (14 pools in the range 2–11) and Iguape virus (at least 49 pools in the range 2–47), and both were present in all countries. Bovine viral diarrhea virus (BVDV) hits were also common and were found in 23 pools distributed among all countries except Russia (Figure 2b), although with very few reads in the range 1–4. Specific hits against BVDV were detected in Sweden (six pools), Norway (10 pools), Finland (three pools), and Iceland (four pools). The only other flavivirus hits were a single West Nile virus read from Norway (Region A) and two classical swine fever virus (CSFV) reads from Russia (Region A).
3.3. Herpesviridae
Herpesviridae hits fell into three subfamilies (Alpha-, Beta- and Gammaherpesvirinae). Gammaherpesvirinae read counts were the most common, with hits in at least 30 pools distributed among all countries (Figure 2c) and generally with a high number of reads (range 1–54,453), especially in nasal swabs from Region C in Norway (sampling 2, minimum total read count 186,432 hits). Alphaherpesvirinae reads were detected in at least 39 pools and in all countries, although with lower read counts than Gammaherpesvirinae (range 1–7). Betaherpesvirinae sequence reads were also detected in a total of 18 pools in all countries, with high read counts again in nasal swabs from Region C in Norway (sampling 2; range 2–276). Most hits were against ruminant herpesviruses (e.g., ovine herpesvirus 2 (16 pools in the range 1–54,453), bovine herpesvirus 6 (13 pools in the range 1–36,279), or alcephaline herpesvirus 1 (11 pools in the range 2–36,279) and 2 (14 pools in the range 1–36,279), including reindeer gammaherpesvirus and cervid herpesvirus 3, with two sequence read hits in one pool each.
3.4. Papillomaviridae
Papillomavirus hits were detected in all countries, and in both rectal and nasal swab pools. Read counts were low both in rectal (range 1–182) and in nasal swab pools (range 1–89). Interestingly, three hits against the reindeer papillomavirus were detected in one nasal swab pool in Iceland and one in Norway. Several hits against other ruminant papillomaviruses (e.g., bovine papillomaviruses or cervus elaphus papillomaviruses) were detected in 26 pools in Sweden, Finland, and Russia.
3.5. Paramyxoviridae
Paramyxoviridae reads were found in pools from all countries, but usually with only a few reads and often most similar to human respirovirus 1, with two sequence reads detected in 10 different pools. Norway was an exception, as two nasal swabs pools contained in total 37 sequence reads most like human respirovirus 3 (15 sequence reads in one pool), bovine respirovirus 3 (18 sequence reads in two pools), and caprine respirovirus 3 (four sequence reads in two pools).
3.6. Parvoviridae
Most hits belonging to the Parvoviridae family were associated with the red-crowned crane parvovirus, with read counts in the range 2–109 detected in 48 pools, but several pools also contained reads that hit various viruses of the genus Bocaparvovirus. Parvoviridae sequence reads were detected in 31 rectal swab pools (read count range 2–109) and in 22 nasal swab pools (read count range 2–48) in all countries.
3.7. Peribunyaviridae
Pools from all countries except Finland contained reads assigned to an Orthobunyavirus species with read counts detected in 34 pools in the range 2–23 (Figure 2d). In addition, three Simbu virus reads were found in one pool from Sweden and one from Russia, and six Ngari virus reads were found in four pools from Iceland, Norway, and Sweden.
3.8. Picobirnaviridae
Picobirnavirus hits were widespread in the pools and were found in 72 pools from all countries, with read counts in the range 1–31. The most common host species of these hits were marmot (eleven pools in the range 1–6), humans and other primates (32 pools in the range 1–31), and dromedary (eleven pools in the range 2–6).
3.9. Picornaviridae
Picornaviridae sequences were relatively rare and belonged to a diverse set of viruses. Sequence reads were detected in rectal swab pools from Norway, Sweden, Finland, and Russia (Figure 2e), and were most prominently identified as viruses from the genus Kobuvirus (17 pools in the range 2–53). As exceptions, one sequence read for human rhinovirus A was detected in one rectal swab pool from Sweden, and one rectal swab pool from Finland and one from Russia showed 46 sequence read hits to hepatoviruses (e.g., human hepatovirus A or hedgehog and rodent hepatoviruses). The only nasal swab pools in which picornavirus sequences were detected were from Region B in Russia, with one pool with sequence reads matching bovine rhinitis A (38 reads) and B (nine reads) virus, as well as foot-and-mouth disease virus type A (FMDV; six reads), all members of the genus Apthovirus.
3.10. Poxviridae
Low numbers of sequence reads belonging to the family Poxviridae were found in 11 rectal swab pools from all countries and in 13 nasal swab pools from all countries except Russia (Figure 2f). Most sequence reads matched orf virus (ORFV, genus Parapoxvirus;17 pools in the range 1–4), but sequence reads matching ruminant poxviruses of other genera (e.g., cowpox virus (CPXV), goat poxvirus (GPV), and white-tail deer poxvirus) were also detected in six pools with sequence reads in the range 1–4.
3.11. Small Circular DNA Viruses
As found in many other studies of fecal microbiome [37,41], many reads from small circular DNA viruses were observed in the present study, with most sequences belonging to the families Circoviridae (e.g., CRESS virus), Genomoviridae (e.g., Alces alces faeces assoc. genomovirus) and Smacoviridae (e.g., ovine faeces assoc. smacovirus 1 and bovine faeces assoc. smacovirus) (Supplementary Materials). In particular, small circular DNA viruses were especially prevalent in Norwegian rectal swab pools, with read counts detected in at least 14 out of 21 pools, but similar read counts were also identified in rectal swab pools from Sweden, Finland, and Iceland, and in nasal swab pools from Sweden and Norway. The clinical significance of these types of viruses has not yet been established.
3.12. Other Viruses
A variety of other viruses were detected in NGS analysis (Supplementary Materials). Adenoviridae hits were present in four pools (range 2–129) from Finland, Norway, and Sweden, and mostly belonged to ruminant viruses such as bovine adenoviruses (three pools with sequence reads in the range 4–129) or deer mastadenovirus B (one pool with two sequence reads). Astroviruses are frequently found in stool samples from many mammals, and ruminant astroviruses (e.g., bovine, deer, or yak astroviruses) were detected in at least eight rectal swab pools from Finland, Norway, and Sweden (range 1–44). Ruminant calicivirus hits (e.g., bovine calicivirus) were also detected in two pools from Norway (range 2–38). Reads for bovine rotavirus A and other reoviruses (e.g., human rotavirus A) were detected in seven rectal swab pools from Norway and Sweden, but with low numbers of hits (range 1–12). Interestingly, Reoviridae sequences were also detected in one nasal swab pool from Sweden and two from Norway, with two sequence read hits matching bluetongue virus in one of the Norwegian pools. Hits for human polyomavirus 12 and other polyomaviruses were only detected in one nasal swab pool from Norway. A variety of unclassified viruses, such as statovirus and Hainan astro-like virus 2, were also detected.
4. Discussion
Next-generation sequencing screening of viral pathogens in domestic animals and wildlife is an important tool to identify exposure to certain pathogens and help understand the etiology of diseases, but also to prevent possible disease outbreaks and identify emerging viral diseases in previously unexposed populations. In this study, Eurasian tundra reindeer in Iceland, Fennoscandia, and Yakutia, Russia, were screened for the presence of viruses. The sample set collected is unique in terms of the number of animals per country and the number of countries and sampling sites, representing a wide geographical coverage and spanning two winter seasons. Semi-domesticated reindeer are only available for sampling during the few times they are gathered during the reindeer herding year. Thus, these are presumably healthy animals that are gathered for tagging, selection of slaughter animals, etc. Sick animals in such herds will either be taken care of (caught, treated, or euthanized) or maybe never identified (survive and get healthy again, or die, usually never found due to scavengers, sometimes killed by predators). Sampling reindeer during regular herding practices is carried out under field conditions, and contamination of the nostrils and rectum of the animals with environmental or human material can occur during this procedure. Therefore, some of the viral material identified during this study may have been introduced during the animal handling and/or sampling procedure (e.g., human respiroviruses, papillomaviruses or herpesviruses, or red-crane parvovirus). Whether the sequence reads represent environmental/human contamination or a reindeer-specific virus needs to be elucidated. However, even in the case of contamination, the presence of this viruses may as well happen without the direct involvement of the sampler, due to the direct handling of the animals by reindeer herders during gathering, marking, and slaughtering. Even though sampled animals were considered healthy upon examination, a large variety of nucleic acid sequences of viral origin were detected in nasal and rectal swab pools from all countries studied. Therefore, it is possible that apparently healthy semi-domesticated reindeer may have a role as a pathogen reservoir for both domestic animals and wildlife, but also contribute to the transmission by meat and milk consumption, contact, and so on, of zoonotic pathogens to humans (e.g., Hepatitis E virus or ORFV) [9,42,43].
The method employed to detect the presence of viral nucleic acid sequences was to compare the sequence reads with the NCBI GenBank nt database (using the BLASTn algorithm) and collect the cases where the sequence reads were most similar to a viral sequence deposited in GenBank (‘best hit’), irrespective of the host species of this viral sequence. This method enables a first screening of large amounts of data, but has several drawbacks. For example, if the virus sequenced is lacking in the database, some other distantly or closely related virus will be the best hit, or there will be no hit at all. This incompleteness of the database will limit the precision of virus discovery. Other sources of false classification are parts of the host genome or microbial nucleic acids that are absent from the database. In such cases, these unknown nucleic acid sequences present in the sample may end up as viral sequences. In addition, the large nt database is in part uncurated and may contain erroneous sequences, giving rise to false hits. Other issues are contamination of reagents with various genomic material and the difficulty in distinguishing between-sample leakage of reads and between-run carry-over contamination that may occur on the Illumina MiSeq platform used in the present work. With these limitations in mind, we chose to report all virus hits obtained from the sample pools, under the condition that at least two reads hit the same or taxonomically closely related viruses. Findings that only relate to viral sequences from host species taxonomically very distant from reindeer, or otherwise less likely to infect reindeer, should be considered highly uncertain. Furthermore, samples were pooled, due to limited available funding, representing dilution of viruses in each pool. However, all individual samples have been preserved, making it possible to explore interesting pools in future studies.
In the present study, human viruses (e.g., Lassa mammarenavirus, dengue virus) and non-reindeer-specific ruminant viruses (e.g., ruminant gammaherpesviruses, bovine papillomavirus) may have been overrepresented in comparison with reindeer-specific viruses, due to the lack of reindeer-specific viral sequences in the NCBI database. Therefore, it can be assumed that several of the hits found belong to specific reindeer viruses or other viral species that can infect reindeer. In fact, there is reason to believe that the sequence reads hits for Gammaherpesvirinae indicate a host-specific reindeer virus (rangiferine gammaherpesvirus 1) previously identified in semi-domesticated and wild reindeer in Norway [44,45]. The same applies for papillomaviruses, which are in general host-specific, with one or several papillomavirus species associated with a single host but sharing homologue sequences in parts of their genome [26].
The climate in the Arctic and sub-Arctic region is changing faster than the global average [46]. General knowledge on climate change effects and adaptation strategies has increased significantly in recent years, but there is still a substantial information gap regarding the influence of climate change on infectious diseases. In a One Health perspective, zoonotic infections are a particular concern, and we need more knowledge of what is present in the wild environment. Both animal and human health will most likely be affected by changes in the distribution and virulence of zoonotic pathogens caused by climate change, but also by other anthropogenic drivers and new animal hosts. Further, a population of humans or animals not previously exposed to a particular disease is immunologically naïve, so an outbreak of that disease in a new area (i.e., high-latitude regions) will likely have more severe effects.
The changing climate will give opportunities for climate-sensitive infectious diseases to establish or occur sporadically in new areas [47]. Vector-borne diseases are a particular concern in this regard. Arthropod vectors (e.g., ticks, mosquitoes, and midges) and reservoir animals (e.g., rodents, birds, and wild ungulates) for infectious diseases might both extend their distribution northwards as a result of changes in ecosystems associated with climate warming [48,49]. The rate of development, persistence, and multiplication of most arthropods and microorganisms is directly affected by microclimatic conditions, especially temperature. Warmer temperatures affecting activity and population dynamics of vectors may increase transmission of pathogens and result in spread to new environments. Warmer temperatures at high latitudes may also result in a longer vegetation period, making it easier for arthropod hosts to reproduce and thus develop denser populations [50].
Sequence reads indicating viruses from the family Arenaviridae were detected in reindeer from all countries studied. In general, most arenaviruses are only present in the southern hemisphere, with lymphocytic choriomeningitis virus being the only one described in Europe [51]. No arenavirus has yet been described in any Rangifer species. The widespread detection of sequence hits with high read counts against viruses from the family Arenaviridae raises the question of whether there is an unknown widespread arenavirus in reindeer. Alternative explanations are that the hits belong to one or more circulating viruses with similar genome sequences, or that the reads are homologs to the reindeer genome itself. Incidental integration of non-retrovirus RNA viruses, such as arenaviruses, has been described [52], and it is possible that such integration may have happened in the reindeer genome in the past, with the subsequent detected hits. In either case, further investigations to clarify this matter are necessary, since some arenaviruses are known to cause severe viral hemorrhagic fevers in humans through contact with infected rodents [53], and since the arenaviruses detected may be pathogenic to reindeer.
Blast hits for the family Flaviviridae were detected in reindeer from all countries studied. Most of the sequences matched dengue virus and Iguape virus (genus Flavivirus). However, it is highly unlikely that these viruses are circulating in Arctic reindeer populations. Another member of the genus Flavivirus with positive hits was West Nile virus (WNV), with a single sequence read hit from one nasal swab pool in Norway. Different wild mammals present in the Arctic are known to be flavivirus hosts [54], but flavivirus infections in reindeer have only been described for WNV [12]. Most known flaviviruses are arthropod-borne viruses, with mosquitoes and ticks as intermediate hosts. The arthropod-borne nature of WNV and other flaviviruses, and the fact that they can circulate, be introduced by, and maintained in migratory birds as reservoirs [55], make flaviviruses a risk to the Arctic reindeer population. In recent years, the mosquito Culex modestus Ficalbi 1889 has been identified as one of the main bridge vectors of WNV between birds and mammals, and it appears to have spread in northern and central Europe [56,57]. Climate change, diverse feeding habits, and increased vector competence may have made Cx. modestus more robust to high latitudes [58,59]. However, in Sweden, seropositivity for WNV has been detected only in nonresident birds, which is not considered indicative of local transmission [60].
Classical swine fever virus (CSFV, Pestivirus C) is closely related to BVDV and BDV, but only pigs and wild boars are considered natural reservoirs of this virus [61]. Experimental infection of several ruminants has been reported, but there is no evidence of natural infection of reindeer or other cervids under natural conditions. According to the World Organisation for Animal Health [62], the Nordic countries are officially CSFV-free, but the status in Russia is uncertain, with outbreaks reported in 2014. The two sequence reads against CSFV were detected in Russia (Region A), which may indicate circulation of CSFV in that area. However, the sequence reads could also belong to a different pestivirus species.
The remaining hits for Flaviviridae belonged to BVDV (pestivirus A and B; genus Pestivirus). Most cattle farms in the Nordic countries are currently considered BVDV-free, especially in the reindeer husbandry areas, after successful BVD eradication programs in the 1990s [63]. However, BVDV hits were detected in 23 pools, from Finland (three pools), Norway (10 pools), Sweden (six pools), and Iceland (four pools). To date, only one reindeer pestivirus has been isolated (pestivirus reindeer-1, V60-Krefeld) and sequenced [64]. The lack of genome sequences in the databases hampers identification of reindeer-specific pestivirus sequences by NGS. These findings, together with data from a previous serological screening [14], hint at the possibility that a reindeer-specific pestivirus, presumably closely related to BDV, is circulating among wild and semi-domesticated reindeer populations and may be responsible for these hits [15].
Several herpesviruses are known to infect and cause disease in Eurasian tundra reindeer (Table 1). One of the most common reindeer pathogens is the cervid herpesvirus 2 (CvHV2; subfamily Alphaherpesvirinae, genus Varicellovirus), which is enzootic in semi-domesticated reindeer, with seroprevalences reported to be ~50% [21,65,66]. Surprisingly, no hits against CvHV2 were detected in the present study. However, several hits against Alphaherpesvirinae were detected in 39 pools in all countries studied, suggesting that CvHV2 was in fact the virus generating the sequence hits. Once again, underrepresentation of a reindeer-specific virus may be the reason for the lack of hits if the sequences belong to highly conserved genes among herpesviruses, such as the UL24 gene or the glycoprotein B or H genes [67,68]. Sequence read hits for viruses from the subfamily Gammaherpesvirinae were common, with sequence reads in a total of 30 pools representing all countries. Most of the Gammaherpesvirinae viruses belonged to the malignant catarrhal fever virus group (MCFV; genus Macavirus), e.g., ovine herpesvirus 2 or alcephaline herpesvirus 1 and 2, and undetermined gammaherpesviruses. Read counts were especially high in Norway, with most hits generated from nasal swab pools from Region C in sampling 2. Those pools had a maximum sequence read count of 54,453 against ovine herpesvirus 2 (OvHV-2) and a minimum amount of 186,450 sequence hits for Gammaherpesvirinae in general. Betaherpesviruses are not often considered when discussing semi-domesticated reindeer health. This study detected Betaherpesvirinae sequences in all countries examined, including hits for cervid herpesvirus 3, a betaherpesvirus first identified in the eyes of semi-domesticated reindeer in Norway [27].
This study detected blast hits against an orthobunyavirus (family Peribunyaviridae) in all countries, with the exception of Finland. Sequence hits were in general low (2–4) except for Sweden, which had a maximum sequence read count of 23. Orthobunyaviruses have a wide geographic and host range, although individual viruses may be restricted to a small number of host species [69]. Adverse veterinary outcomes include fetal abnormalities and abortion storms among livestock (e.g., Schmallenberg virus; SBV). SBV, transmitted by biting midges (Culicoides spp.), first emerged in Europe in 2011 and in Sweden in late 2012. The virus then spread rapidly north beyond the Arctic Circle, occurring in high prevalence after the vector season in 2012 [70]. However, the virus has not been detected in Swedish domestic animals or circulating among wild cervids since the vector season in 2014 [71]. Even though SBV has not been detected in semi-domestic or wild reindeer in their natural range, the presence of seropositive reindeer in zoological parks in Germany demonstrates the susceptibility of reindeer to infection [40]. Northern Fennoscandia has a long vector-free winter season compared with ecosystems in central and southern Europe. Virus transmission and spread are possible at temperatures around 15 °C [72], and in northern Fennoscandia daily mean temperatures at this level are usually limited to May–August [73]. Virus persistence depends on the winter survival of adult midges, which must have access to an immunologically naïve ruminant population. If SBV is introduced to the reindeer population in Sweden or in one of the other countries studied, the effects may be serious. However, based on northern latitude climate conditions, it can be assumed that this region has an unfavorable climate for overwintering SBV vectors. In addition, midge activity and the reproductive season of Swedish wild cervids are seasonal and biological mismatches for the virus, which may explain why SBV has so far had little impact on Swedish wild ruminant health. These animals are thus highly unlikely to be reservoirs of this virus. Thus, the findings in the present study indicate that an unknown orthobunyavirus, different to SBV, may be circulating in the reindeer populations studied.
Papillomaviruses are considered species-specific, and to date only rangifer tarandus papillomavirus 1 (reindeer papillomavirus) has been isolated from semi-domesticated reindeer [26]. However, two other rangifer papillomaviruses have recently been characterized, in Norwegian reindeer [27] and Western Arctic caribou [36]. Papillomavirus sequence reads were detected in nasal and rectal swab pools in all countries in the present study. However, only one hit against the reindeer papillomavirus was detected, in a nasal swab pool from Iceland.
All sequence reads from the family Paramyxoviridae belonged to the genus Respirovirus. Human respirovirus 1 sequence reads were the most common among sequences from this genus and were detected mostly in nasal swab pools from all countries, with 15 sequence reads from human respirovirus 3 also detected in a nasal swab pool from Norway (Region C). Both viruses are considered human parainfluenza viruses, known pathogens of the respiratory tract which cause acute respiratory disease [74]. Bovine and caprine respirovirus 3 are also parainfluenza viruses which cause disease in ruminants, and sequence reads for these viruses were detected in two nasal swab pools from Norway (Region C). A previous serological screening for antibodies against bovine parainfluenza 3 reported 53% seroprevalence in Swedish reindeer [35]. All viruses in the Respirovirus genus seem to exhibit considerable genetic and antigenic similarity, and thus the presence of a reindeer-specific respirovirus cannot be discarded as a possibility.
Once again, overrepresentation bias towards other more common papillomaviruses or respiroviruses may have influenced the results of the sequence matches, with several other ruminant and human viruses in several swab pools instead of reindeer-specific viruses. On the other hand, one should not discard the possibility of a novel reindeer papillomavirus or respirovirus, or the possibility of human papillomaviruses and respiroviruses being present in the swab pools due to contamination during sampling or processing of the swabs.
While the majority of sequences belonging to the family Parvoviridae matched red-crowned crane parvovirus, hits against several bocaparvoviruses were also detected. Parvovirus infections can be associated with a variety of clinical signs, ranging from asymptomatic infections to severe disease, depending on the species [75]. Evidence of the presence of a caribou-specific parvovirus has been reported [36]. Whether the sequence reads represent environmental contamination or a reindeer-specific parvovirus needs to be further investigated. In several other projects, we also observed red-crowned crane parvovirus hits (unpublished data), so the validity of these should be regarded as highly uncertain.
Picobirnavirus is the only genus in the family Picobirnaviridae. Sequence reads from this genus were detected in nasal and rectal swabs from all countries studied. Several species of picobirnaviruses have been described as infecting mammals, but they have not been clearly linked to disease [76]. Only one species has so far been isolated from ruminants, roe deer picobirnavirus [76], which was detected in two rectal swab pools from Russia (Region C). However, most of the sequence read hits in this study were identified as marmot, human and other primates, and dromedary picobirnaviruses.
Most Picornaviridae sequences were detected in rectal swab pools and matched viruses of the genus Kobuvirus (e.g., Aichivirus A and B, or caprine and bovine kobuvirus). Kobuviruses are known to infect the gastrointestinal tract of several mammal species, causing gastroenteritis and diarrhea. Only three ruminant Kobuvirus species have so far been isolated, bovine (Aichivirus B1 and D), caprine (Aichivirus C2), and ovine (Aichivirus B3) kobuviruses [77], but kobuvirus RNA has also been detected in roe deer [78]. The widespread distribution of kobuviruses detected in rectal swab pools from reindeer in Fennoscandia and Yakutia, Russia, may indicate that at least one virus in this genus infects these reindeer populations. Additional studies are needed to determine whether this virus is a novel kobuvirus and to establish the epidemiological and clinical importance of kobuviruses in semi-domesticated and wild Eurasian tundra reindeer. One sequence read for human rhinovirus A (genus Enterovirus) and 46 sequences reads for hepatovirus A (e.g., human hepatitis A virus and other hepatoviruses) were also detected in rectal swab pools. Sequence reads matching viruses from the genus Apthovirus were only detected in one nasal swab pool, from Yakutia, Russia (Region C), with 47 sequence hits for bovine rhinitis virus A and B and six sequence reads for FMDV, a known and important pathogen which causes foot-and-mouth disease (FMD) in cattle and other domestic ruminants. FMD is a notifiable disease and is currently absent from the Nordic countries and most of the European Union, which has protocols in place to avoid the spread of FMDV. Russia is also mainly considered FMD-free, but outbreaks of FMD have recently been reported in far-east Russia [79]. Although FMD has been reported in reindeer and other wild and semi-domesticated ungulates, it apparently fails to establish in wildlife and it is most likely maintained in livestock, with sporadic spread to wild and semi-domestic ungulates [80,81].
Poxviridae sequences were detected in rectal swab pools from all countries studied here and in nasal swab pools from all countries except Russia. Most sequence reads matched ORFV, which is a member of the Parapoxvirus genus causing contagious ecthyma in small ruminants, reindeer, and many wildlife species, and a zoonotic infection [25]. ORFV-specific genome sequences have been detected by PCR in reindeer with no clinical signs of contagious ecthyma, indicating that the virus may circulate among reindeer without presenting as regular disease outbreaks [82]. In our experience, it is very common to observe a limited amount of ORFV reads in samples from various ungulates, including reindeer, using the NGS technology (unpublished observations). This may, in fact, indicate presence of the virus, since ORFV may have a broad host range among wild ungulates [83]. However, since the whole genome sequence of several Parapoxvirus species are available in GenBank, the matching NGS reads may also reflect similarities between certain immunomodulatory components of the virus and the host [84]. One example is the viral interleukin ortholog (vIL-10), which needs to have close similarity to the interleukin-10 of the host if the virus is to achieve effective replication [85]. Thus, the finding of poxvirus sequences in the present screening needs to be further substantiated on nucleotide sequence level.
New climatic conditions and landscape alterations have also contributed to the presence and altered distribution of other ungulates (e.g., roe deer or wild boar) [86,87] that can act as reservoirs of several viruses (e.g., CSFV, FMDV, ORFV, bluetongue virus, or SBV) that may be transmitted to semi-domesticated reindeer in the same areas [88,89]. At the same time, the detection of sequence reads belonging to some of those viruses in apparently healthy reindeer may indicate that after transmission to semi-domesticated reindeer, this species may have a role as reservoir in the subsequent transmission to other domestic and wild animals in the area, but also humans [9,40,41]. However, the possible role of semi-domesticated reindeer as a reservoir needs to be further investigated and cannot be inferred from the current data.
This screening of Eurasian tundra reindeer for viruses by NGS identified several viral families and species that can affect human and animal health in all countries and sampling sites studied. However, only a few of these virus families and species are recognized as being pathogenic for reindeer. Although the NGS screening method has limitations with regard to identifying pathogenicity and a potential causative role for a virus to cause a certain disease, it proved useful in suggesting potential pathogens present in Eurasian tundra reindeer as the host species. This first screening involved a significant number of reindeer samples, representing a broad geographic region and five countries. The results obtained should be further analyzed by addressing the gene sequences generated and conducting phylogenetic studies.
5. Conclusions
This screening of Eurasian tundra reindeer for viruses by NGS identified numerous viral families, including several species that can impair the health of reindeer, wildlife, livestock, and humans. A One Health perspective on further studies of these risks is vital. Climate change and other anthropogenic drivers will expand the future distribution of infectious diseases to new areas, ecosystems, and hosts.
This study showed that a large variety of virus species are circulating in the reindeer populations in all five countries studied. Only a few of these virus species are currently recognized as being pathogenic for reindeer. Some of the hits identified may belong to reindeer-specific pathogens that are underrepresented in GenBank (e.g., CvHV2, reindeer gammaherpesvirus, and parvovirus), thus generating ‘best hits’ with similar viruses associated with other hosts. However, several hits may belong to novel reindeer viruses (e.g., kobuvirus, picobirnavirus, arenavirus) with unknown impacts on reindeer populations. These novel viruses could represent a potential health risk for reindeer, other animal species, and humans, so further studies are needed to identify their pathogenic potential.
Supplementary Materials
The following tables are available online at https://www.mdpi.com/
article/10.3390/ijerph18126561/s1. Complete dataset with viral Blastn hits from rectal and nasal swabs collected from semi-domesticated and wild Eurasian tundra reindeer (Rangifer tarandus tarandus) in Sweden, Norway, Finland, Iceland and Russia in different geographical locations and during two separate sampling periods.
Author Contributions
Conceptualization, A.A., A.O. and M.T.; software, M.L., Å.H.; validation, A.A., M.T.; formal analysis, M.L.; investigation, A.O., J.K., J.S.R., M.T., T.R., U.R. and V.F.; resources, A.A., A.O., J.K., J.S.R., M.T., T.R., U.R. and V.F.; writing—original draft preparation, A.O., J.S.R. and M.L.; writing—review and editing, A.A., J.K., M.T., T.R., U.R., V.F. and Å.H.; supervision, A.A., T.R.; project administration, A.A., A.O. and M.T.; funding acquisition, A.A., J.K., M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NordForsk Nordic Centre of Excellence in Arctic Research, grant number 76413 (CLINF; www.clinf.org). The sampling in Norway and Iceland was partly funded by FRAM, High North Research Centre for Climate and the Environment, through the Terrestrial Flagship, grant number 362256, and the Reindeer Development Fund, Norway. The publication charges for this article have been funded by a grant from the publication fund of UiT The Arctic University of Norway.
Institutional Review Board Statement
Ethical review and approval were waived for this study in Finland, Iceland, Norway and Sweden, since samples were obtained from slaughtered/dead animals (Finland, Iceland and Sweden) or during a general health surveillance of the herds when animals were gathered and handled for other herding purposes, and the study was therefore not classified as an animal experiment. Animal handling procedures and sample collection in Russia were performed in accordance with regulations set by the Russian Authorization Board (FS/U.VN-03/163733/ 07.04.2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is available as Supplementary Material and can be accessed online at https://www.mdpi.com/article/10.3390/ijerph18126561/s1.
Acknowledgments
All reindeer herders from the different sampling sites, personnel at the slaughterhouses in Finland, Norway, Sweden, and Russian Federation, and reindeer guides in Iceland are gratefully acknowledged for their involvement in the study. The authors thank Mervi Honkatukia and Heli Lindeberg (Natural Resources Institute Finland; Luke, Finland), Johanna Rautiainen (Lammasmaailma Oy, Finland), Eva Marie Breines, Emily Magnuson and Sandra Núñez Egido (UiT The Arctic University of Norway, Tromsø, Norway) and Ingebjørg H. Nymo and Torill Mørk (Norwegian Veterinary Institute, Tromsø, Norway) for technical assistance during sampling. Skarphéðinn G. Þórisson and Ràn Thorarinsdottir (East Iceland Nature Research Centre, Iceland) are acknowledged for facilitating sampling of wild reindeer on Iceland. Gunnar Andersson (National Veterinary Institute, Sweden) is gratefully acknowledged for designing the map of sampling sites in each participating country.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zell, R. Global climate change and the emergence/re-emergence of infectious diseases. Int. J. Med. Microbiol. Suppl. 2004, 293, 16–26. [Google Scholar] [CrossRef]
- Xu, L.; Myneni, R.; Chapin, F.S., III; Callaghan, T.V.; Pinzon, J.E.; Tucker, C.J.; Zhu, Z.; Bi, J.; Ciais, P.; Tømmervik, H.; et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Chang. 2013, 3, 581–586. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Jones, K.; Patel, N.G.; Levy, M.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Thórisson, S. The history of reindeer in Iceland and reindeer study 1979–1981. Rangifer 1984, 4, 22–38. [Google Scholar] [CrossRef][Green Version]
- Forbes, B.C.; Kumpula, T.; Meschtyb, N.; Laptander, R.; Macias-Fauria, M.; Zetterberg, P.; Verdonen, M.; Skarin, A.; Kim, K.-Y.; Boisvert, L.N.; et al. Sea ice, rain-on-snow and tundra reindeer nomadism in Arctic Russia. Biol. Lett. 2016, 12, 20160466. [Google Scholar] [CrossRef]
- Risvoll, C.; Hovelsrud, G.K. Pasture access and adaptive capacity in reindeer herding districts in Nordland, Northern Norway. Polar J. 2016, 6, 87–111. [Google Scholar] [CrossRef]
- Sánchez Romano, J.; Leijon, M.; Hagström, Å.; Jinnerot, T.; Rockström, U.K.; Tryland, M. Chlamydia pecorum associated with an outbreak of infectious keratoconjunctivitis in semi-domesticated reindeer in Sweden. Front. Vet. Sci. 2019, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Tryland, M.; Nymo, I.H.; Sánchez Romano, J.; Mørk, T.; Klein, J.; Rockström, U. Infectious disease outbreak associated with supplementary feeding of semi-domesticated reindeer. Front. Vet. Sci. 2019, 6, 126. [Google Scholar] [CrossRef]
- Dudley, J.P.; Hoberg, E.P.; Jenkins, E.J.; Parkinson, A.J. Climate change in the North American Arctic: A one health perspective. EcoHealth 2015, 12, 713–725. [Google Scholar] [CrossRef]
- Macdonald, E.; Handeland, K.; Blystad, H.; Bergsaker, M.; Fladberg, M.; Gjerset, B.; Nilsen, O.; Os, H.; Sandbu, S.; Stokke, E.; et al. Public health implications of an outbreak of rabies in arctic foxes and reindeer in the Svalbard archipelago, Norway, September 2011. Eurosurveillance 2011, 16, 19985. [Google Scholar] [CrossRef][Green Version]
- Palmer, M.V.; Stoffregen, W.C.; Rogers, U.G.; Hamir, A.N.; Richt, J.A.; Pedersen, D.D.; Waters, W.R. West Nile virus infection in reindeer (Rangifer Tarandus). J. Vet. Diagn. Investig. 2004, 16, 219–222. [Google Scholar] [CrossRef]
- Larska, M. Pestivirus infection in reindeer (Rangifer Tarandus). Front. Microbiol. 2015, 6, 1187. [Google Scholar] [CrossRef]
- Omazic, A.; Aurosell, C.; Fedorov, V.; Hagström, Å.; Kantanen, J.; Leijon, M.; Mørk, T.; Nordtun, C.S.; Nymo, I.H.; Þórisson, S.G.; et al. Seroprevalence of pestivirus in Eurasian tundra reindeer in Finland, Sweden, Norway, Iceland and Russian Federation. Infect. Ecol. Epidemiol. 2019, 9, 1682223. [Google Scholar] [CrossRef]
- Das Neves, C.G.; Wensman, J.J.; Nymo, I.H.; Skjerve, E.; Alenius, S.; Tryland, M. Pestivirus infections in semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus): A retrospective cross-sectional serological study in Finnmark county, Norway. Viruses 2019, 12, 29. [Google Scholar] [CrossRef]
- das Neves, C.G.; Roth, S.; Rimstad, E.; Thiry, E.; Tryland, M. Cervid herpesvirus 2 infection in reindeer: A review. Vet. Microbiol. 2010, 143, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.L.; das Neves, C.G.; Finstad, G.F.; Beckmen, K.B.; Skjerve, E.; Nymo, I.H.; Tryland, M. Evidence of alphaherpesvirus infections in Alaskan caribou and reindeer. BMC Vet. Res. 2012, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.M.; Curry, P.; Elkin, B.; Russell, D.; Veitch, A.; Branigan, M.; Campbell, M.; Croft, B.; Cuyler, C.; Côté, S.D.; et al. Multi-pathogen serological survey of migratory caribou herds: A snapshot in time. PLoS ONE 2019, 14, e0219838. [Google Scholar] [CrossRef]
- Tryland, M.; Das Neves, C.G.; Sunde, M.; Mørk, T. Cervid herpesvirus 2, the primary agent in an outbreak of infectious keratoconjunctivitis in semidomesticated reindeer. J. Clin. Microbiol. 2009, 47, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Tryland, M.; Romano, J.S.; Marcin, N.; Nymo, I.H.; Josefsen, T.D.; Sørensen, K.K.; Mørk, T. Cervid herpesvirus 2 and not Moraxella bovoculi caused keratoconjunctivitis in experimentally inoculated semi-domesticated Eurasian tundra reindeer. Acta Vet. Scand. 2017, 59, 23. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romano, J.; Mørk, T.; Laaksonen, S.; Ågren, E.; Nymo, I.H.; Sunde, M.; Tryland, M. Infectious keratoconjunctivitis in semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus): Microbiological study of clinically affected and unaffected animals with special reference to cervid herpesvirus 2. BMC Vet. Res. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romano, J.; Sørensen, K.K.; Larsen, A.K.; Mørk, T.; Tryland, M. Ocular histopathological findings in semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus) with infectious keratoconjunctivitis after experimental inoculation with cervid herpesvirus 2. Viruses 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2008, 154, 171–177. [Google Scholar] [CrossRef]
- Li, H.; McGuire, T.C.; Müller-Doblies, U.U.; Crawford, T.B. A simpler, more sensitive competitive inhibition enzyme-linked immunosorbent assay for detection of antibody to malignant catarrhal fever viruses. J. Vet. Diagn. Investig. 2001, 13, 361–364. [Google Scholar] [CrossRef]
- Tryland, M.; Das Neves, C.G.; Klein, J.; Mørk, T.; Hautaniemi, M.; Wensman, J.J. Viral infections and diseases. In Reindeer and Caribou—Health and Disease; Tryland, M., Kutz, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 273–3034. [Google Scholar]
- Moreno-López, J.; Mörner, T.; Pettersson, U. Papillomavirus DNA associated with pulmonary fibromatosis in European elks. J. Virol. 1986, 57, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.L.; Schapendonk, C.M.E.; Van Leeuwen, M.; Kuiken, T.; Bodewes, R.; Raj, V.S.; Haagmans, B.L.; Das Neves, C.G.; Tryland, M.; Osterhaus, A. Identification and characterization of two novel viruses in ocular infections in reindeer. PLoS ONE 2013, 8, e69711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Büttner, M.; Von Einem, C.; McInnes, C.; Oksanen, A. Clinical findings and diagnosis of a severs parapoxvirus epidemic in Finnish reindeer. Tierarztl. Prax. 1995, 23, 614–618. [Google Scholar]
- Tryland, M.; Oksanen, A.; Aschfalk, A.; Josefsen, T.D. Parapoxvirus infection in Norwegian semi-domesticated reindeer (Rangifer tarandus tarandus). Vet. Rec. 2001, 149, 394–395. [Google Scholar] [CrossRef]
- Kummeneje, K.; Krogsrud, J. Contagious ecthyma (orf) in reindeer (Rangifer tarandus). Vet. Rec. 1979, 105, 60–61. [Google Scholar] [CrossRef]
- Nordkvist, M. Munvårtsjuka-En ny rensjukdom? Rennäringsnytt 1973, 8, 6–8. [Google Scholar]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Woldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasites Vectors 2014, 7, 11. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef]
- Ogryzkov, S.E. The pathology of foot and mouth disease in reindeer. Trudy II Vses. Konf. Patol. Anat. Zhivotnykh Mosk. Vet Akad. 1964, 420–425. [Google Scholar]
- Rehbinder, C.; Belák, S.; Nordkvist, M. A serological, retrospective study in reindeer on five different viruses. Rangifer 1992, 12, 191. [Google Scholar] [CrossRef][Green Version]
- Schürch, A.C.; Schipper, D.; Bijl, M.A.; Dau, J.; Beckmen, K.B.; Schapendonk, C.M.E.; Raj, V.S.; Osterhaus, A.D.M.E.; Haagmans, B.L.; Tryland, M.; et al. Metagenomic survey for viruses in Western Arctic Caribou, Alaska, through iterative assembly of taxonomic units. PLoS ONE 2014, 9, e105227. [Google Scholar] [CrossRef]
- Johansson, Ö.; Ullman, K.; Lkhagvajav, P.; Wiseman, M.; Malmsten, J.; Leijon, M. Detection and genetic characterization of viruses present in free-ranging snow leopards using next-generation sequencing. Front. Vet. Sci. 2020, 7, 645. [Google Scholar] [CrossRef]
- Blomström, A.-L.; Widén, F.; Hammer, A.-S.; Belák, S.; Berg, M. Detection of a novel astrovirus in brain tissue of mink suffering from shaking mink syndrome by use of viral metagenomics. J. Clin. Microbiol. 2010, 48, 4392–4396. [Google Scholar] [CrossRef] [PubMed]
- HTStream. 2020. Available online: https://s4hts.github.io/HTStream/ (accessed on 30 March 2021).
- National Center for Biotechnology Information (NCBI). 1988. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 30 March 2021).
- Li, L.; Kapoor, A.; Slikas, B.; Bamidele, O.S.; Wang, C.; Shaukat, S.; Alam Masroor, M.; Wilson, M.L.; Ndjango, J.-B.N.; Peeters, M.; et al. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J. Virol. 2010, 84, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romano, J.; Grund, L.; Obiegala, A.; Nymo, I.H.; Ancin-Murguzur, F.J.; Li, H.; Król, N.; Pfeffer, M.; Tryland, M. A multi-pathogen screening of captive reindeer (Rangifer tarandus) in Germany based on serological and molecular assays. Front. Vet. Sci. 2019, 6, 461. [Google Scholar] [CrossRef]
- Slukinova, O.S.; Kyuregyan, K.K.; Karlsen, A.A.; Potemkin, I.A.; Kichatova, V.S.; Semenov, S.I.; Stepanov, K.M.; Rumyantseva, T.D.; Mikhailov, M.I. Serological evidence of Hepatitis E virus circulation among reindeer and reindeer herders. Vector Borne Zoonotic Dis. 2021. [Google Scholar] [CrossRef]
- Das Neves, C.G.; Ihlebæk, H.M.; Skjerve, E.; Hemmingsen, W.; Li, H.; Tryland, M. Gammaherpesvirus infection in semidomesticated reindeer (Rangifer Tarandus Tarandus): A cross-sectional, serologic study in Northern Norway. J. Wildl. Dis. 2013, 49, 261–269. [Google Scholar] [CrossRef]
- das Neves, C.G.; Sacristán, C.; Madslien, K.; Tryland, M. Gammaherpesvirus in cervid species from Norway: Characterization of a new virus in wild and semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus). Viruses 2020, 12, 876. [Google Scholar] [CrossRef] [PubMed]
- Box, J.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.-J.W.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Brooks, D.R. Evolution in action: Climate change, biodiversity dynamics and emerging infectious disease. Philos. Trans. R. Soc. B. Biol. Sci. 2015, 370, 20130553. [Google Scholar] [CrossRef]
- Pauchard, A.; Milbau, A.; Albihn, A.; Alexander, J.M.; Burgess, T.; Daehler, C.C.; Englund, G.; Essl, F.; Evengård, B.; Greenwood, G.B.; et al. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: New challenges for ecology and conservation. Biol. Invasions 2016, 18, 345–353. [Google Scholar] [CrossRef]
- Omazic, A.; Bylund, H.; Boqvist, S.; Högberg, A.; Björkman, C.; Tryland, M.; Evengård, B.; Koch, A.; Berggren, C.; Malogolovkin, A.; et al. Identifying climate-sensitive infectious diseases in animals and humans in Northern regions. Acta Vet. Scand. 2019, 61, 1–12. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Eisen, L.; Comstedt, P.; Mejlon, H.A.; Lindgren, E.; Bergström, S.; Olsen, B. Risk indicators for the tick Ixodes ricinus and Borrelia burgdorferi sensu lato in Sweden. Med. Vet. Entomol. 2009, 23, 226–237. [Google Scholar] [CrossRef]
- Hugot, J.; Gonzalez, J.-P.; Denys, C. Evolution of the old world Arenaviridae and their rodent hosts: Generalized host-transfer or association by descent? Infect. Genet. Evol. 2001, 1, 13–20. [Google Scholar] [CrossRef]
- Desfarges, S.; Ciuffi, A. Viral integration and consequences on host gene expression. In Viruses: Essential Agents of Life; Springer: Berlin, Germany, 2012; pp. 147–175. [Google Scholar]
- Charrel, R.; de Lamballerie, X. Zoonotic aspects of arenavirus infections. Vet. Microbiol. 2010, 140, 213–220. [Google Scholar] [CrossRef]
- Root, J.J.; Bosco-Lauth, A.M. West Nile virus associations in wild mammals: An update. Viruses 2019, 11, 459. [Google Scholar] [CrossRef]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, Influenza A and Enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef]
- Bakonyi, T.; Haussig, J.M. West Nile virus keeps on moving up in Europe. Eurosurveillance 2020, 25, 2001938. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. BioMed Res. Int. 2015, 2015, 1–20. [Google Scholar] [CrossRef]
- Fyodorova, M.V.; Savage, H.M.; Lopatina, J.V.; Bulgakova, T.A.; Ivanitsky, A.V.; Platonova, O.V.; Platonov, A.E. Evaluation of potential West Nile virus vectors in Volgograd region, Russia, 2003 (Diptera: Culicidae): Species composition, bloodmeal host utilization, and virus infection rates of mosquitoes. J. Med. Entomol. 2006, 43, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Lindström, A.; Lilja, T. First finding of the West Nile virus vector Culex modestus Ficalbi 1889 (Diptera; Culicidae) in Sweden. J. Eur. Mosq. Control. Assoc. 2018, 36, 1–2. [Google Scholar]
- Jourdain, E.; Olsen, B.; Lundkvist, A.; Hubálek, Z.; Šikutová, S.; Waldenström, J.; Karlsson, M.; Wahlström, M.; Jozan, M.; Falk, K.I. Surveillance for West Nile virus in wild birds from northern Europe. Vector Borne Zoonotic Dis. 2011, 11, 77–79. [Google Scholar] [CrossRef]
- OIE. Classical Swine Fever. 2020. Available online: https://www.oie.int/en/disease/classical-swine-fever/ (accessed on 30 March 2021).
- Titov, I.; Tsybanov, S.; Malogolovkin, A. Genotyping of classical swine fever virus using high-resolution melt analysis. J. Virol. Methods 2015, 224, 53–57. [Google Scholar] [CrossRef]
- Lindberg, A.L.; Alenius, S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Vet. Microbiol. 1999, 64, 197–222. [Google Scholar] [CrossRef]
- Becher, P.; Orlich, M.; Kosmidou, A.; König, M.; Baroth, M.; Thiel, H.-J. Genetic diversity of pestiviruses: Identification of novel groups and implications for classification. Virology 1999, 262, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, C.G.; Thiry, J.; Skjerve, E.; Yoccoz, N.G.; Rimstad, E.; Thiry, E.; Tryland, M. Alphaherpesvirus infections in semidomesticated reindeer: A cross-sectional serological study. Vet. Microbiol. 2009, 139, 262–269. [Google Scholar] [CrossRef]
- Kautto, A.H.; Alenius, S.; Mossing, T.; Becher, P.; Belák, S.; Larska, M. Pestivirus and alphaherpesvirus infections in Swedish reindeer (Rangifer tarandus tarandus L.). Vet. Microbiol. 2012, 156, 64–71. [Google Scholar] [CrossRef]
- Thiry, J.; Keuser, V.; Muylkens, B.; Meurens, F.; Gogev, S.; Vanderplasschen, A.; Thiry, E. Ruminant alphaherpesviruses related to bovine herpesvirus 1. Vet. Res. 2006, 37, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Kong, X. Characterization of the genes encoding UL24, TK and gH proteins from duck enteritis virus (DEV): A proof for the classification of DEV. Virus Genes 2006, 33, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.R.; Adkins, S.; Alkhovskiy, S.; Beer, M.; Blair, C.; Calisher, C.H.; Drebot, M.; Lambert, A.J.; De Souza, W.M.; Marklewitz, M.; et al. ICTV virus taxonomy profile: Peribunyaviridae. J. Gen. Virol. 2020, 101, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chenais, E.; Stahl, K.; Frössling, J.; Blomqvist, G.; Näslund, K.; Svensson, L.; Renström, L.; Mieziewska, K.; Elvander, M.; Valarcher, J.F. Schmallenberg virus beyond latitude 65° N. Transbound. Emerg. Dis. 2013, 62, e11–e18. [Google Scholar] [CrossRef]
- Malmsten, A.; Malmsten, J.; Blomqvist, G.; Näslund, K.; Vernersson, C.; Hägglund, S.; Dalin, A.-M.; Ågren, E.O.; Valarcher, J.-F. Serological testing of Schmallenberg virus in Swedish wild cervids from 2012 to 2016. BMC Vet. Res. 2017, 13, 84. [Google Scholar] [CrossRef][Green Version]
- European Food Safety Authorities. “Schmallenberg” virus: Analysis of the epidemiological data and assessment of impact. EFSA J. 2012, 10, 2768. [Google Scholar]
- Clima Temps. Sweden Climate Graph. Available online: http://www.sweden.climatemps.com/#brief (accessed on 29 March 2021).
- Rima, B.; Balkema-Buschmann, A.; Dundon, W.G.; Duprex, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.; Lee, B.; Rota, P.; et al. ICTV virus taxonomy profile: Paramyxoviridae. J. Gen. Virol. 2019, 100, 1593–1594. [Google Scholar] [CrossRef]
- Berns, K.I.; Parrish, C.R. Parvoviridae. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 2437–2477. [Google Scholar]
- Delmas, B.; Attoui, H.; Ghosh, S.; Malik, Y.S.; Mundt, E.; Vakharia, V.N. ICTV report consortium ICTV virus taxonomy profile: Picobirnaviridae. J. Gen. Virol. 2019, 100, 133–134. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Di Felice, E.; Robetto, S.; Guidetti, C.; Orusa, R.; Martella, V.; Marsilio, F. Molecular detection of kobuviruses in European roe deer (Capreolus capreolus) in Italy. Arch. Virol. 2015, 160, 2083–2086. [Google Scholar] [CrossRef]
- FAO. Foot and Mouth Disease Situation—March 2019; European Commission for the Control of Foot and Mouth Disease: Rome, Italy, 2019. [Google Scholar]
- EFSA Panel on Animal Health and Welfare. Scientific opinion on foot and mouth disease in Thrace. EFSA J. 2012, 10, 2635. [Google Scholar]
- Rahman, A.-U.; Dhama, K.; Ali, Q.; Raza, M.A.; Chaudhry, U.; Shabbir, M.Z. Foot and mouth disease in a wide range of wild hosts: A potential constraint in disease control efforts worldwide particularly in disease-endemic settings. Acta Trop. 2020, 210, 105567. [Google Scholar] [CrossRef]
- Tryland, M. Asymptomatic parapoxvirus infections in semi-domesticated reindeer (Rangifer tarandus tarandus). In Proceedings of the XIVth International Poxvirus and Iridovirus Workshop, Lake Placid, NY, USA, 20–25 September 2002; pp. 20–25. [Google Scholar]
- Tryland, M.; Beckmen, K.B.; Burek-Huntington, K.A.; Breines, E.M.; Klein, J. Orf virus infection in Alaskan mountain goats, Dall’s sheep, muskoxen, caribou and Sitka black-tailed deer. Acta Vet. Scand. 2018, 60, 1–11. [Google Scholar] [CrossRef]
- Haig, D.M. Orf virus infection and host immunity. Curr. Opin. Infect. Dis. 2006, 19, 127–131. [Google Scholar] [CrossRef]
- Fleming, S.B.; McCaughan, C.A.; Andrews, A.E.; Nash, A.D.; Mercer, A.A. A homolog of interleukin-10 is encoded by the poxvirus Orf virus. J. Virol. 1997, 71, 4857–4861. [Google Scholar] [CrossRef]
- Danilov, P.I.; Panchenko, D.V.; Tirronen, K.F. The European roe deer (Capreolus capreolus L.) at the northern boundary of its range in Eastern Fennoscandia. Russ. J. Ecol. 2017, 48, 459–465. [Google Scholar] [CrossRef]
- Rosvold, J.; Andersen, R. Wild boar in Norway—Is climate a limiting factor? NTNU Vitensk. Rapp. Zool. 2008, 1, 1–23. [Google Scholar]
- Rossi, S.; Viarouge, C.; Faure, E.; Gilot-Fromont, E.; Gache, K.; Gibert, P.; Verheyden, H.; Hars, J.; Klein, F.; Maillard, D.; et al. Exposure of wildlife to the Schmallenberg virus in France (2011–2014): Higher, faster, stronger (than bluetongue). Transbound. Emerg. Dis. 2015, 64, 354–363. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Segalés, J.; Gortázar, C. A review of viral diseases of the European wild boar: Effects of population dynamics and reservoir rôle. Vet. J. 2008, 176, 158–169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).