Iron Oxide Particles Alter Bacterial Uptake and the LPS-Induced Inflammatory Response in Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Particle Preparation
2.2. Cell Culture
2.3. Peripheral Blood Mononuclear Cell (PBMC) Isolation and Culture
2.4. Cell Exposure Trials
2.5. Cytotoxicity
2.6. Inflammatory Cytokine Production
2.7. Bacterial Isolates and Culture
2.8. Bacterial Exposure
2.9. Flow Cytometry
2.10. Statistical Analysis
3. Results
3.1. Effect of Silica and Iron Oxide PM on Inflammation
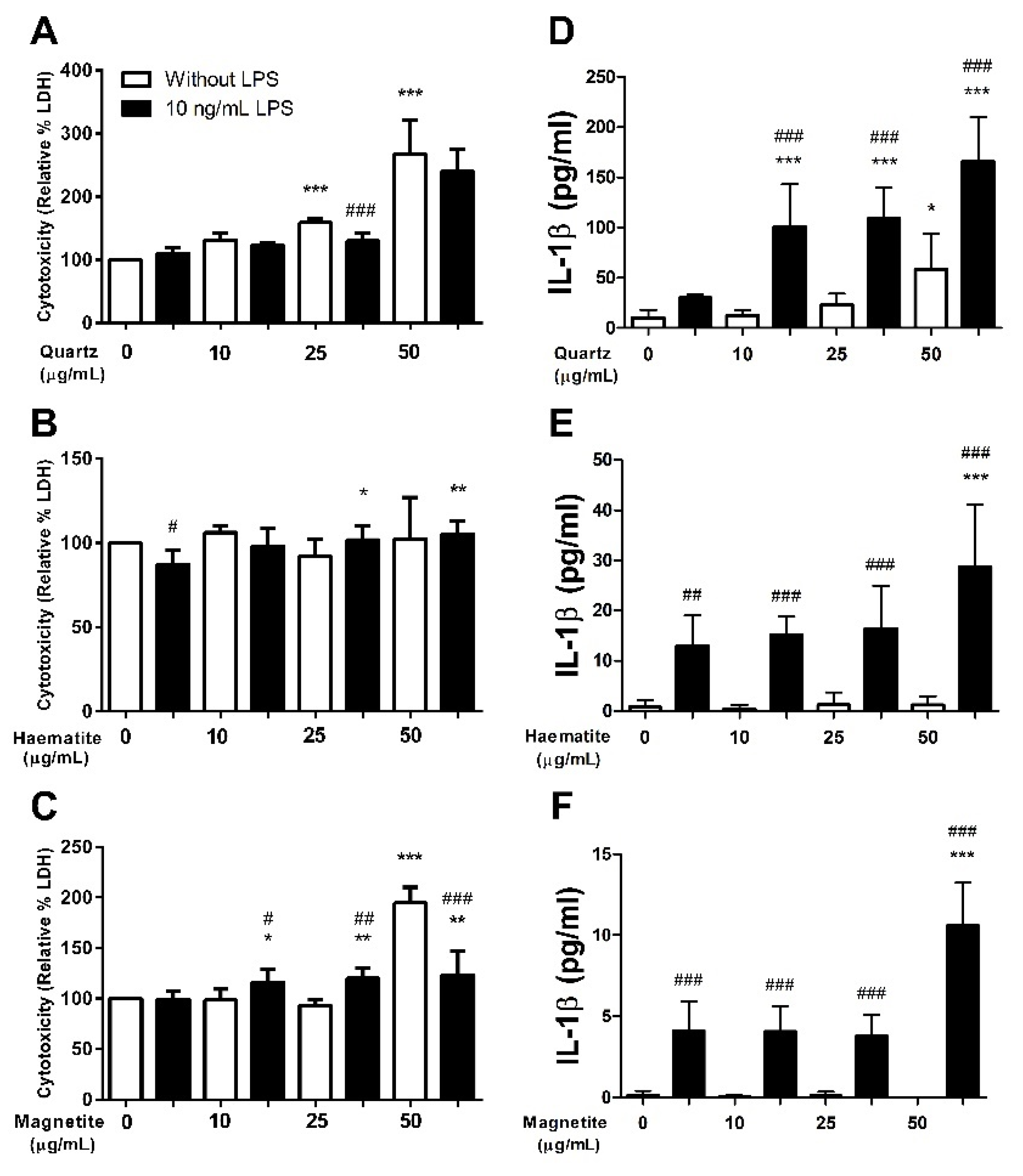
3.1.1. Cytotoxicity
3.1.2. Interleukin-1β
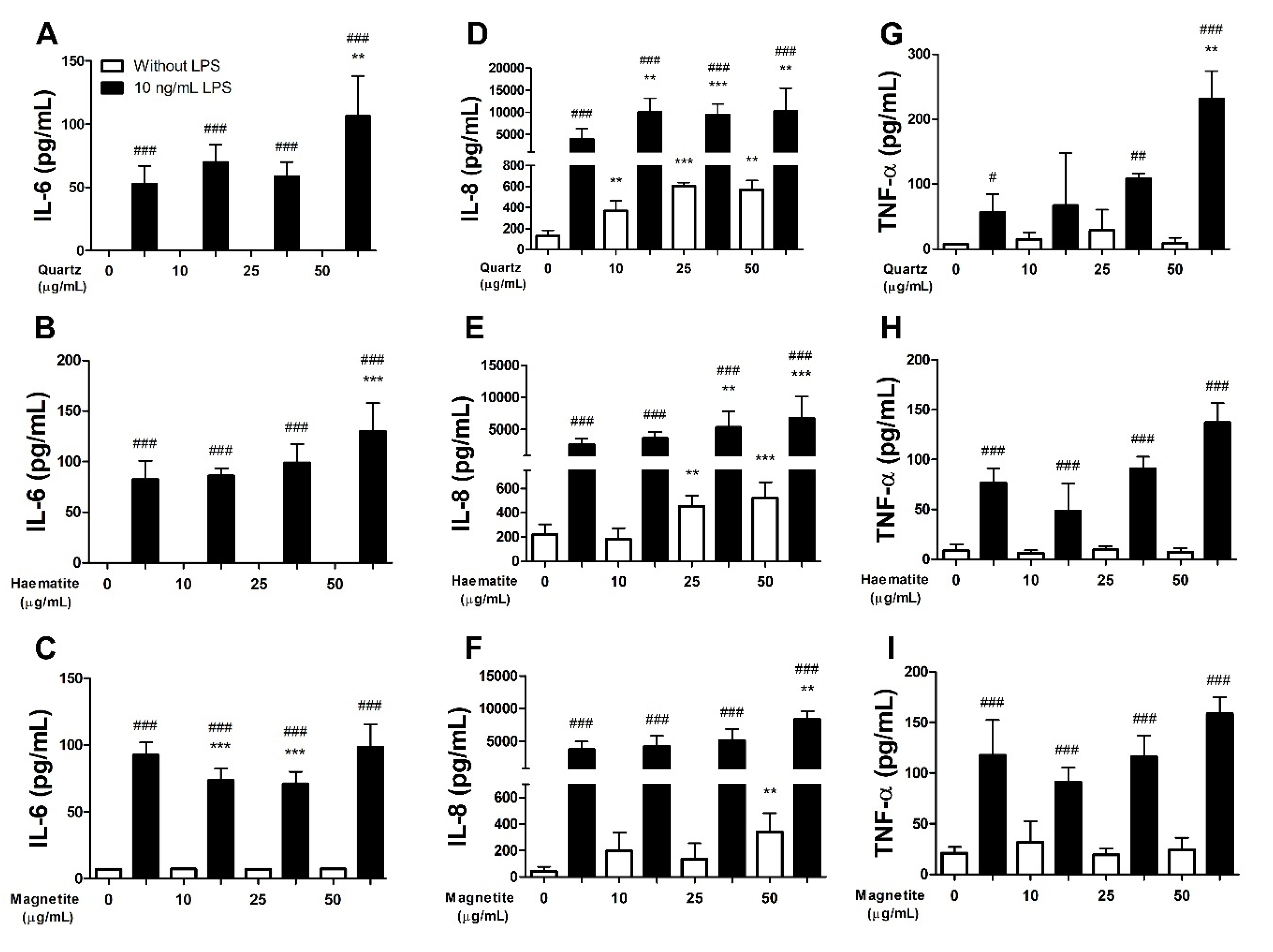
3.1.3. Interleukin-6
3.1.4. Interleukin-8
3.1.5. Tumour Necrosis Factor-α
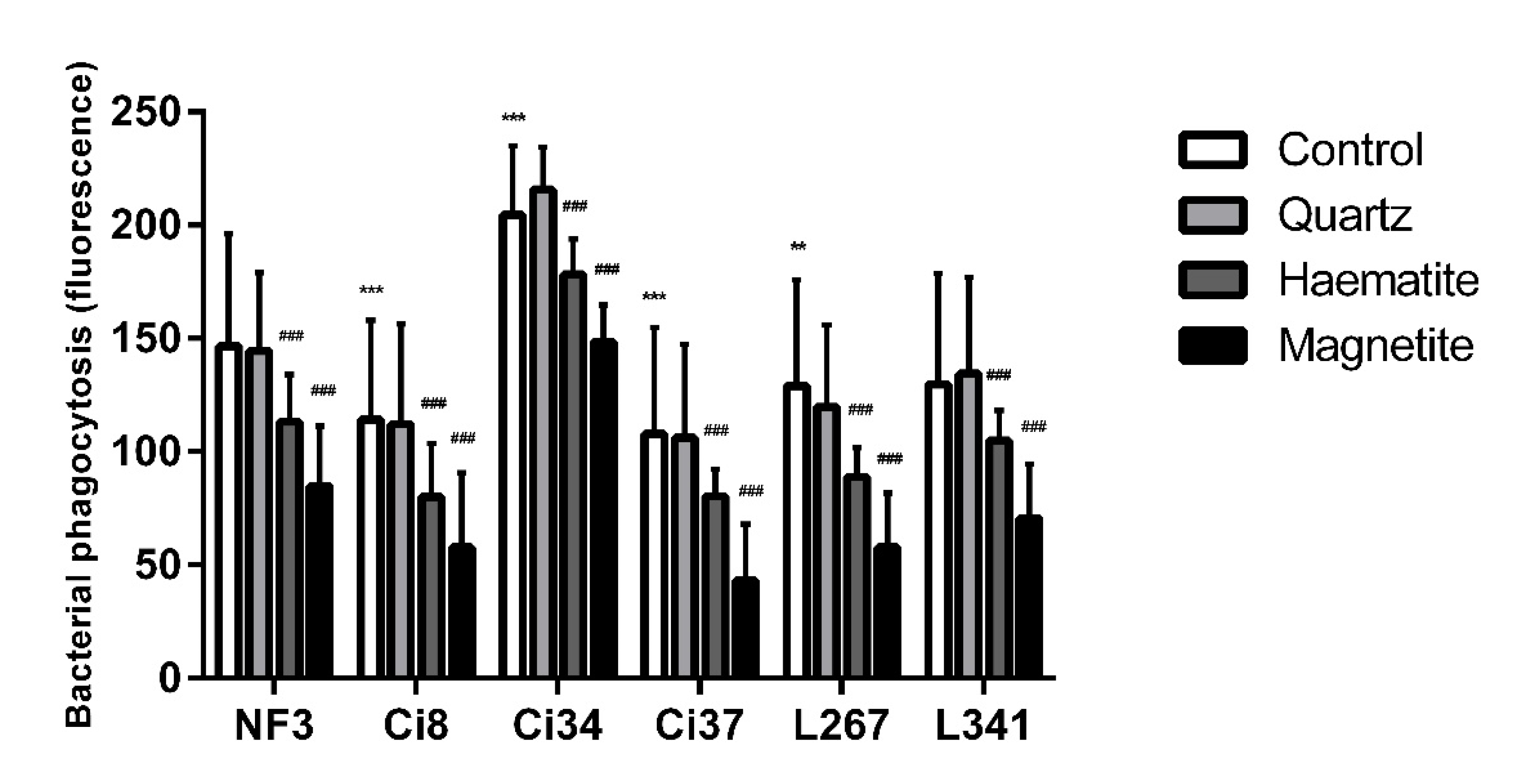
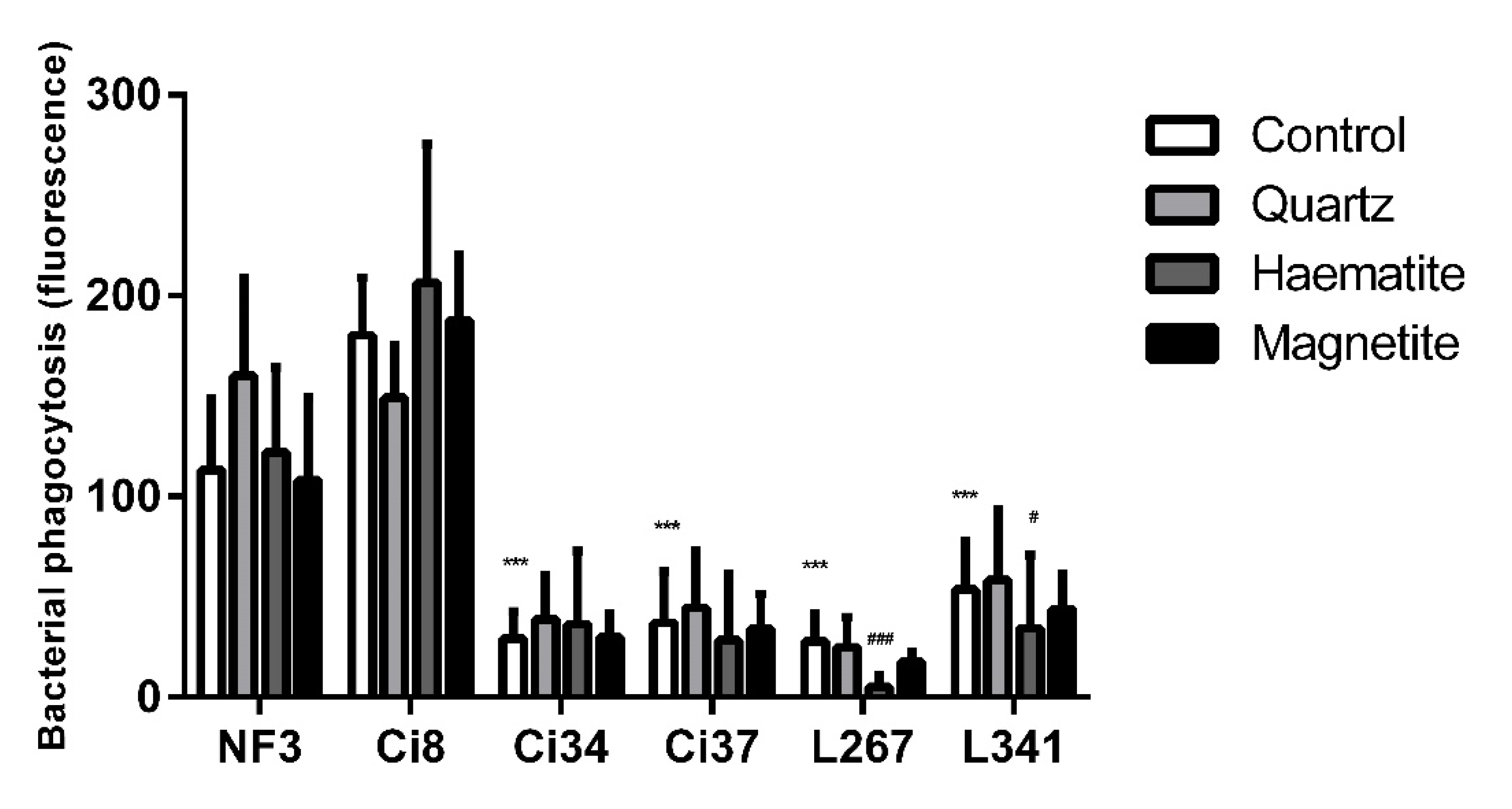
3.2. The Effect of Particles on NTHi Phagocytosis
3.3. Combined Effect of Quartz and Iron Oxide
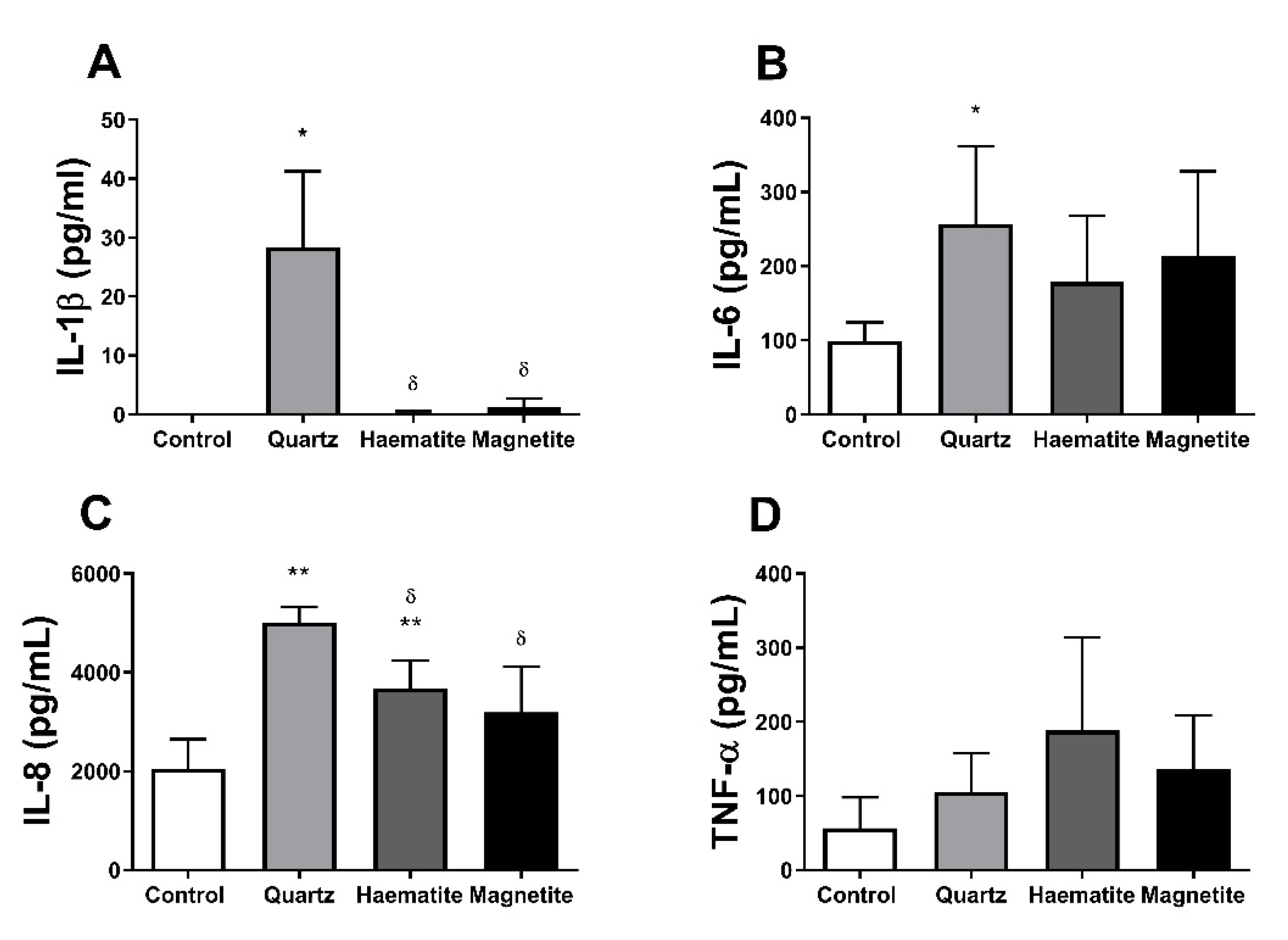
3.4. Responses in PBMCs
3.4.1. Cytotoxicity
3.4.2. Cytokine Production
3.4.3. NTHi Phagocytosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loebinger, M.R.; Wells, A.U.; Hansell, D.M.; Chinyanganya, N.; Devaraj, A.; Meister, M.; Wilson, R. Mortality in bronchiectasis: A long-term study assessing the factors influencing survival. Eur. Respir. J. 2009, 34, 843. [Google Scholar] [CrossRef] [PubMed]
- Blackall, S.R.; Hong, J.B.; King, P.; Wong, C.; Einsiedel, L.; Remond, M.G.W.; Woods, C.; Maguire, G.P. Bronchiectasis in indigenous and non-indigenous residents of Australia and New Zealand. Respirology 2018, 23, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Grimwood, K.; Mulholland, E.K.; Torzillo, P.J. Bronchiectasis in indigenous children in remote Australian communities. Med. J. Aust. 2002, 177, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Aboriginal and Torres Strait Islander Peoples: Smoking trends, Australia, 1994 to 2014-15; Australian Bureau of Statistics: Canberra, Australia, 2017.
- Melody, S.M.; Bennett, E.; Clifford, H.D.; Johnston, F.H.; Shepherd, C.C.J.; Alach, Z.; Lester, M.; Wood, L.J.; Franklin, P.; Zosky, G.R. A cross-sectional survey of environmental health in remote Aboriginal communities in Western Australia. Int. J. Environ. Health Res. 2016, 26, 525–535. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, K.A.; Torzillo, P.J.; Chang, A.B. Hospitalisation of Indigenous children in the Northern Territory for lower respiratory illness in the first year of life. Med. J. Aust. 2010, 192, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Clifford, H.; Pearson, G.; Franklin, P.; Walker, R.; Zosky, G. Environmental health challenges in remote Aboriginal Australian communities: Clean air, clean water and safe housing. Aust. Indig. Health Bull. 2015, 15, 1–13. [Google Scholar]
- Clifford, H.; Teo, T.; Kirkham, L.A.; Thornton, R.; Zosky, G.; Pickering, J. Dust exposure impacts haemophilus influenzae attachment and invasion of human airway epithelial cells. Eur. Respir. J. 2016, 48, PA4266. [Google Scholar]
- Shepherd, C.C.J.; Clifford, H.D.; Mitrou, F.; Melody, S.M.; Bennett, E.J.; Johnston, F.H.; Knibbs, L.D.; Pereira, G.; Pickering, J.L.; Teo, T.H.; et al. The contribution of geogenic particulate matter to lung disease in indigenous children. Int. J. Environ. Res. Public Health 2019, 16, 2636. [Google Scholar] [CrossRef]
- Goeminne, P.C.; Cox, B.; Finch, S.; Loebinger, M.R.; Bedi, P.; Hill, A.T.; Fardon, T.C.; de Hoogh, K.; Nawrot, T.S.; Chalmers, J.D. The impact of acute air pollution fluctuations on bronchiectasis pulmonary exacerbation: A case-crossover analysis. Eur. Respir. J. 2018, 52, 1702557. [Google Scholar] [CrossRef]
- Goeminne, P.C.; Bijnens, E.; Nemery, B.; Nawrot, T.S.; Dupont, L.J. Impact of traffic related air pollution indicators on non-cystic fibrosis bronchiectasis mortality: A cohort analysis. Respir. Res. 2014, 15, 108. [Google Scholar] [CrossRef]
- Pinto, E.H.; Longo, P.L.; Camargo, C.C.B.D.; Dal Corso, S.; Lanza, F.D.C.; Stelmach, R.; Athanazio, R.; Fernandes, K.P.S.; Mayer, M.P.A.; Bussadori, S.K.; et al. Assessment of the quantity of microorganisms associated with bronchiectasis in saliva, sputum and nasal lavage after periodontal treatment: A study protocol of a randomised controlled trial. Bmj Open 2016, 6, e010564. [Google Scholar] [CrossRef] [PubMed]
- Pizzutto, S.J.; Hare, K.M.; Upham, J.W. Bronchiectasis in children: Current concepts in immunology and microbiology. Front. Pediatrics 2017, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Carville, K.; Bowman, J.; Jacoby, P.; Riley, T.V.; Leach, A.J.; Lehmann, D. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatric Infect. Dis. J. 2006, 25, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Angrill, J.; Agustí, C.; de Celis, R.; Rañó, A.; Gonzalez, J.; Solé, T.; Xaubet, A.; Rodriguez-Roisin, R.; Torres, A. Bacterial colonisation in patients with bronchiectasis: Microbiological pattern and risk factors. Thorax 2002, 57, 15. [Google Scholar] [CrossRef] [PubMed]
- Clifford, H.D.; Perks, K.L.; Zosky, G.R. Geogenic PM10 exposure exacerbates responses to influenza infection. Sci. Total Environ. 2015, 533, 275–282. [Google Scholar] [CrossRef]
- Williams, L.J.; Tristram, S.G.; Zosky, G.R. Inorganic particulate matter modulates non-typeable Haemophilus influenzae growth: A link between chronic bacterial infection and geogenic particles. Environ. Geochem. Health 2019, 42, 2137–2145. [Google Scholar] [CrossRef]
- Geiser, M.; Casaulta, M.; Kupferschmid, B.; Schulz, H.; Semmler-Behnke, M.; Kreyling, W. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am. J. Respir. Cell Mol. Biol. 2008, 38, 371–376. [Google Scholar] [CrossRef]
- Lehnert, B.E. Pulmonary and thoracic macrophage subpopulations and clearance of particles from the lung. Environ. Health Perspect. 1992, 97, 17–46. [Google Scholar] [CrossRef]
- Marti-Lliteras, P.; Regueiro, V.; Morey, P.; Hood, D.W.; Saus, C.; Sauleda, J.; Agusti, A.G.; Bengoechea, J.A.; Garmendia, J. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect. Immun. 2009, 77, 4232–4242. [Google Scholar] [CrossRef]
- Singh, N.K.; Kunde, D.A.; Tristram, S.G. Effect of epithelial cell type on in vitro invasion of non-typeable Haemophilus influenzae. J. Microbiol. Methods 2016, 129, 66–69. [Google Scholar] [CrossRef]
- Munford, R.S. Sensing gram-negative bacterial lipopolysaccharides: A human disease determinant? Infect. Immun. 2008, 76, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Ovrevik, J.; Lag, M.; Schwarze, P.; Refsnes, M. p38 and Src-ERK1/2 pathways regulate crystalline silica-induced chemokine release in pulmonary epithelial cells. Toxicol. Sci. 2004, 81, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Jundi, K.; Greene, C.M. Transcription of interleukin-8: How altered regulation can affect cystic fibrosis lung disease. Biomolecules 2015, 5, 1386–1398. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Gómez, D.M.; Urcuqui-Inchima, S.; Hernandez, J.C. Silica nanoparticles induce NLRP3 inflammasome activation in human primary immune cells. Innate Immun. 2017, 23, 697–708. [Google Scholar] [CrossRef]
- Peeters, P.M.; Eurlings, I.M.J.; Perkins, T.N.; Wouters, E.F.; Schins, R.P.F.; Borm, P.J.A.; Drommer, W.; Reynaert, N.L.; Albrecht, C. Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Part. Fibre Toxicol. 2014, 11, 58. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sha, R.; Yang, L.; Zhao, X.; Zhu, Y.; Gao, J.; Zhang, Y.; Wen, L.-P. Impact of morphology on iron oxide nanoparticles-induced inflammasome activation in macrophages. ACS Appl. Mater. Interfaces 2018, 10, 41197–41206. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.J.; Zosky, G.R. The inflammatory effect of iron oxide and silica particles on lung epithelial cells. Lung 2019, 197, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Iosifidis, T.; Perks, K.; Ditcham, W.G.F.; Devadason, S.G.; Siah, W.S.; Devine, B.; Maley, F.; Cook, A. The concentration of iron in real-world geogenic PM10 is associated with increased inflammation and deficits in lung function in mice. PLoS ONE 2014, 9, e90609. [Google Scholar] [CrossRef] [PubMed]
- Andreakos, E.; Sacre, S.M.; Smith, C.; Lundberg, A.; Kiriakidis, S.; Stonehouse, T.; Monaco, C.; Feldmann, M.; Foxwell, B.M. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood 2004, 103, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.; Baldwin, A.S. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol. Cell. Biol. 1993, 13, 7191–7198. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, K.A. Regulation of interleukin-8 gene expression. J. Interferon Cytokine Res. 1999, 19, 429–438. [Google Scholar] [CrossRef]
- Hipp, M.S.; Urbich, C.; Mayer, P.; Wischhusen, J.; Weller, M.; Kracht, M.; Spyridopoulos, I. Proteasome inhibition leads to NF-kappaB-independent IL-8 transactivation in human endothelial cells through induction of AP-1. Eur. J. Immunol. 2002, 32, 2208–2217. [Google Scholar] [CrossRef]
- Bannach, F.G.; Gutierrez-Fernandez, A.; Parmer, R.J.; Miles, L.A. Interleukin-6-induced plasminogen gene expression in murine hepatocytes is mediated by transcription factor CCAAT/enhancer binding protein beta (C/EBPbeta). J. Thromb. Haemost. 2004, 2, 2205–2212. [Google Scholar] [CrossRef]
- Young, D.P.; Kushner, I.; Samols, D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. J. Immunol. 2008, 181, 2420–2427. [Google Scholar] [CrossRef]
- Hershko, D.D.; Robb, B.W.; Luo, G.; Hasselgren, P.O. Multiple transcription factors regulating the IL-6 gene are activated by cAMP in cultured Caco-2 cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R1140–R1148. [Google Scholar] [CrossRef]
- Hattori, T.; Ohoka, N.; Hayashi, H.; Onozaki, K. C/EBP homologous protein (CHOP) up-regulates IL-6 transcription by trapping negative regulating NF-IL6 isoform. FEBS Lett. 2003, 541, 33–39. [Google Scholar] [CrossRef]
- Hungness, E.S.; Luo, G.J.; Pritts, T.A.; Sun, X.; Robb, B.W.; Hershko, D.; Hasselgren, P.O. Transcription factors C/EBP-beta and -delta regulate IL-6 production in IL-1beta-stimulated human enterocytes. J. Cell. Physiol. 2002, 192, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hungness, E.S.; Pritts, T.A.; Luo, G.J.; Sun, X.; Penner, C.G.; Hasselgren, P.O. The transcription factor activator protein-1 is activated and interleukin-6 production is increased in interleukin-1beta-stimulated human enterocytes. Shock 2000, 14, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Tuyt, L.M.; Dokter, W.H.; Birkenkamp, K.; Koopmans, S.B.; Lummen, C.; Kruijer, W.; Vellenga, E. Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-kappa B-dependent IL-6 expression in human monocytes. J. Immunol. 1999, 162, 4893–4902. [Google Scholar] [PubMed]
- Miyazawa, K.; Mori, A.; Yamamoto, K.; Okudaira, H. Transcriptional roles of CCAAT/enhancer binding protein-beta, nuclear factor-kappaB, and C-promoter binding factor 1 in interleukin (IL)-1beta-induced IL-6 synthesis by human rheumatoid fibroblast-like synoviocytes. J. Biol. Chem. 1998, 273, 7620–7627. [Google Scholar] [CrossRef]
- Inoue, K.; Takano, H.; Yanagisawa, R.; Sakurai, M.; Shimada, A.; Morita, T.; Sato, M.; Yoshino, S.; Yoshikawa, T. Role of interleukin-6 in toll-like receptor 4 and 2 expressions induced by lipopolysaccharide in the lung. Immunopharmacol. Immunotoxicol. 2007, 29, 63–68. [Google Scholar] [CrossRef]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of silica particle size on macrophage inflammatory responses. PLoS ONE 2014, 9, e92634. [Google Scholar] [CrossRef]
- Knapp, S.; Leemans, J.C.; Florquin, S.; Branger, J.; Maris, N.A.; Pater, J.; van Rooijen, N.; van der Poll, T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 2003, 167, 171–179. [Google Scholar] [CrossRef]
- Kooguchi, K.; Hashimoto, S.; Kobayashi, A.; Kitamura, Y.; Kudoh, I.; Wiener-Kronish, J.; Sawa, T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect. Immun. 1998, 66, 3164–3169. [Google Scholar] [CrossRef]
- Hodge, S.; Upham, J.W.; Pizzutto, S.; Petsky, H.L.; Yerkovich, S.; Baines, K.J.; Gibson, P.; Simpson, J.L.; Buntain, H.; Chen, A.C.H.; et al. Is alveolar macrophage phagocytic dysfunction in children with protracted bacterial bronchitis a forerunner to bronchiectasis? Chest 2016, 149, 508–515. [Google Scholar] [CrossRef]
- Zosky, G.R.; Boylen, C.E.; Wong, R.S.; Smirk, M.N.; Gutierrez, L.; Woodward, R.C.; Siah, W.S.; Devine, B.; Maley, F.; Cook, A. Variability and consistency in lung inflammatory responses to particles with a geogenic origin. Respirology 2014, 19, 58–66. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, L.J.; Tristram, S.G.; Zosky, G.R. Iron Oxide Particles Alter Bacterial Uptake and the LPS-Induced Inflammatory Response in Macrophages. Int. J. Environ. Res. Public Health 2021, 18, 146. https://doi.org/10.3390/ijerph18010146
Williams LJ, Tristram SG, Zosky GR. Iron Oxide Particles Alter Bacterial Uptake and the LPS-Induced Inflammatory Response in Macrophages. International Journal of Environmental Research and Public Health. 2021; 18(1):146. https://doi.org/10.3390/ijerph18010146
Chicago/Turabian StyleWilliams, Lewis J., Stephen G. Tristram, and Graeme R. Zosky. 2021. "Iron Oxide Particles Alter Bacterial Uptake and the LPS-Induced Inflammatory Response in Macrophages" International Journal of Environmental Research and Public Health 18, no. 1: 146. https://doi.org/10.3390/ijerph18010146
APA StyleWilliams, L. J., Tristram, S. G., & Zosky, G. R. (2021). Iron Oxide Particles Alter Bacterial Uptake and the LPS-Induced Inflammatory Response in Macrophages. International Journal of Environmental Research and Public Health, 18(1), 146. https://doi.org/10.3390/ijerph18010146





