Short-Term Exposure to Fine Particulate Matter and Hospitalizations for Acute Lower Respiratory Infection in Korean Children: A Time-Series Study in Seven Metropolitan Cities
Abstract
1. Introduction
2. Methods
2.1. Number of Daily Children’s ALRI Hospitalization
2.2. PM2.5 Modeling Data
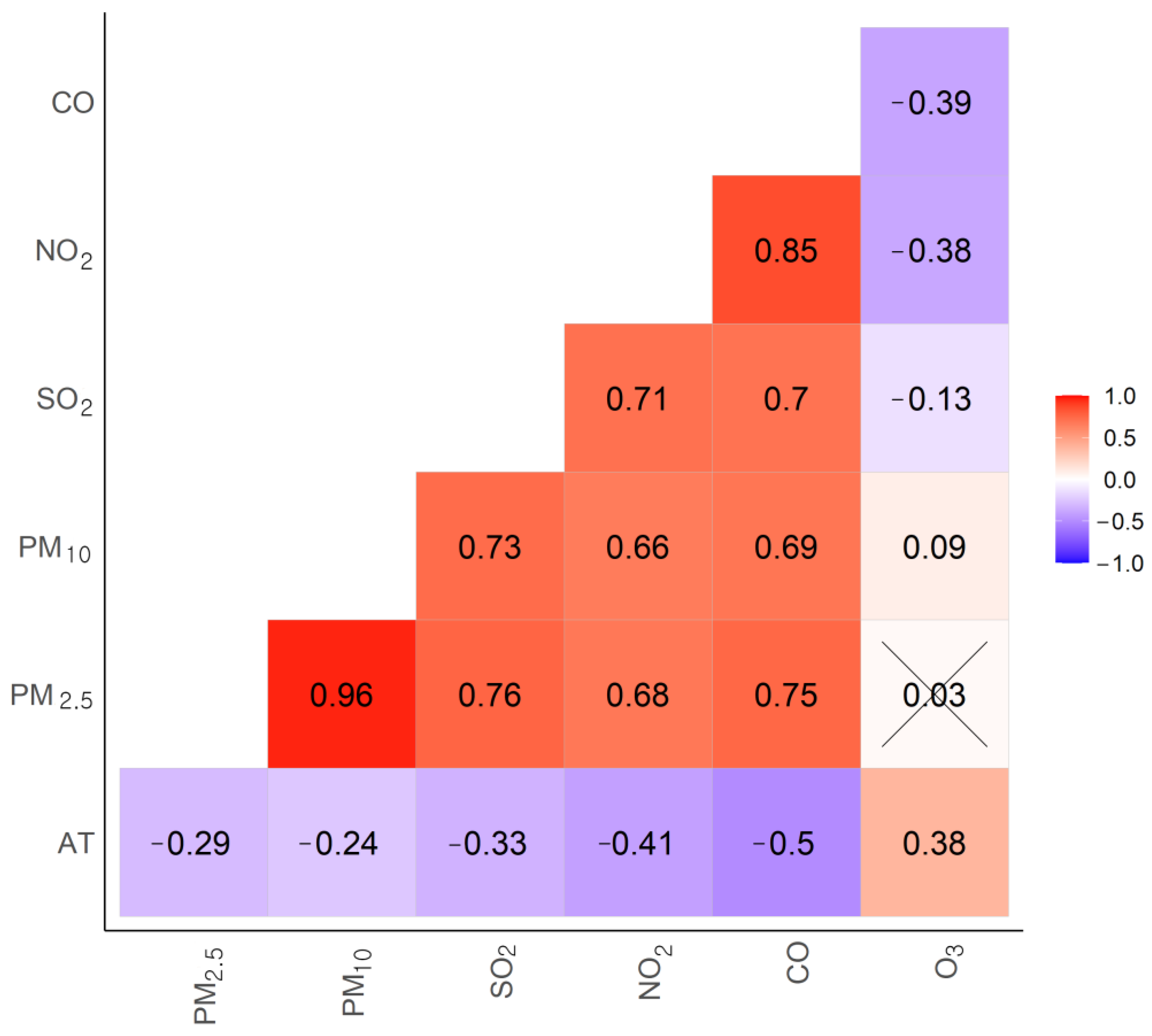
2.3. Air Pollution Monitoring Data and Environmental Variables
2.4. Statistical Analysis
2.5. Two-Pollutant Model
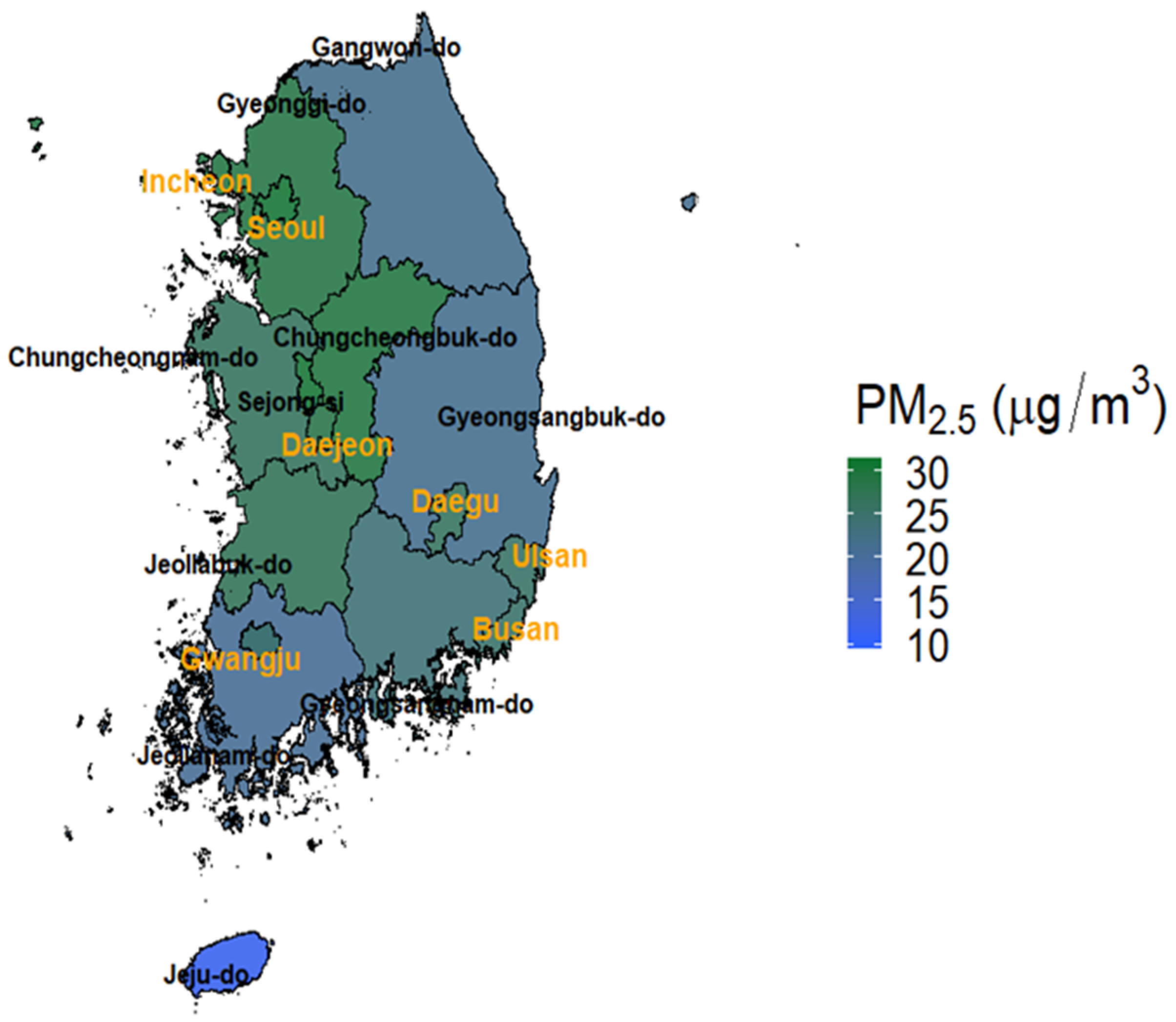
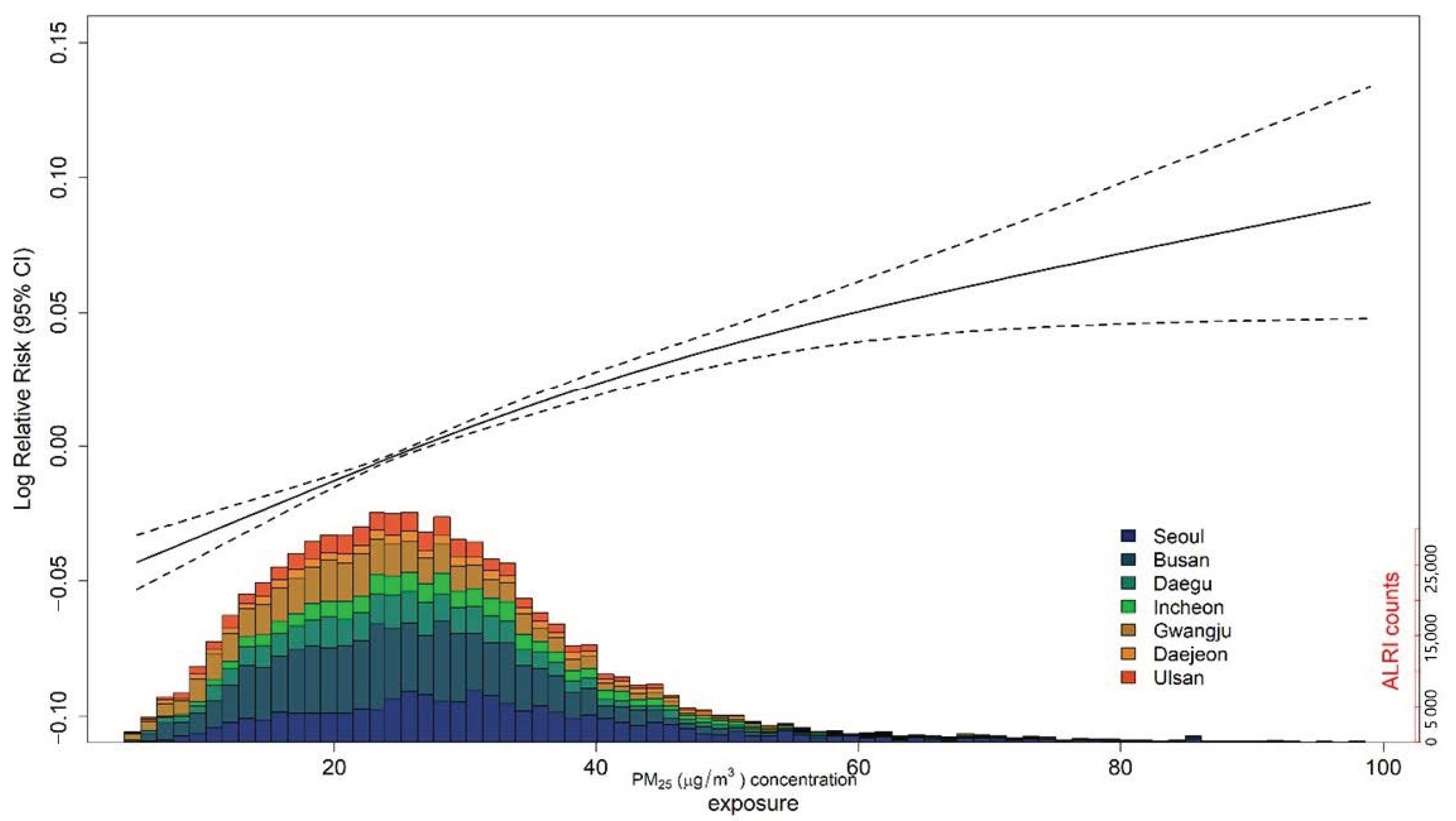
3. Results
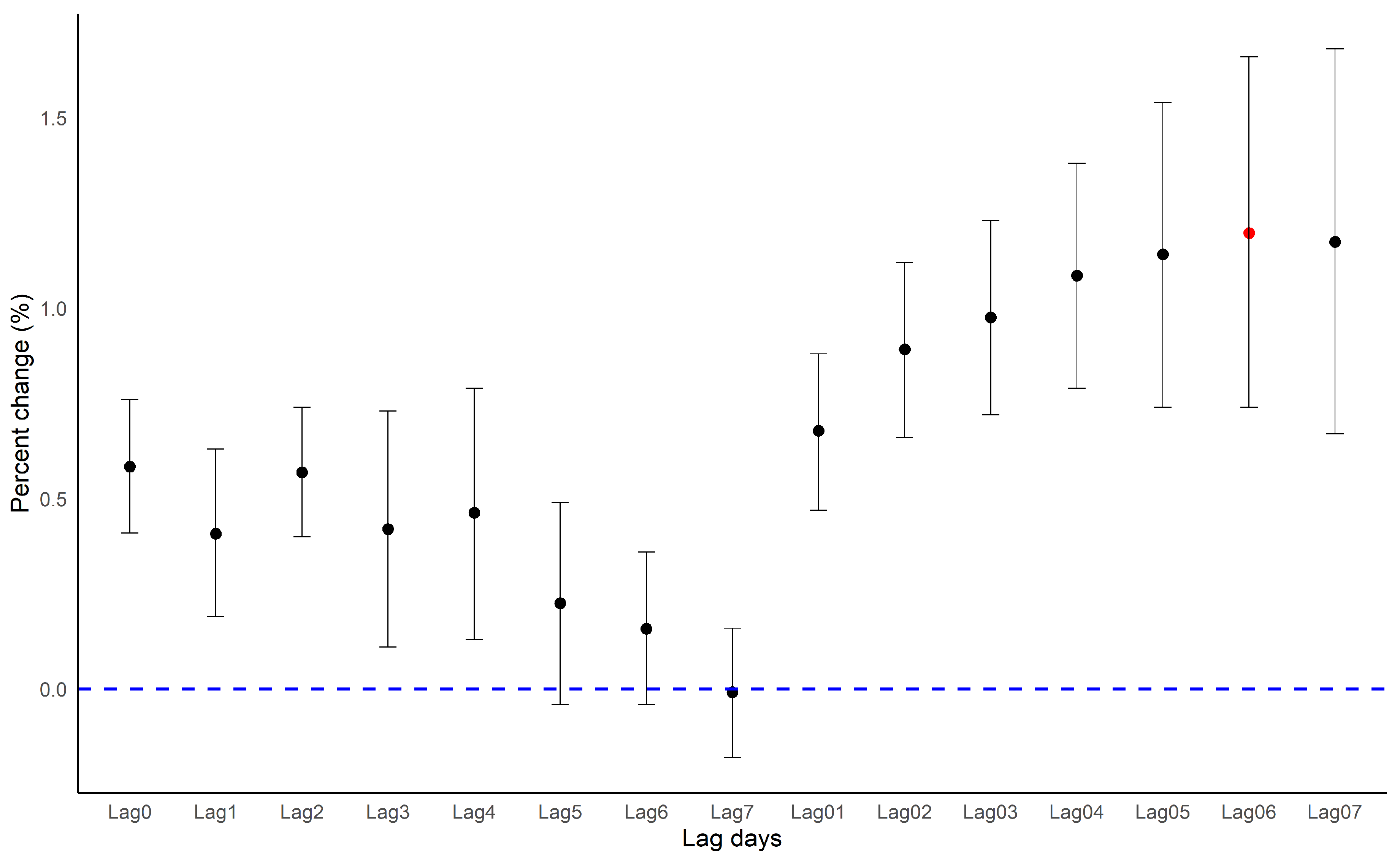
3.1. Main Results
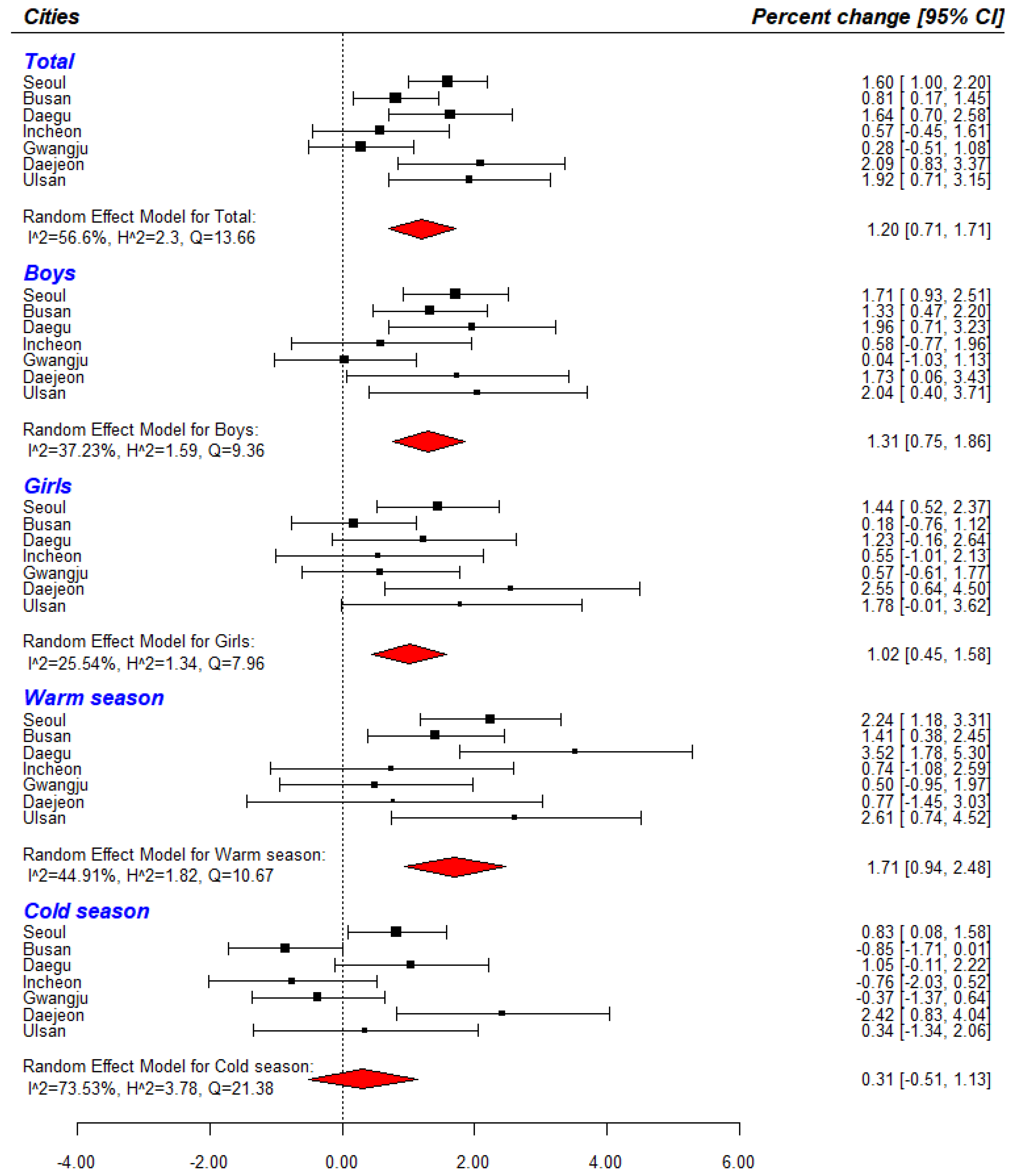
3.2. Subgroup Analysis
3.3. Two-Pollutants Model
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM2.5 | particulate matter with a diameter equal to or less than 2.5 μm |
| ALRI | acute lower respiratory infection |
| GAM | generalized additive model |
| RR | relative risk |
| CI | confidence interval |
References
- Apte, J.S.; Marshall, J.D.; Cohen, A.J.; Brauer, M. Addressing Global Mortality from Ambient PM2.5. Environ. Sci. Technol. 2015, 49, 8057–8066. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Roth, L.; Malig, B.; Marty, M. The effects of fine particle components on respiratory hospital admissions in children. Environ. Health Perspect. 2009, 117, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.Q.; Larson, T.V.; Hanley, Q.S.; Rebolledo, V.; Dumler, K.; Checkoway, H.; Wang, S.Z.; Lin, D.; Pierson, W.E. Pulmonary function changes in children associated with fine particulate matter. Environ. Res. 1993, 63, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J. Particulate matter exposure in children: Relevance to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Tecer, L.H.; Alagha, O.; Karaca, F.; Tuncel, G.; Eldes, N. Particulate matter (PM(2.5), PM(10-2.5), and PM(10)) and children’s hospital admissions for asthma and respiratory diseases: A bidirectional case-crossover study. J. Toxicol. Environ. Health A 2008, 71, 512–520. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Mehta, S.; Shin, H.; Burnett, R.; North, T.; Cohen, A.J. Ambient particulate air pollution and acute lower respiratory infections: A systematic review and implications for estimating the global burden of disease. Air Qual. Atmos. Health 2013, 6, 69–83. [Google Scholar] [CrossRef]
- Colley, J.R.; Douglas, J.W.; Reid, D.D. Respiratory disease in young adults: Influence of early childhood lower respiratory tract illness, social class, air pollution, and smoking. Br. Med. J. 1973, 3, 195–198. [Google Scholar] [CrossRef]
- Spears, D.; Dey, S.; Chowdhury, S.; Scovronick, N.; Vyas, S.; Apte, J. The association of early-life exposure to ambient PM2.5 and later-childhood height-for-age in India: An observational study. Environ. Health 2019, 18, 62. [Google Scholar] [CrossRef]
- Horne, B.D.; Joy, E.A.; Hofmann, M.G.; Gesteland, P.H.; Cannon, J.B.; Lefler, J.S.; Blagev, D.P.; Korgenski, E.K.; Torosyan, N.; Hansen, G.I.; et al. Short-Term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. Am. J. Respir. Crit. Care Med. 2018, 198, 759–766. [Google Scholar] [CrossRef]
- GBD. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2015, 17, 1133–1161. [Google Scholar] [CrossRef]
- Bruce, N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: A systematic review and meta-analysis. Bull. World Health Organ. 2008, 86, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic. Biol. Med. 1996, 21, 669–681. [Google Scholar] [CrossRef]
- Greenwell, L.L.; Moreno, T.; Jones, T.P.; Richards, R.J. Particle-induced oxidative damage is ameliorated by pulmonary antioxidants. Free Radic. Biol. Med. 2002, 32, 898–905. [Google Scholar] [CrossRef]
- Donaldson, K.; Beswick, P.H.; Gilmour, P.S. Free radical activity associated with the surface of particles: A unifying factor in determining biological activity? Toxicol. Lett. 1996, 88, 293–298. [Google Scholar] [CrossRef]
- Zheng, P.W.; Wang, J.B.; Zhang, Z.Y.; Shen, P.; Chai, P.F.; Li, D.; Jin, M.J.; Tang, M.L.; Lu, H.C.; Lin, H.B.; et al. Air pollution and hospital visits for acute upper and lower respiratory infections among children in Ningbo, China: A time-series analysis. Environ. Sci. Pollut. Res. Int. 2017, 24, 18860–18869. [Google Scholar] [CrossRef]
- Darrow, L.A.; Klein, M.; Flanders, W.D.; Mulholland, J.A.; Tolbert, P.E.; Strickland, M.J. Air pollution and acute respiratory infections among children 0-4 years of age: An 18-year time-series study. Am. J. Epidemiol. 2014, 180, 968–977. [Google Scholar] [CrossRef]
- Karr, C.; Lumley, T.; Shepherd, K.; Davis, R.; Larson, T.; Ritz, B.; Kaufman, J. A case-crossover study of wintertime ambient air pollution and infant bronchiolitis. Environ. Health Perspect. 2006, 114, 277–281. [Google Scholar] [CrossRef]
- Health Effects Institute. State of Global Air 2017: A Special Report on Global Exposure to Air Pollution and Its Disease Burden; Health Effect Institute: Boston, MA, USA, 2017. [Google Scholar]
- Park, J.-E.; Lee, S.-Y.; Kang, J.H. Common Disease Codes in Pediatric Inpatients (1997–2008). Korean J. Pediatric Infect. Dis. 2011, 18. [Google Scholar] [CrossRef]
- Kim, K.N.; Kim, S.; Lim, Y.H.; Song, I.G.; Hong, Y.C. Effects of short-term fine particulate matter exposure on acute respiratory infection in children. Int. J. Hyg. Environ. Health 2020, 229, 113571. [Google Scholar] [CrossRef]
- Luong, L.T.M.; Dang, T.N.; Thanh Huong, N.T.; Phung, D.; Tran, L.K.; Van Dung, D.; Thai, P.K. Particulate air pollution in Ho Chi Minh city and risk of hospital admission for acute lower respiratory infection (ALRI) among young children. Environ. Pollut. 2020, 257. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.C.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef]
- Oh, J.; Lee, S.; Kim, M.H.; Kwag, Y.; Kim, H.S.; Kim, S.; Ye, S.; Ha, E. The impact of PM2.5 on acute otitis media in children (aged 0–3): A time series study. Environ. Int. 2020, 145, 106133. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Kim, S.; Lim, Y.H.; Bae, H.J.; Hong, Y.C. Spatial and Temporal Trends of Number of Deaths Attributable to Ambient PM2.5 in the Korea. J. Korean Med Sci. 2018, 33, e193. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Klein, M.; Peel, J.L.; Sarnat, S.E.; Sarnat, J.A. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J. Expo. Sci. Environ. Epidemiol. 2007, 17, S29–S35. [Google Scholar] [CrossRef]
- Seo, J.; Kim, J.Y.; Youn, D.; Lee, J.Y.; Kim, H.; Lim, Y.B.; Kim, Y.; Jin, H.C. On the multiday haze in the Asian continental outflow: The important role of synoptic conditions combined with regional and local sources. Atmos. Chem. Phys. 2017, 17, 9311–9332. [Google Scholar] [CrossRef]
- Zanobetti, A.; Schwartz, J. Temperature and Mortality in Nine US Cities. Epidemiology 2008, 19, 563–570. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Schwartz, J.; Dockery, D.W. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air 2014, 24, 103–112. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef]
- Alessandrini, E.; Sajani, S.Z.; Scotto, F.; Miglio, R.; Marchesi, S.; Lauriola, P. Emergency ambulance dispatches and apparent temperature: A time series analysis in Emilia-Romagna, Italy. Environ. Res. 2011, 111, 1192–1200. [Google Scholar] [CrossRef]
- Rucker, G.; Schwarzer, G.; Carpenter, J.R.; Schumacher, M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Bootsma, M.C.; Rovers, M.M.; Bonten, M.J. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.N. Which is the Preferred Measure of Heterogeneity in Meta-Analysis and Why? A Revisit. Biostat. Biom. Open Access J. 2017, 1. [Google Scholar] [CrossRef]
- Kulinskaya, E.; Dollinger, M.B. An accurate test for homogeneity of odds ratios based on Cochran’s Q-statistic. BMC Med. Res. Methodol. 2015, 15, 49. [Google Scholar] [CrossRef]
- Ito, K.; Thurston, G.D.; Silverman, R.A. Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J. Expo. Sci. Environ. Epidemiol. 2007, 17, S45–S60. [Google Scholar] [CrossRef]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology (Sunnyvale) 2016, 6. [Google Scholar] [CrossRef]
- Braga, A.L.; Saldiva, P.H.; Pereira, L.A.; Menezes, J.J.; Conceicao, G.M.; Lin, C.A.; Zanobetti, A.; Schwartz, J.; Dockery, D.W. Health effects of air pollution exposure on children and adolescents in Sao Paulo, Brazil. Pediatr. Pulmonol. 2001, 31, 106–113. [Google Scholar] [CrossRef]
- Segala, C.; Poizeau, D.; Mesbah, M.; Willems, S.; Maidenberg, M. Winter air pollution and infant bronchiolitis in Paris. Environ. Res. 2008, 106, 96–100. [Google Scholar] [CrossRef]
- Barnett, A.G.; Williams, G.M.; Schwartz, J.; Neller, A.H.; Best, T.L.; Petroeschevsky, A.L.; Simpson, R.W. Air pollution and child respiratory health: A case-crossover study in Australia and New Zealand. Am. J. Respir. Crit. Care Med. 2005, 171, 1272–1278. [Google Scholar] [CrossRef]
- Ismail, S.N.S.; Abdul Rahman, S.R.; Sahani, M.; Firuz, R.; Latif, M.T. A case crossover analysis of primary air pollutants association on acute respiratory infection (ARI) among children in urban region of Klang valley, Malaysia. Ann. Trop. Med. Public Health 2017, 10. [Google Scholar] [CrossRef]
- Ha, E.H.; Lee, J.T.; Kim, H.; Hong, Y.C.; Lee, B.E.; Park, H.S.; Christiani, D.C. Infant Susceptibility of Mortality to Air Pollution in Seoul, South Korea. Pediatrics 2003, 111, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.T.; Schindler, C.; Dien, T.M.; Probst-Hensch, N.; Perez, L.; Kunzli, N. Acute effects of ambient air pollution on lower respiratory infections in Hanoi children: An eight-year time series study. Environ. Int. 2018, 110, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. Role of environmental stress in the physiological response to chemical toxicants. Environ. Res. 2003, 92, 1–7. [Google Scholar] [CrossRef]
- Kalkstein, L.S.; Greene, J.S. An evaluation of climate/mortality relationships in large U.S. cities and the possible impacts of a climate change. Environ. Health Perspect. 1997, 105, 84–93. [Google Scholar] [CrossRef]
- Gold, D.R.; Litonjua, A.; Schwartz, J.; Lovett, E.; Larson, A.; Nearing, B.; Allen, G.; Verrier, M.; Cherry, R.; Verrier, R. Ambient Pollution and Heart Rate Variability. Circulation 2000, 101, 1267–1273. [Google Scholar] [CrossRef]
- Creason, J.; Neas, L.; Walsh, D.; Williams, R.O.N.; Sheldon, L.; Liao, D.; Shy, C. Particulate matter and heart rate variability among elderly retirees: The Baltimore 1998 PM study. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 116–122. [Google Scholar] [CrossRef]
- Ren, C.; O’Neill, M.S.; Park, S.K.; Sparrow, D.; Vokonas, P.; Schwartz, J. Ambient Temperature, Air Pollution, and Heart Rate Variability in an Aging Population. Am. J. Epidemiol. 2011, 173, 1013–1021. [Google Scholar] [CrossRef]
- Ren, C.; Tong, S. Temperature modifies the health effects of particulate matter in Brisbane, Australia. Int. J. Biometeorol. 2006, 51, 87–96. [Google Scholar] [CrossRef]
- Kenney, M.J.; Ganta, C.K. Autonomic Nervous System and Immune System Interactions. Compr Physiol 2014, 4, 1177–1200. [Google Scholar]
- Gouveia, N.; Fletcher, T. Time series analysis of air pollution and mortality: Effects by cause, age and socioeconomic status. J. Epidemiol. Community Health 2000, 54, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.A.T.; Liu, X.; Shi, L.; Zu, K.; Goodman, J. Applying Nonparametric Methods to Analyses of Short-Term Fine Particulate Matter Exposure and Hospital Admissions for Cardiovascular Diseases among Older Adults. Int. J. Environ. Res. Public Health 2017, 14, 1051. [Google Scholar] [CrossRef] [PubMed]
- Cesari, D.; De Benedetto, G.E.; Bonasoni, P.; Busetto, M.; Dinoi, A.; Merico, E.; Chirizzi, D.; Cristofanelli, P.; Donateo, A.; Grasso, F.M.; et al. Seasonal variability of PM2.5 and PM10 composition and sources in an urban background site in Southern Italy. Sci. Total Environ. 2018, 612, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cheng, J.; Lv, J.; Wu, L.; Wu, J. Comparison of chemical compositions in air particulate matter during summer and winter in Beijing, China. Environ. Geochem. Health 2016, 39, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, E.H.; Schauer, J.J.; Yi, S.-M.; Heo, J. Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ. Int. 2018, 117, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Woodward, A.; Hou, X.Y.; Zhu, T.; Zhang, J.L.; Brown, H.; Yang, J.; Qin, R.N.; Gao, J.H.; Gu, S.H.; et al. Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci. Total Environ. 2017, 575, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Lepeule, J.; Litonjua, A.A.; Gasparrinie, A.; Koutrakis, P.; Sparrows, D.; Vokonas, P.S.; Schwartz, J. Lung function association with outdoor temperature and relative humidity and its interaction with air pollution in the elderly. Environ. Res. 2018, 165, 110–117. [Google Scholar] [CrossRef]
- Zhu, L.; Ge, X.; Chen, Y.; Zeng, X.; Pan, W.; Zhang, X.; Ben, S.; Yuan, Q.; Xin, J.; Shao, W.; et al. Short-term effects of ambient air pollution and childhood lower respiratory diseases. Sci. Rep. 2017, 7, 4414. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Sigaud, S.; Goldsmith, C.A.; Zhou, H.; Yang, Z.; Fedulov, A.; Imrich, A.; Kobzik, L. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol. Appl. Pharmacol. 2007, 223, 1–9. [Google Scholar] [CrossRef]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Salonen, R.O.; Pennanen, A.S.; Sillanpaa, M.; Halinen, A.I.; Happo, M.S.; Hillamo, R.; Brunekreef, B.; Katsouyanni, K.; Sunyer, J.; et al. Heterogeneities in inflammatory and cytotoxic responses of RAW 264.7 macrophage cell line to urban air coarse, fine, and ultrafine particles from six European sampling campaigns. Inhal. Toxicol. 2007, 19, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, B.-U.; Kim, H.C.; Kim, S. A Multiscale Tiered Approach to Quantify Contributions: A Case Study of PM2.5 in South Korea during 2010–2017. Atmosphere 2020, 11, 141. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, D.-R.; Koo, Y.-S.; Lee, J.-B.; Park, H.-J. Analysis of Domestic and Foreign Contributions using DDM in CMAQ during Particulate Matter Episode Period of February 2014 in Seoul. J. Korean Soc. Atmos. Environ. 2016, 32, 82–99. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Arah, O.A. Bias Formulas for Sensitivity Analysis of Unmeasured Confounding for General Outcomes, Treatments, and Confounders. Epidemiology 2011, 22, 42–52. [Google Scholar] [CrossRef]
- Peng, R.D.; Dominici, F. Statistical Methods for Environmental Epidemiology with R: A Case Study in Air Pollution and Health; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
| Category | Total | Seoul | Busan | Daegu | Incheon | Gwangju | Daejeon | Ulsan |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Boys | 397,333 | 19,855 | 85,099 | 116,538 | 61,456 | 34,358 | 49,815 | 30,212 |
| Girls | 316,255 | 15,059 | 62,126 | 96,798 | 50,740 | 26,248 | 40,514 | 24,740 |
| Seasons | ||||||||
| Warm | 334,766 | 66,075 | 102,548 | 41,269 | 29,095 | 52,887 | 16,566 | 26,326 |
| Cold | 378,822 | 81,150 | 110,788 | 49,060 | 31,511 | 59,309 | 18,378 | 28,626 |
| Total | 713,588 | 147,225 | 213,336 | 90,329 | 60,606 | 112,196 | 34,944 | 54,952 |
| Category | Total | Season | Cities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Warm | Cold | Seoul | Busan | Daegu | Incheon | Gwangju | Daejeon | Ulsan | ||
| PM2.5 (μg/m3) | 29.0 (7.6) | 24.2 (13.4) | 30.1 (17.2) | 30.6 (8.3) | 25.3 (6.1) | 26.4 (6.9) | 29.1 (8.3) | 24.8 (7.9) | 28.6 (9.2) | 25.6 (6.2) |
| PM10 (μg/m3) | 46.9 (23.7) | 43.1 (23.9) | 50.6 (28.5) | 47.7 (29.4) | 47.3 (24.0) | 47.0 (25.7) | 52.6 (28.9) | 43.0 (26.7) | 43.1 (24.8) | 47.4 (24.9) |
| SO2 (ppm) | 0.005 (0.002) | 0.005 (0.003) | 0.006 (0.002) | 0.005 (0.002) | 0.006 (0.002) | 0.004 (0.002) | 0.007 (0.002) | 0.004 (0.001) | 0.004 (0.002) | 0.008 (0.003) |
| NO2 (ppm) | 0.024 (0.009) | 0.021 (0.009) | 0.028 (0.011) | 0.033 (0.012) | 0.021 (0.007) | 0.023 (0.011) | 0.028 (0.012) | 0.020 (0.008) | 0.021 (0.009) | 0.023 (0.008) |
| CO (ppm) | 0.500 (0.163) | 0.419 (0.114) | 0.581 (0.221) | 0.544 (0.210) | 0.398 (0.108) | 0.481 (0.202) | 0.588 (0.218) | 0.501 (0.180) | 0.492 (0.212) | 0.494 (0.141) |
| O3 (ppm) | 0.025 (0.010) | 0.031 (0.011) | 0.019 (0.009) | 0.021 (0.011) | 0.028 (0.010) | 0.025 (0.012) | 0.024 (0.011) | 0.027 (0.012) | 0.024 (0.013) | 0.026 (0.010) |
| Mean apparent temperature (°C) | 13.9 (11.5) | 22.8 (7.7) | 4.9 (6.8) | 12.7 (11.9) | 15.2 (10.2) | 14.5 (11.2) | 12.7 (12.2) | 14.3 (11.9) | 13.1 (12.2) | 14.5 (10.7) |
| Mean temperature (°C) | 13.9 (9.7) | 21.4 (5.2) | 6.3 (6.9) | 12.8 (10.8) | 15.1 (8.3) | 14.7 (9.7) | 12.6 (10.1) | 14.4 (9.6) | 13.2 (10.2) | 14.4 (8.8) |
| Mean relative humidity (%) | 64.6 (16.7) | 69.7 (15.3) | 59.5 (16.4) | 60.2 (14.9) | 62.4 (18.3) | 57.8 (16.9) | 71.7 (16.2) | 67.5 (13.7) | 67.8 (14.4) | 64.9 (17.6) |
| Mean dew point (°C) | 6.5 (11.6) | 14.9 (7.3) | −1.9 (8.7) | 4.5 (12.1) | 7.2 (11.6) | 5.3 (11.8) | 7.1 (11.8) | 7.7 (10.7) | 6.6 (11.4) | 7.1 (11.6) |
| Cities | Single Pollutant (95% C.I) | Two-Pollutant (95% C.I) | ||
|---|---|---|---|---|
| PM2.5 | PM2.5 + SO2 | PM2.5 + NO2 | PM2.5 + O3 | |
| Seoul | 1.60 (1.00, 2.20) | 1.58 (0.80, 2.37) | 1.84 (1.09, 2.60) | 1.56 (0.96, 2.16) |
| Busan | 0.81 (0.17, 1.45) | −0.96 (−1.71, −0.19) | 0.31 (−0.48, 1.10) | 0.94 (0.28, 1.60) |
| Daegu | 1.64 (0.70, 2.58) | 1.60 (0.45, 2.77) | 1.15 (0.00. 2.32) | 1.55 (0.60, 2.50) |
| Incheon | 0.57 (−0.45, 1.61) | −0.98 (−2.26. 0.32) | 0.62 (−0.61, 1.87) | 0.45 (−0.59, 1.50) |
| Gwangju | 0.28 (−0.51, 1.08) | −0.09 (−1.04, 0.86) | 0.14 (−0.79, 1.08) | −0.01 (−0.84, 0.83) |
| Daejeon | 2.09 (0.83, 3.37) | 2.70 (1.09, 4.34) | 3.01 (1.55, 4.49) | 1.74 (0.44, 3.05) |
| Ulsan | 1.92 (0.71, 3.15) | 1.45 (0.00, 2.92) | 1.40 (−0.16, 2.99) | 1.91 (0.66, 3.17) |
| Total | 1.20 (0.71, 1.71) | 0.69 (−0.34, 1.74) | 1.14 (0.43, 1.85) | 1.11 (0.60, 1.64) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Han, C.; Lee, D.-W.; Jang, Y.; Choi, Y.-J.; Bae, H.J.; Kim, S.; Ha, E.; Hong, Y.-C.; Lim, Y.-H. Short-Term Exposure to Fine Particulate Matter and Hospitalizations for Acute Lower Respiratory Infection in Korean Children: A Time-Series Study in Seven Metropolitan Cities. Int. J. Environ. Res. Public Health 2021, 18, 144. https://doi.org/10.3390/ijerph18010144
Oh J, Han C, Lee D-W, Jang Y, Choi Y-J, Bae HJ, Kim S, Ha E, Hong Y-C, Lim Y-H. Short-Term Exposure to Fine Particulate Matter and Hospitalizations for Acute Lower Respiratory Infection in Korean Children: A Time-Series Study in Seven Metropolitan Cities. International Journal of Environmental Research and Public Health. 2021; 18(1):144. https://doi.org/10.3390/ijerph18010144
Chicago/Turabian StyleOh, Jongmin, Changwoo Han, Dong-Wook Lee, Yoonyoung Jang, Yoon-Jung Choi, Hyun Joo Bae, Soontae Kim, Eunhee Ha, Yun-Chul Hong, and Youn-Hee Lim. 2021. "Short-Term Exposure to Fine Particulate Matter and Hospitalizations for Acute Lower Respiratory Infection in Korean Children: A Time-Series Study in Seven Metropolitan Cities" International Journal of Environmental Research and Public Health 18, no. 1: 144. https://doi.org/10.3390/ijerph18010144
APA StyleOh, J., Han, C., Lee, D.-W., Jang, Y., Choi, Y.-J., Bae, H. J., Kim, S., Ha, E., Hong, Y.-C., & Lim, Y.-H. (2021). Short-Term Exposure to Fine Particulate Matter and Hospitalizations for Acute Lower Respiratory Infection in Korean Children: A Time-Series Study in Seven Metropolitan Cities. International Journal of Environmental Research and Public Health, 18(1), 144. https://doi.org/10.3390/ijerph18010144






