The Urban River Syndrome: Achieving Sustainability Against a Backdrop of Accelerating Change
Abstract
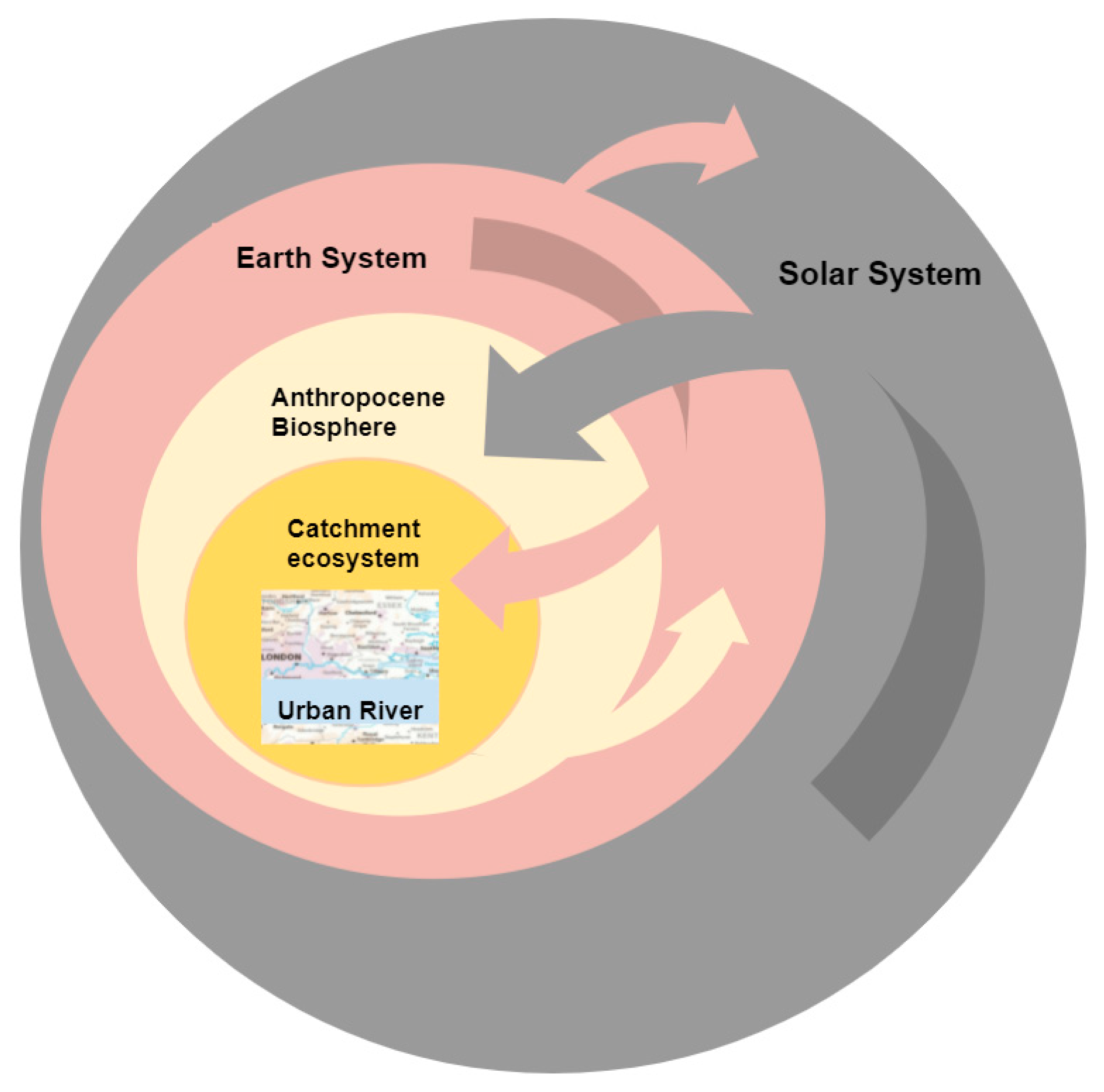
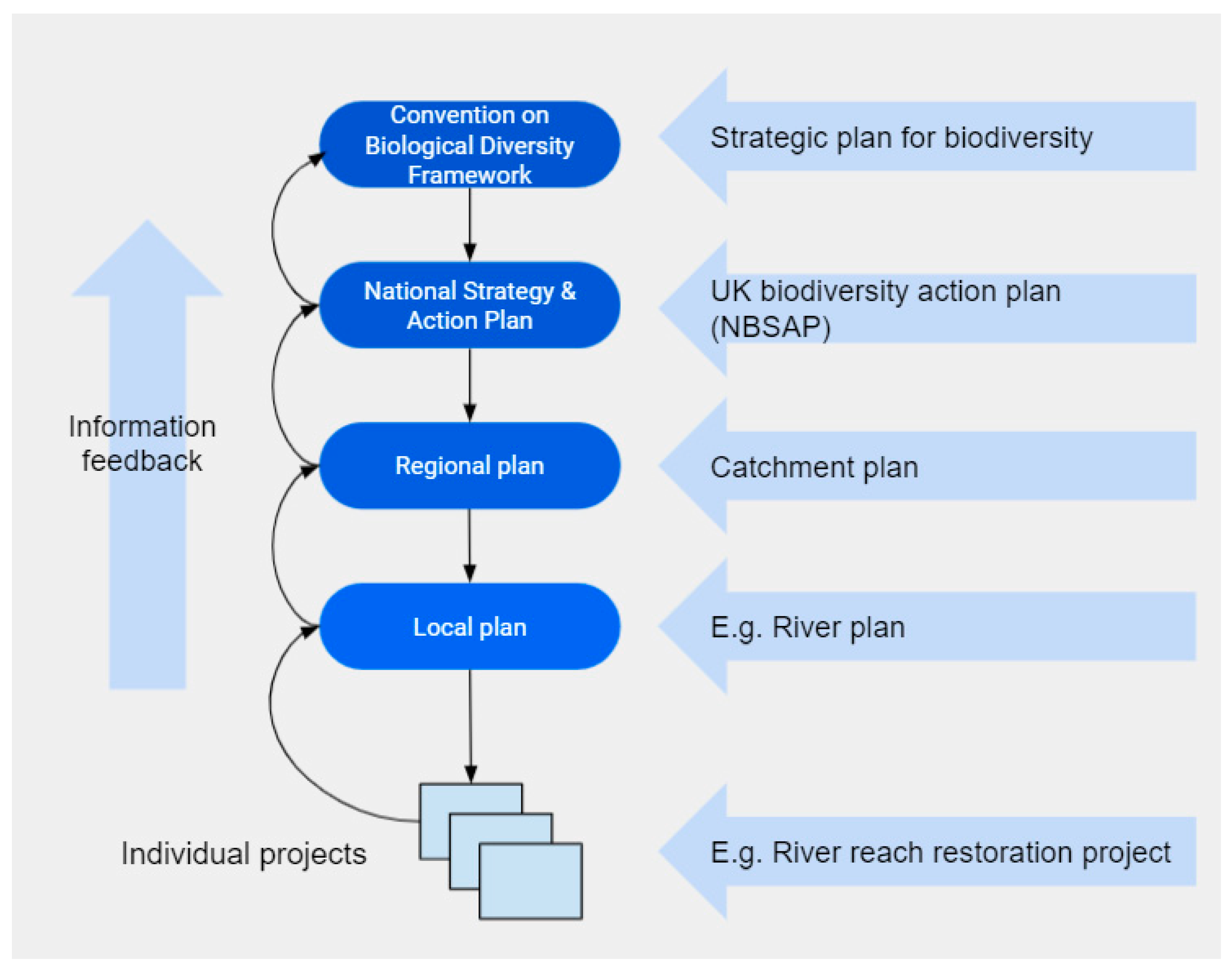
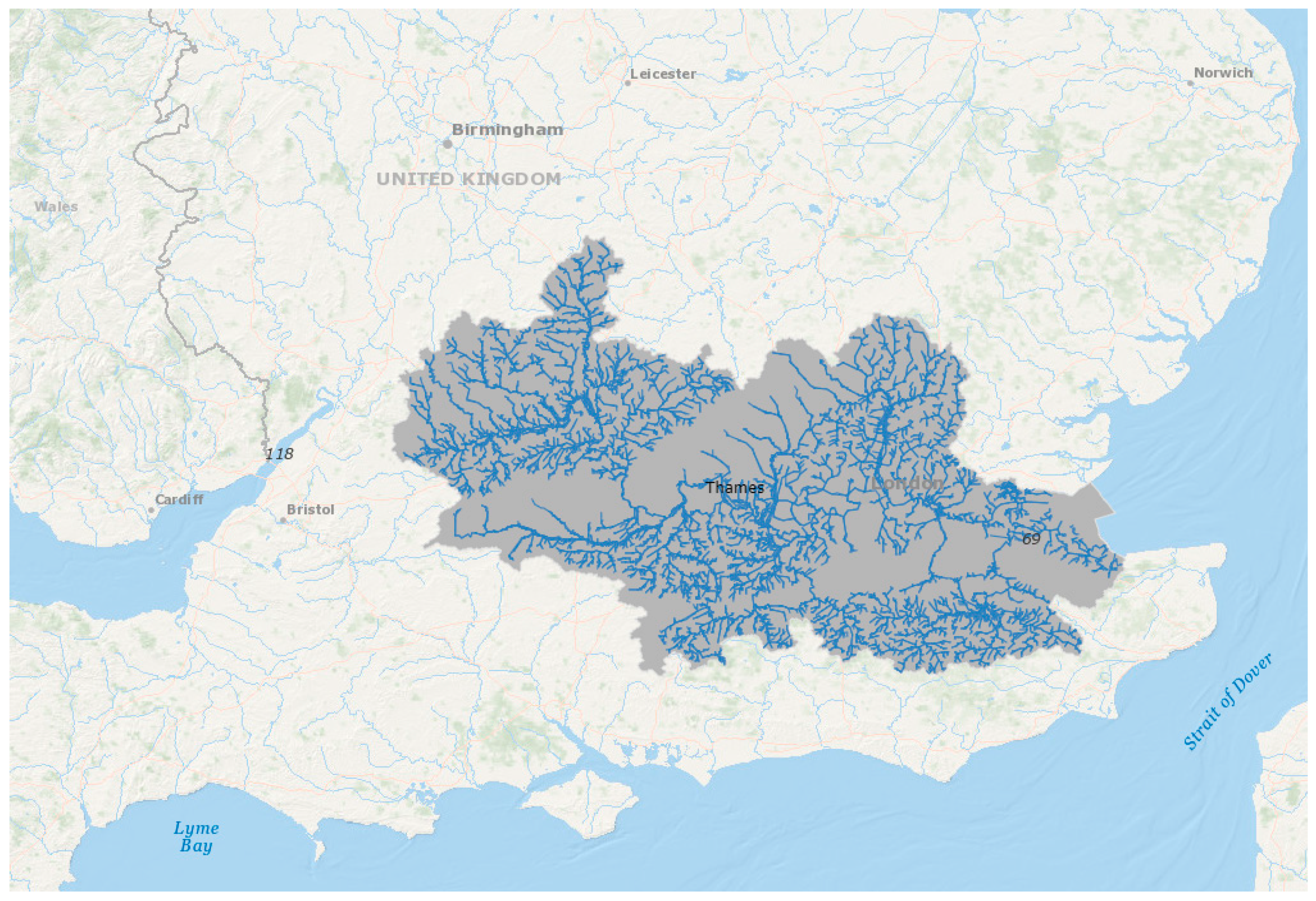
1. Introduction
2. The Urban River Syndrome—Ecosystems Previously Transformed
3. Ecosystem Adaptation and Non-Native Species in the Urban Thames
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Downs, P.W.; Piégay, H. Catchment-scale cumulative impact of human activities on river channels in the late Anthropocene: Implications, limitations, prospect. Geomorphology 2019, 338, 88–104. [Google Scholar] [CrossRef]
- Wood, L.B.; Ager, D.V. The Restoration of the Tidal Thames; Hilger: Bristol, UK, 1982. [Google Scholar]
- Hughes, A. The Riverine Ecosystem Synthesis: Towards Conceptual Cohesiveness in River Science; Academic Press: London, UK, 2012. [Google Scholar]
- Doretto, A.; Piano, E.; Larson, C.E. The river continuum concept: Lessons from the past and perspectives for the future. Can. J. Fish. Aquat. Sci. 2020, 77, 1856–1864. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Wu, J. Quantifying the speed, growth modes, and landscape pattern changes of urbanization: A hierarchical patch dynamics approach. Landsc. Ecol. 2013, 28, 1875–1888. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Res. Appl. 2006, 22, 123–147. [Google Scholar] [CrossRef]
- Liu, J.; Mooney, H.; Hull, V.; Davis, S.J.; Gaskell, J.; Hertel, T.; Lubchenco, J.; Seto, K.C.; Gleick, P.; Kremen, C.; et al. Systems integration for global sustainability. Science 2015, 347, 1258832. [Google Scholar] [CrossRef]
- Di Baldassarre, G.; Brandimarte, L.; Beven, K. The seventh facet of uncertainty: Wrong assumptions, unknowns and surprises in the dynamics of human–water systems. Hydrol. Sci. J. 2016, 61, 1748–1758. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Bates, N.R.; Johnson, R.J. Acceleration of ocean warming, salinification, deoxygenation and acidification in the surface subtropical North Atlantic Ocean. Commun. Earth Environ. 2020, 1, 1–12. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- Greater London Authority. Road Runoff Water Quality Study. 2019. Available online: https://www.london.gov.uk/sites/default/files/road_runoff_water_quality_study_exec_summary_dec_19.pdf (accessed on 11 June 2021).
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Forshay, K.J.; Furlong, E.T.; Groves, J.F.; Hladik, M.L.; et al. Urban Stormwater: An Overlooked Pathway of Extensive Mixed Contaminants to Surface and Groundwaters in the United States. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef]
- Kuhlemann, L.M.; Tetzlaff, D.; Soulsby, C. Urban water systems under climate stress: An isotopic perspective from Berlin, Germany. Hydrol. Process. 2020, 34, 3758–3776. [Google Scholar] [CrossRef]
- Spromberg, J.A.; Baldwin, D.H.; Damm, S.E.; Mcintyre, J.K.; Huff, M.; Sloan, C.A.; Anulacion, B.F.; Davis, J.W.; Scholz, N.L. EDITOR’S CHOICE: Coho salmon spawner mortality in western US urban watersheds: Bioinfiltration prevents lethal storm water impacts. J. Appl. Ecol. 2016, 53, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Martínez, V.M.; Pech, D.; Sures, B.; Purucker, S.T.; Poulin, R. Can parasites really reveal environmental impact? Trends Parasitol. 2010, 26, 44–51. [Google Scholar] [CrossRef]
- Boardman, J. Extreme rainfall and its impact on cultivated landscapes with particular reference to Britain. Earth Surf. Process. Landf. 2015, 40, 2121–2130. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Bruce Johnsen, D. Salmon, science, and reciprocity on the Northwest Coast. Ecol. Soc. 2009, 14, 43. [Google Scholar] [CrossRef]
- Rivera-Núñez, T.; Fargher, L. The concept of “palimpsest” in a reconceptualization of bidiversity conservation. Environ. Conserv. 2020, 48, 1–4. [Google Scholar] [CrossRef]
- Thompson, V.D.; Rick, T.; Garland, C.J.; Thomas, D.H.; Smith, K.Y.; Bergh, S.; Sanger, M.; Tucker, B.; Lulewicz, I.; Semon, A.M.; et al. Ecosystem stability and Native American oyster harvesting along the Atlantic coast of the United States. Sci. Adv. 2020, 6, eaba9652. [Google Scholar] [CrossRef]
- Lichatowich, J. Salmon, People, and Place: A Biologist’s Search for Salmon Recovery; Oregon State University: Corvallis, ON, USA, 2013; ISBN 9780870717253. [Google Scholar]
- Bennett, E.M.; Biggs, R.; Peterson, G.D.; Gordon, L.J. Patchwork Earth: Navigating pathways to just, thriving, and sustainable futures. One Earth 2021, 4, 172–176. [Google Scholar] [CrossRef]
- Stebbings, H.; Braganza, A. Exploring Continuous Organizational Transformation: Morphing through Network Interdependence. J. Chang. Manag. 2009, 9, 27–48. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The urban stream syndrome: Current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Booth, D.B.; Roy, A.H.; Smith, B.; Capps, K.A. Global perspectives on the urban stream syndrome. Freshw. Sci. 2016, 35, 412–420. [Google Scholar] [CrossRef]
- Emmerik, T.; Schwarz, A. Plastic debris in rivers. WIREs Water 2020, 7, e1398. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Toxicol. 2009, 28, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Richmond, E.K.; Rosi, E.J.; Walters, D.M.; Fick, J.; Hamilton, S.K.; Brodin, T.; Sundelin, A.; Grace, M.R. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat. Commun. 2018, 9, 4491. [Google Scholar] [CrossRef]
- Yadav, M.K.; Short, M.D.; Aryal, R.; Gerber, C.; van den Akker, B.; Saint, C.P. Occurrence of illicit drugs in water and wastewater and their removal during wastewater treatment. Water Res. 2017, 124, 713–727. [Google Scholar] [CrossRef] [PubMed]
- López-García, E.; Mastroianni, N.; Ponsà-Borau, N.; Barceló, D.; Postigo, C.; López de Alda, M. Drugs of abuse and their metabolites in river sediments: Analysis, occurrence in four Spanish river basins and environmental risk assessment. J. Hazard. Mater. 2021, 401, 123312. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, M.; Sepp, T.; Ujvari, B.; Ewald, P.W.; Thomas, F. Human activities might influence oncogenic processes in wild animal populations. Nat. Ecol. Evol. 2018, 2, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.; Lerebours, A.; Thomas, F.; Fort, J.; Kreitsberg, R.; Gentes, S.; Meitern, R.; Saks, L.; Ujvari, B.; Giraudeau, M.; et al. Linking pollution and cancer in aquatic environments: A review. Environ. Int. 2021, 149, 106391. [Google Scholar] [CrossRef] [PubMed]
- Thaler, E.A.; Larsen, I.J.; Yu, Q. The Extent of Soil Loss across the US Corn Belt. Proc. Natl. Acad. Sci. 2021, 118, e1922375118. [Google Scholar] [CrossRef] [PubMed]
- Comte, L.; Grenouillet, G. Do stream fish track climate change? Assessing distribution shifts in recent decades. Ecography 2013, 36, 1236–1246. [Google Scholar] [CrossRef]
- Jackson, M.C.; Grey, J. Accelerating rates of freshwater invasions in the catchment of the River Thames. Biol. Invasions 2013, 15, 945–951. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Hunter, P.D.; Lankester, T.; Hubbard, S.; Spyrakos, E.; Tyler, A.N.; Présing, M.; Horváth, H.; Lamb, A.; Balzter, H.; et al. Validation of Envisat MERIS algorithms for chlorophyll retrieval in a large, turbid and optically-complex shallow lake. Remote Sens. Environ. 2015, 157, 158–169. [Google Scholar] [CrossRef]
- Fei, S.; Morin, R.S.; Oswalt, C.M.; Liebhold, A.M. Biomass losses resulting from insect and disease invasions in US forests. Proc. Natl. Acad. Sci. USA 2019, 116, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.; Stentiford, G.D.; Wang, H.-C.C.; Koskella, B.; Tyler, C.R. The Pathobiome in Animal and Plant Diseases. Trends Ecol. Evol. 2019, 34, 996–1008. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Becnel, J.J.; Weiss, L.M.; Keeling, P.J.; Didier, E.S.; Williams, B.A.P.P.; Bjornson, S.; Kent, M.L.; Freeman, M.A.; Brown, M.J.F.F.; et al. Microsporidia—Emergent Pathogens in the Global Food Chain. Trends Parasitol. 2016, 32, 336–348. [Google Scholar] [CrossRef]
- Johnson, B.M.; Kemp, B.M.; Thorgaard, G.H. Increased mitochondrial DNA diversity in ancient Columbia River basin Chinook salmon Oncorhynchus tshawytscha. PLoS ONE 2018, 13, e0190059. [Google Scholar] [CrossRef]
- Sommerwerk, N.; Wolter, C.; Freyhof, J.; Tockner, K. Components and drivers of change in European freshwater fish faunas. J. Biogeogr. 2017, 44, 1781–1790. [Google Scholar] [CrossRef]
- Wang, W.; Xu, N.; Zhang, L.; Andersen, K.H.; Klaminder, J. Anthropogenic forcing of fish boldness and its impacts on ecosystem structure. Glob. Chang. Biol. 2021, 27, 1239–1249. [Google Scholar] [CrossRef]
- Osterback, A.M.K.; Frechette, D.M.; Shelton, A.O.; Hayes, S.A.; Bond, M.H.; Shaffer, S.A.; Moore, J.W. High predation on small populations: Avian predation on imperiled salmonids. Ecosphere 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Ahlstrom, C.A.; van Toor, M.L.; Woksepp, H.; Chandler, J.C.; Reed, J.A.; Reeves, A.B.; Waldenström, J.; Franklin, A.B.; Douglas, D.C.; Bonnedahl, J.; et al. Evidence for continental-scale dispersal of antimicrobial resistant bacteria by landfill-foraging gulls. Sci. Total Environ. 2021, 764, 144551. [Google Scholar] [CrossRef]
- Black, J.J.; Baumann, P.C. Carcinogens and cancers in freshwater fishes. Environ. Health Perspect. 1991, 90, 27–33. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Q.; Man, S.; Zeng, X.; Yu, Y.; Pang, Y.; Sheng, G.; Fu, J. Tissue concentrations, bioaccumulation, and biomagnification of synthetic musks in freshwater fish from Taihu Lake, China. Environ. Sci. Pollut. Res. 2013, 20, 311–322. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wong, M.H. Mercury accumulation in freshwater fish with emphasis on the dietary influence. Water Res. 2000, 34, 4234–4242. [Google Scholar] [CrossRef]
- Sham, R.C.; Tao, L.S.R.; Mak, Y.K.Y.; Yau, J.K.C.; Wai, T.C.; Ho, K.K.Y.; Zhou, G.-J.; Li, Y.; Wang, X.; Leung, K.M.Y. Occurrence and trophic magnification profile of triphenyltin compounds in marine mammals and their corresponding food webs. Environ. Int. 2020, 137, 105567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Q.; Du, X.; Yin, G.; Qiu, Y.; Ye, L.; Zhu, Z.; Zhao, J. Occurrence and trophic magnification of polybrominated diphenyl ethers (PBDEs) and their methoxylated derivatives in freshwater fish from Dianshan Lake, Shanghai, China. Environ. Pollut. 2016, 219, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Kliemann, B.C.K.; Delariva, R.L.; de Arruda Amorim, J.P.; da Silva Ribeiro, C.; da Silva, B.; Silveira, R.V.; Ramos, I.P. Dietary changes and histophysiological responses of a wild fish (Geophagus cf. proximus) under the influence of tilapia cage farm. Fish. Res. 2018, 204, 337–347. [Google Scholar] [CrossRef]
- Nardoto, G.B.; Murrieta, R.S.S.; Prates, L.E.G.; Adams, C.; Garavello, M.E.P.E.; Schor, T.; De Moraes, A.; Rinaldi, F.D.; Gragnani, J.G.; Moura, E.A.F.; et al. Frozen chicken for wild fish: Nutritional transition in the Brazilian Amazon region determined by carbon and nitrogen stable isotope ratios in fingernails. Am. J. Hum. Biol. 2011, 23, 642–650. [Google Scholar] [CrossRef]
- Popper, A.N.; Hawkins, A.D.; Thomsen, F. Taking the Animals’ Perspective Regarding Anthropogenic Underwater Sound. Trends Ecol. Evol. 2020, 35, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C. Ecological Light Pollution—Review. Ecol. Soc. Am. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Jackson, M.D.; Keyzers, R.A.; Linklater, W.L. Single compounds elicit complex behavioural responses in wild, free-ranging rats OPEN. Sci. Rep. 2018, 8, 12588. [Google Scholar] [CrossRef] [PubMed]
- McFrederick, Q.S.; Fuentes, J.D.; Roulston, T.; Kathilankal, J.C.; Lerdau, M. Effects of air pollution on biogenic volatiles and ecological interactions. Oecologia 2009, 160, 411–420. [Google Scholar] [CrossRef]
- Jacox, M.G.; Alexander, M.A.; Bograd, S.J.; Scott, J.D. Thermal displacement by marine heatwaves. Nature 2020, 584, 82–86. [Google Scholar] [CrossRef]
- Ellis, E.C. Ecology in an anthropogenic biosphere. Ecol. Monogr. 2015, 85, 287–331. [Google Scholar] [CrossRef]
- Selvam, B.P.; Natchimuthu, S.; Arunachalam, L.; Bastviken, D. Methane and carbon dioxide emissions from inland waters in India—Implications for large scale greenhouse gas balances. Glob. Chang. Biol. 2014, 20, 3397–3407. [Google Scholar] [CrossRef] [PubMed]
- Morritt, D.; Stefanoudis, P.V.; Pearce, D.; Crimmen, O.A.; Clark, P.F. Plastic in the Thames: A river runs through it. Mar. Pollut. Bull. 2014, 78, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Stippkugel, A.; Lenz, M.; Wirtz, K.; Engel, A. Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181203. [Google Scholar] [CrossRef]
- Payne, A.E.; Demory, M.-E.; Leung, L.R.; Ramos, A.M.; Shields, C.A.; Rutz, J.J.; Siler, N.; Villarini, G.; Hall, A.; Ralph, F.M. Responses and impacts of atmospheric rivers to climate change. Nat. Rev. Earth Environ. 2020, 1, 143–157. [Google Scholar] [CrossRef]
- Environment Agency The State of the Environment: Soil. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/805926/State_of_the_environment_soil_report.pdf (accessed on 28 March 2021).
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Terando, A.J.; et al. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming winter temperatures. Glob. Chang. Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef]
- Hawkins, A.; Popper, A. Assessing the impacts of underwater sounds on fishes and other forms of marine life. Acoust Today 2014, 10, 30–41. [Google Scholar]
- Wiernicki, C.J.; Liang, D.; Bailey, H.; Secor, D.H. The Effect of Swim Bladder Presence and Morphology on Sound Frequency Detection for Fishes. Rev. Fish. Sci. Aquac. 2020, 28, 459–477. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; den Boer-Visser, A. Cities Change the Songs of Birds. Curr. Biol. 2006, 16, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Kunc, H.P.; Schmidt, R. The effects of anthropogenic noise on animals: A meta-analysis. Biol. Lett. 2019, 15, 20190649. [Google Scholar] [CrossRef]
- Van der Sleen, P.; Lugo-Carvajal, A.; Zuanon, J.; Holmgren, M. Cats singing in the dark? Spawning aggregations of sound-producing fish in Amazonian floodplain forests. Environ. Biol. Fishes 2020, 103, 1265–1267. [Google Scholar] [CrossRef]
- Lee, J.J. The sound in the silence: Discovering a fish’s soundscape. Science 2012, 337, 1290–1291. [Google Scholar] [CrossRef]
- Roca, I.T.; Magnan, P.; Proulx, R. Use of acoustic refuges by freshwater fish: Theoretical framework and empirical data in a three-species trophic system. Freshw. Biol. 2018, 65, 45–54. [Google Scholar] [CrossRef]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef]
- Taylor, M.H. Lunar Synchronization of Fish Reproduction. Trans. Am. Fish. Soc. 2011, 113, 484–493. [Google Scholar] [CrossRef]
- Ekness, P.; Randhir, T.O. Effect of climate and land cover changes on watershed runoff: A multivariate assessment for storm water management. J. Geophys. Res. Biogeosciences 2015. [Google Scholar] [CrossRef]
- Ekins, P.; Global, C.; Outlook, E.; Gupta, J.; Global, C.; Outlook, E.; UN Environment; United Nations Environment Programme (UNEP). Global Environment Outlook—GEO-6: Healthy Planet, Healthy People; UNEP: Nairobi, Kenya, 2019. [Google Scholar]
- UNEP. Global Chemicals Outlook II: From Legacies to Innovative Solutions; UNEP: Nairobi, Kenya, 2019. [Google Scholar]
- Daughton, C.G. The Matthew Effect and widely prescribed pharmaceuticals lacking environmental monitoring: Case study of an exposure-assessment vulnerability. Sci. Total Environ. 2014, 466–467, 315–325. [Google Scholar] [CrossRef]
- Anna, S.; Sofia, B.; Christina, R.; Magnus, B. The dilemma in prioritizing chemicals for environmental analysis: Known versus unknown hazards. Environ. Sci. Process. Impacts 2016, 18, 1042–1049. [Google Scholar] [CrossRef]
- Scott, P.D.; Bartkow, M.; Blockwell, S.J.; Coleman, H.M.; Khan, S.J.; Lim, R.; McDonald, J.A.; Nice, H.; Nugegoda, D.; Pettigrove, V.; et al. An assessment of endocrine activity in Australian rivers using chemical and in vitro analyses. Environ. Sci. Pollut. Res. 2014, 21, 1295112967. [Google Scholar] [CrossRef] [PubMed]
- Pochodylo, A.L.; Helbling, D.E. Emerging investigators series: Prioritization of suspect hits in a sensitive suspect screening workflow for comprehensive micropollutant characterization in environmental samples. Environ. Sci. Water Res. Technol. 2016, 3, 54–65. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Penuelas, J. Changes in the environmental microbiome in the Anthropocene. Glob. Chang. Biol. 2020, 26, 3175–3177. [Google Scholar] [CrossRef]
- Garcia-Armisen, T.; Vercammen, K.; Passerat, J.; Triest, D.; Servais, P.; Cornelis, P. Antimicrobial resistance of heterotrophic bacteria in sewage-contaminated rivers. Water Res. 2011, 45, 788–796. [Google Scholar] [CrossRef]
- Hu, X.Y.; Logue, M.; Robinson, N. Antimicrobial resistance is a global problem—A UK perspective. Eur. J. Integr. Med. 2020, 36, 101136. [Google Scholar] [CrossRef]
- Vane, C.H.; Beriro, D.J.; Turner, G.H. Rise and fall of mercury (Hg) pollution in sediment cores of the Thames Estuary, London, UK. Earth Environ. Sci. Trans. R. Soc. Edinb. 2015, 105, 285–296. [Google Scholar] [CrossRef][Green Version]
- Johnstone, K.M.; Rainbow, P.S.; Clark, P.F.; Smith, B.D.; Morritt, D. Trace metal bioavailabilities in the Thames estuary: Continuing decline in the 21st century. J. Mar. Biol. Assoc. UK 2016, 96, 205–216. [Google Scholar] [CrossRef]
- Botelho, M.T.; Fuller, N.; Vannuci-Silva, M.; Yang, G.; Richardson, K.; Ford, A.T. Unusual male size vs sperm count relationships in a coastal marine amphipod indicate reproductive impairment by unknown toxicants. Aquat. Toxicol. 2021, 233, 105793. [Google Scholar] [CrossRef]
- Nevitt, G.A.; Bonadonna, F. Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biol. Lett. 2005, 1, 303–305. [Google Scholar] [CrossRef]
- Stacey, N. Hormones, pheromones and reproductive behavior. Fish Physiol. Biochem. 2003, 28, 229–235. [Google Scholar] [CrossRef]
- McIntyre, J.K.; Baldwin, D.H.; Beauchamp, D.A.; Scholz, N.L. Low-level copper exposures increase visibility and vulnerability of juvenile coho salmon to cutthroat trout predators. Ecol. Appl. 2012, 22, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Polverino, G.; Martin, J.M.; Bertram, M.G.; Soman, V.R.; Tan, H.; Brand, J.A.; Mason, R.T.; Wong, B.B.M. Psychoactive pollution suppresses individual differences in fish behaviour. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202294. [Google Scholar] [CrossRef] [PubMed]
- Kurelec, B. The Genotoxic Disease Syndrome. Mar. Environ. Res. 1993, 35, 341–348. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Q.; Chen, L.; Yang, S. Features of an intersex Chinese mitten crab, (Eriocheir Japonica Sinensis)(Decapoda, Brachyura). Crustaceana 2005, 78, 371–377. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Stephens, A.E.A.A.; Jepson, P.D.; Jobling, S.; Johnson, A.C.; Matthiessen, P.; Sumpter, J.P.; Tyler, C.R.; McLean, A.R. A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182416. [Google Scholar] [CrossRef]
- Tyler, C.R.; Jobling, S. Roach, sex, and gender-bending chemicals: The feminization of wild fish in English rivers. Bioscience 2008, 58, 1051–1059. [Google Scholar] [CrossRef]
- Jobling, S.; Coey, S.; Whitmore, J.G.; Kime, D.E.; Van Look, K.J.W.; McAllister, B.G.; Beresford, N.; Henshaw, A.C.; Brighty, G.; Tyler, C.R.; et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol. Reprod. 2002, 67, 515–524. [Google Scholar] [CrossRef]
- Jaeger, K.L.; Olden, J.D.; Pelland, N.A. Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc. Natl. Acad. Sci. USA 2014, 111, 13894–13899. [Google Scholar] [CrossRef]
- Nogales, B.; Lanfranconi, M.P.; Piña-Villalonga, J.M.; Bosch, R. Anthropogenic perturbations in marine microbial communities. FEMS Microbiol. Rev. 2010, 35, 275–298. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Yu, X.; Chen, G.; Yu, Z. Patterns in the composition of microbial communities from a subtropical river: Effects of environmental, spatial and temporal factors. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Olden, J.D.; Comte, L.; Giam, X. The homogocene: A research prospectus for the study of biotic homogenisation. NeoBiota 2018, 36, 23–36. [Google Scholar] [CrossRef]
- Measham, N. Riverfly Census 2015. Salmon Trout Conserv. UK 2015. Available online: https://www.salmon-trout.org/wp-content/uploads/2017/08/2015-Report_Riverfly-Census.pdf (accessed on 28 March 2021).
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Bierkens, M.F.P.; Huijbregts, M.A.J.; Schipper, A.M. Threats of global warming to the world’s freshwater fishes. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.; Correa, C.; Marzluff, J.M.; Hendry, A.P.; Palkovacs, E.P.; Gotanda, K.M.; Hunt, V.M.; Apgar, T.M.; Zhou, Y. Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl. Acad. Sci. USA 2017, 114, 8951–8956. [Google Scholar] [CrossRef]
- Sih, A.; Ferrari, M.C.O.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef]
- Casado, J.; Brigden, K.; Santillo, D.; Johnston, P. Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high resolution mass spectrometry. Sci. Total Environ. 2019, 670, 1204–1225. [Google Scholar] [CrossRef] [PubMed]
- Hansen Jesse, L.C.; Obrycki, J.J. Field deposition of Bt transgenic corn pollen: Lethal effects on the monarch butterfly. Oecologia 2000, 125, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Jeger, M.; Bragard, C.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Jaques Miret, J.A.; MacLeod, A.; Navajas Navarro, M.; Niere, B.; Parnell, S.; et al. Risk assessment and reduction options for Ceratocystis platani in the EU. EFSA J. 2016, 14, e04640. [Google Scholar] [CrossRef]
- Soulioti, N.; Tsopelas, P.; Woodward, S. Platypus cylindrus, a vector of Ceratocystis platani in Platanus orientalis stands in Greece. For. Pathol. 2015, 45, 367–372. [Google Scholar] [CrossRef]
- Griffiths, A.M.; Ellis, J.S.; Clifton-Dey, D.; Machado-Schiaffino, G.; Bright, D.; Garcia-Vazquez, E.; Stevens, J.R. Restoration versus recolonisation: The origin of Atlantic salmon (Salmo salar L.) currently in the River Thames. Biol. Conserv. 2011, 144, 2733–2738. [Google Scholar] [CrossRef]
- Des Roches, S.; Pendleton, L.H.; Shapiro, B.; Palkovacs, E.P. Conserving intraspecific variation for nature’s contributions to people. Nat. Ecol. Evol. 2021, 5, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.S.; Brown, J.A.; Lockwood, J.L. Reciprocal human-natural system feedback loops within the invasion process. NeoBiota 2020, 508, 489–508. [Google Scholar] [CrossRef]
- Martin, S.; Thouzeau, G.; Richard, M.; Chauvaud, L.; Jean, F.; Clavier, J. Benthic community respiration in areas impacted by the invasive mollusk Crepidula fornicata. Mar. Ecol. Prog. Ser. 2007, 347, 51–60. [Google Scholar] [CrossRef]
- Bohn, K.; Richardson, C.; Jenkins, S. The invasive gastropod Crepidula fornicata: Reproduction and recruitment in the intertidal at its northernmost range in Wales, UK, and implications for its secondary spread. Mar. Biol. 2012, 159, 2091–2103. [Google Scholar] [CrossRef]
- Kostecki, C.; Rochette, S.; Girardin, R.; Blanchard, M.; Desroy, N.; Le Pape, O. Reduction of flatfish habitat as a consequence of the proliferation of an invasive mollusc. Estuar. Coast. Shelf Sci. 2011, 92, 154–160. [Google Scholar] [CrossRef]
- Bouasria, M.; Khadraoui, F.; Benzaama, M.H.; Touati, K.; Chateigner, D.; Gascoin, S.; Pralong, V.; Orberger, B.; Babouri, L.; El Mendili, Y. Partial substitution of cement by the association of Ferronickel slags and Crepidula fornicata shells. J. Build. Eng. 2021, 33, 101587. [Google Scholar] [CrossRef]
- Cugier, P.; Struski, C.; Blanchard, M.; Mazurié, J.; Pouvreau, S.; Olivier, F.; Trigui, J.R.; Thiébaut, E. Assessing the role of benthic filter feeders on phytoplankton production in a shellfish farming site: Mont Saint Michel Bay, France. J. Mar. Syst. 2010, 82, 21–34. [Google Scholar] [CrossRef]
- Thieltges, D.W. Benefit from an invader: American slipper limpet Crepidula fornicatareduces star fish predation on basibiont European mussels. Hydrobiologia 2005, 541, 241–244. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Spencer, B.E.; Pascoe, P.L. The American Oyster Drill, Urosalpinx Cinerea (Gastropoda): Evidence Of Decline in an Imposex-Affected Population (R. Blackwater, Essex). J. Mar. Biol. Assoc. UK 1991, 71, 827–838. [Google Scholar] [CrossRef]
- Sousa, A.C.A.; Pastorinho, M.R.; Takahashi, S.; Tanabe, S. History on organotin compounds, from snails to humans. Environ. Chem. Lett. 2014, 12, 117–137. [Google Scholar] [CrossRef]

| Ecological Theories | Year Introduced | Recently Reviewed |
|---|---|---|
| The River Continuum Concept (RCC) | 1980 | [4] |
| Hierarchical Patch Dynamics (HPD) | 1940s | [5] |
| Functional Process Zones (FPZ) | 1980 | [6] |
| Aquatic & Riparian Biota | Recent Reports | |
|---|---|---|
| Species range shifts | New | [36] |
| Accelerating non-native species establishment | New | [37] |
| Riparian vegetation degradation and die-back, tree disease, invasive plants, clearance by humans | New | [38,39,40] |
| Increased prevalence of pathogens, including viruses, bacteria, and parasites | New | [41] |
| Reductions in biodiversity because of fish stocking and farming | New | [42,43] |
| Behavioural changes due to domesticated transplants and xenobiotics | New | [44] |
| Ecosystem processes | ||
| Migration between landfills and waste sites by gulls with increasing predation in rivers and estuaries | New | [45,46] |
| Cancers in aquatic animals: birds, fish, bivalves and mammals | New | [47] |
| Toxin bioaccumulation in prey species with trophic amplification (e.g., per- and polyfluoroalkyl substances, PFASs) | New | [48,49,50,51] |
| Changes in diet | New | [52,53] |
| Interference with sound, light and chemical signalling regimes | New | [54,55,56,57] |
| Atmosphere, soils, water, and sediment chemistry | ||
| Global warming (e.g., warmer water) with marine heat waves in the estuary and ocean | Increasing | [58] |
| Anthroposphere | New | [59] |
| NO2, CH4 and CO2 emissions from water bodies, landfills and sewage treatment plants (STPs) | New | [60] |
| Rubbish, including micro- and nanoplastics. Accumulation in animals, soils and the substrate with transport to the ocean | New | [61,62] |
| Hydrology | ||
| Increasing extreme flooding and drought events | Increasing | [63] |
| Channel morphology | ||
| Soil erosion with silt banks forming in the lower reaches | Increasing | [64] |
| Condition | Causative Agent |
|---|---|
| Root and collar rot in alder | Phytophthora alni (Phytopthora) |
| Canker stain of plane trees | Ceratocystis platani (fungus) |
| Acute oak decline | Multiple environmental causes (e.g., pollution and climate change) |
| Horse chestnut leaf miner | Cameraria ohridella (moth) |
| Chalara die-back of Ash | Hymenoscyphus fraxineus (fungus) |
| Massaria disease | Splanchnonema platani (fungus) |
| Oak processionary moth | Thaumetopoea processionea (moth) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, M.; Soloviev, M. The Urban River Syndrome: Achieving Sustainability Against a Backdrop of Accelerating Change. Int. J. Environ. Res. Public Health 2021, 18, 6406. https://doi.org/10.3390/ijerph18126406
Richardson M, Soloviev M. The Urban River Syndrome: Achieving Sustainability Against a Backdrop of Accelerating Change. International Journal of Environmental Research and Public Health. 2021; 18(12):6406. https://doi.org/10.3390/ijerph18126406
Chicago/Turabian StyleRichardson, Martin, and Mikhail Soloviev. 2021. "The Urban River Syndrome: Achieving Sustainability Against a Backdrop of Accelerating Change" International Journal of Environmental Research and Public Health 18, no. 12: 6406. https://doi.org/10.3390/ijerph18126406
APA StyleRichardson, M., & Soloviev, M. (2021). The Urban River Syndrome: Achieving Sustainability Against a Backdrop of Accelerating Change. International Journal of Environmental Research and Public Health, 18(12), 6406. https://doi.org/10.3390/ijerph18126406







